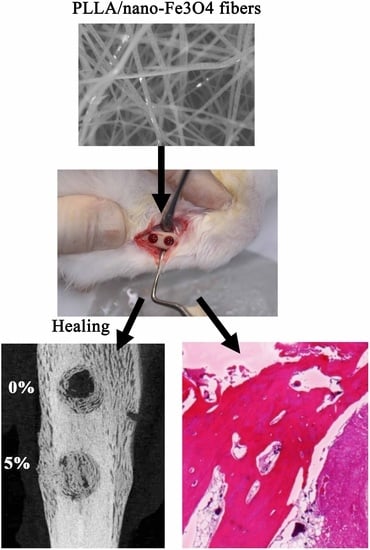
In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospun Nano-Fe3O4/PLLA Composites
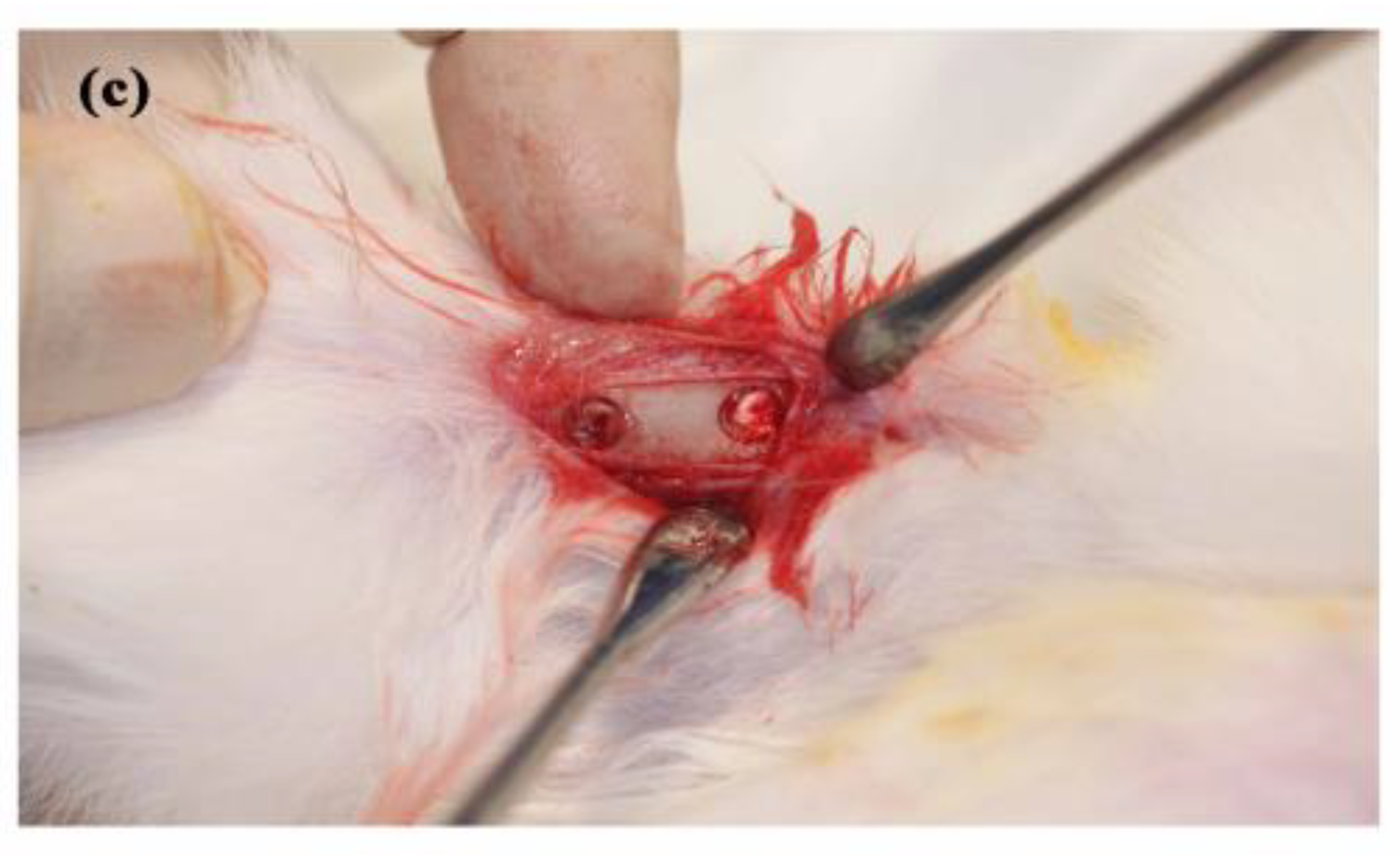
2.2. Animal Experiments
2.3. Micro-CT Measurements
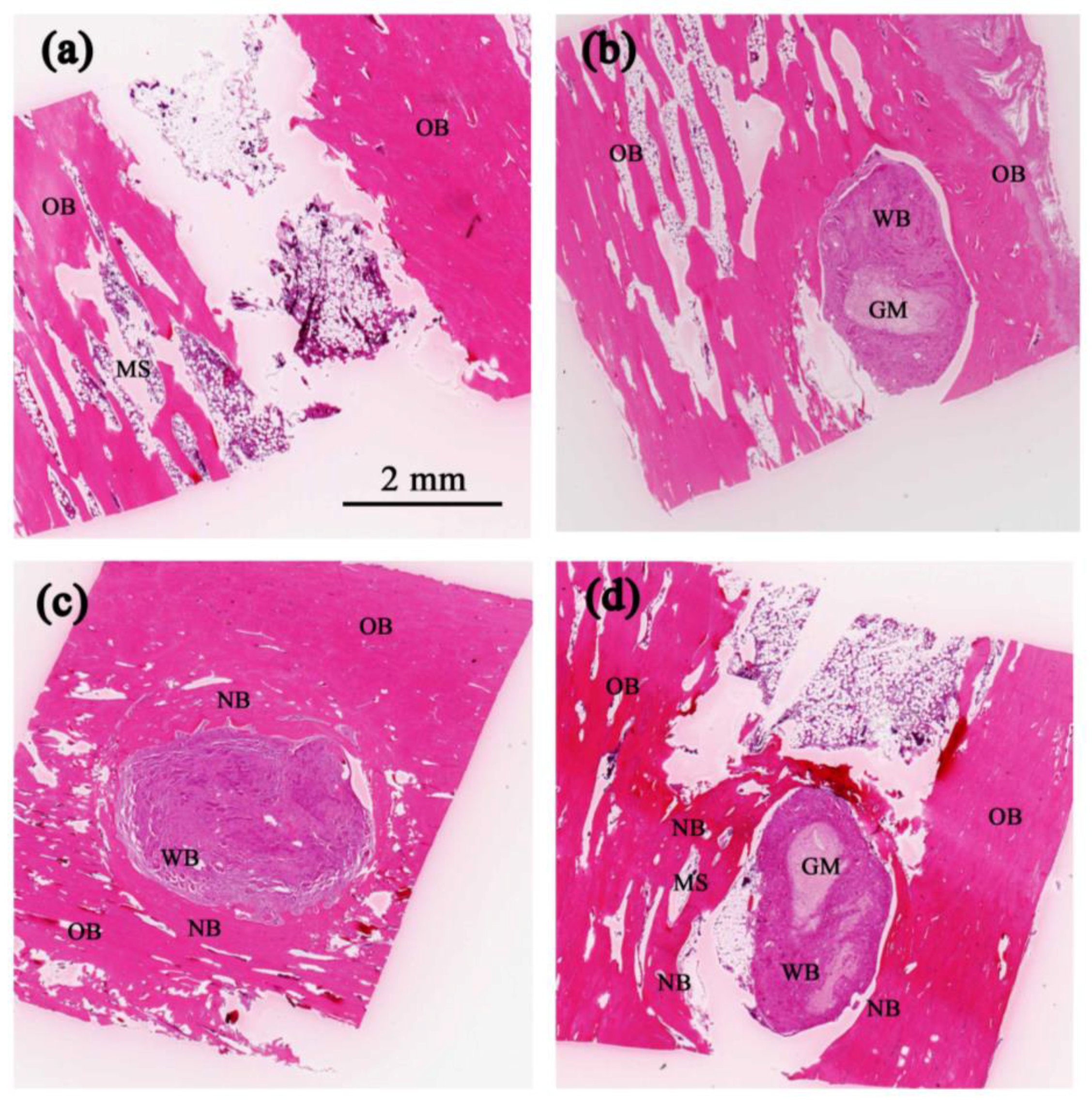
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Yeung, W.K.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, T.; Nerem, R.M. Bioengineered tissues: The science, the technology, and the industry. Orthod. Craniofac. Res. 2005, 8, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Lv, Y.G. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, C.; Farlie, P.; Penington, A.J. Bone Regeneration in a Rabbit Critical-Sized Skull Defect Using Autologous Adipose-Derived Cells. Tissue Eng. Part A 2008, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Pietrzak, W.S.; Blanton, M.W. Allograft and Alloplastic Bone Substitutes: A Review of Science and Technology for the Craniomaxillofacial Surgeon. J. Craniofac. Surg. 2005, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Dudas, J.R.; Marra, K.G.; Cooper, G.M.; Penascino, V.M.; Mooney, M.P.; Jiang, S.; Rubin, J.P.; Losee, J.E. The Osteogenic Potential of Adipose-Derived Stem Cells for the Repair of Rabbit Calvarial Defects. Ann. Plast. Surg. 2006, 56, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Haider, A.; Choi, Y.R.; Kang, I.K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- El-Amin, S.F.; Lu, H.H.; Khan, Y.; Burems, J.; Mitchell, J.; Tuan, R.S.; Laurencin, C.T. Extracellular matrix production by human osteoblasts cultured on biodegradable polymers applicable for tissue engineering. Biomaterials 2003, 24, 1213–1221. [Google Scholar] [CrossRef]
- Rücker, M.; Laschke, M.W.; Junker, D.; Carvalho, C.; Schramm, A.; Mülhaupt, R.; Gellrich, N.C.; Menger, M.D. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials 2006, 27, 5027–5038. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F. Biologic determinants of bone formation for osseointegration: Clues for future clinical improvements. J. Prosthet. Dent. 1998, 80, 439–449. [Google Scholar] [CrossRef]
- Wolf-Brandstetter, C.; Roessler, S.; Storch, S.; Hempel, U.; Gbureck, U.; Nies, B.; Bierbaum, S.; Scharnweber, D. Physicochemical and cell biological characterization of PMMA bone cements modified with additives to increase bioactivity. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.H.; Wang, X.L.; Zhang, G.; He, Y.X.; Leng, Y.; Tang, T.T.; Pan, X.; Qin, L. Biofabrication of a PLGA–TCP-based porous bioactive bone substitute with sustained release of icaritin. J. Tissue Eng. Regen. Med. 2015, 9, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Frohbergh, M.E.; Katsman, A.; Mondrinos, M.J.; Stabler, C.T.; Hankenson, K.D.; Oristaglio, J.T.; Lelkes, P.I. Osseointegrative Properties of Electrospun Hydroxyapatite-Containing Nanofibrous Chitosan Scaffolds. Tissue Eng. Part A 2015, 21, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Pinho, E.D.; Correlo, V.M.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. Biodegradable Nanofibers-Reinforced Microfibrous Composite Scaffolds for Bone Tissue Engineering. Tissue Eng. Part A 2010, 16, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing—Production, characterization, and in vivo behavior. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 Nanoparticles Role in Biomedical Applications. Biochem. Res. Int. 2016, 2016, 7840161. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.L.; Neoh, K.G.; Kang, E.T.; Shuter, B.; Wang, S.C. Solvent-free atom transfer radical polymerization for the preparation of poly(poly(ethyleneglycol) monomethacrylate)-grafted Fe3O4 nanoparticles: Synthesis, characterization and cellular uptake. Biomaterials 2007, 28, 5426–5436. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A novel calcium phosphate ceramic-magnetic nanoparticle composite as a potential bone substitute. Biomed. Mater. 2010, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, Y.; Qi, X.; Kong, H.; Wang, C.Y.; Xu, Z.; Xie, S.S.; Gu, N.; Xu, H.Y. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale 2010, 2, 2565–2569. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Shi, Y.; Duan, S.; Wei, Y.; Cai, Q.; Yang, X. Electrospun magnetic poly(l-lactide) (PLLA) nanofibers by incorporating PLLA-stabilized Fe3O4 nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3498–3505. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Chan, Y.H.; Feng, S.W.; Lo, Y.J.; Teng, N.C.; Huang, H.M. Development and biocompatibility tests of electrospun poly-l-lactide nanofibrous membranes incorporating oleic acid-coated Fe3O4. J. Polym. Eng. 2014, 34, 241–245. [Google Scholar] [CrossRef]
- Shen, L.K.; Fan, K.H.; Wu, T.L.; Huang, H.M.; Leung, T.K.; Chen, C.J.; Chang, W.J. Fabrication and magnetic testing of a poly-l-lactide biocomposite incorporating magnetite nanoparticles. J. Polym. Eng. 2014, 34, 237–240. [Google Scholar] [CrossRef]
- Zhang, D.; Karki, A.B.; Rutman, D.; Young, D.P.; Wang, A.; Cocke, D.; Ho, T.H.; Guo, Z. Electrospun polyacrylonitrile nanocomposite fibers reinforced with Fe3O4 nanoparticles: Fabrication and property analysis. Polymer 2009, 50, 4189–4198. [Google Scholar] [CrossRef]
- Pan, Y.H.; Wang, H.T.; Wu, T.L.; Fan, K.H.; Huang, H.M.; Chang, W.J. Fabrication of Fe3O4/PLLA composites for use in bone tissue engineering. Polym. Compos. 2017, 38, 2881–2888. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lo, Y.J.; Feng, S.W.; Huang, Y.C.; Tsai, H.Y.; Lin, C.T.; Fan, K.H.; Huang, H.M. Bone healing improvements using hyaluronic acid and hydroxyapatite/beta-tricalcium phosphate in combination: An animal study. Biomed. Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef] [PubMed]
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Kim, J.S.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and microstructural analysis of osteopromotive property of allogenic demineralized dentin matrix. Int. J. Oral Maxillofac. Implants 2013, 28, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Ho, K.N.; Chan, Y.H.; Chang, K.J.; Lai, W.Y.; Huang, H.M. Damping factor as a diagnostic parameter for assessment of osseointegration during the dental implant healing process: An experimental study in rabbits. Ann. Biomed. Eng. 2016, 44, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Albertini, G.; Giuliani, A.; Komlev, V.; Moroncini, F.; Pugnaloni, A.; Pennesi, G.; Belicchi, M.; Rubini, C.; Rustichelli, F.; Tasso, R.; et al. Organization of extracellular matrix fibers within polyglycolic acid-polylactic acid scaffolds analyzed using X-ray synchrotron-radiation phase-contrast micro computed tomography. Tissue Eng. Part C 2009, 15, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhan, Y.; Lei, Y.; Zhao, R.; Zhong, J.; Liu, X. Electrospun Magnetic Fibrillar Polyarylene Ether Nitriles Nanocomposites Reinforced with Fe-phthalocyanine/Fe3O4 Hybrid Microspheres. J. Appl. Polym. Sci. 2012, 123, 1732–1739. [Google Scholar] [CrossRef]
- Chang, W.J.; Pan, Y.H.; Tzeng, J.J.; Wu, T.L.; Fong, T.H.; Feng, S.W.; Huang, H.M. Development and testing of X-ray imaging-enhanced poly-l-lactide bone screws. PLoS ONE 2015, 10, e0140354. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.; Song, Y.; Han, B.; Hu, X.; Wang, X.; Lin, Y.; Deng, X. Magnetic biodegradable Fe3O4/CS/PVA nanofibrous membranes for bone regeneration. Biomed. Mater. 2011, 6, 055008. [Google Scholar] [CrossRef] [PubMed]
- Gai, G.; Wang, L.; Dong, X.; Xu, S. Electrospun Fe3O4/PVP//Tb(BA)3phen/PVP magnetic–photoluminescent bifunctional bistrand aligned composite nanofibers bundles. J. Mater. Sci. 2013, 48, 5140–5147. [Google Scholar] [CrossRef]
- Huang, H.M.; Cheng, K.Y.; Chen, C.F.; Ou, K.L.; Lin, C.T.; Lee, S.Y. Design and examination of a stability-detecting device for dental implants. Proc. Inst. Mech. Eng. H 2005, 219, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, Fracture and Bone Repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Malizos, K.N.; Papatheodorou, L.K. The healing potential of the periosteum molecular aspects. Injury 2005, 36, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Bielby, R.; Jones, E.; McGonagle, D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury 2007, 38, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, R.; Sankhla, S.; Gupta, A.; Changani, R.; Gagal, K. Percutaneous autologus bone marrow injection in the treatment of delayed or nonunion. Indian J. Orthop. 2007, 41, 67–71. [Google Scholar] [PubMed]
- Hernandez, C.J.; Majeska, R.J.; Schaffler, M.B. Osteocyte density in woven bone. Bone 2004, 35, 1095–1099. [Google Scholar] [CrossRef] [PubMed]






| Samples | Blank | Neat PLLA | 2% Fe3O4/PLLA | 5% Fe3O4/PLLA |
|---|---|---|---|---|
| 0% | 13% | 43% | 44% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, W.-Y.; Feng, S.-W.; Chan, Y.-H.; Chang, W.-J.; Wang, H.-T.; Huang, H.-M. In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications. Polymers 2018, 10, 804. https://doi.org/10.3390/polym10070804
Lai W-Y, Feng S-W, Chan Y-H, Chang W-J, Wang H-T, Huang H-M. In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications. Polymers. 2018; 10(7):804. https://doi.org/10.3390/polym10070804
Chicago/Turabian StyleLai, Wei-Yi, Sheng-Wei Feng, Ya-Hui Chan, Wei-Jen Chang, Hsin-Ta Wang, and Haw-Ming Huang. 2018. "In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications" Polymers 10, no. 7: 804. https://doi.org/10.3390/polym10070804
APA StyleLai, W.-Y., Feng, S.-W., Chan, Y.-H., Chang, W.-J., Wang, H.-T., & Huang, H.-M. (2018). In Vivo Investigation into Effectiveness of Fe3O4/PLLA Nanofibers for Bone Tissue Engineering Applications. Polymers, 10(7), 804. https://doi.org/10.3390/polym10070804