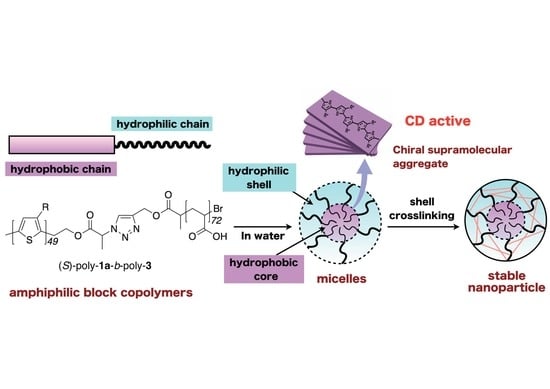
Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Synthesis
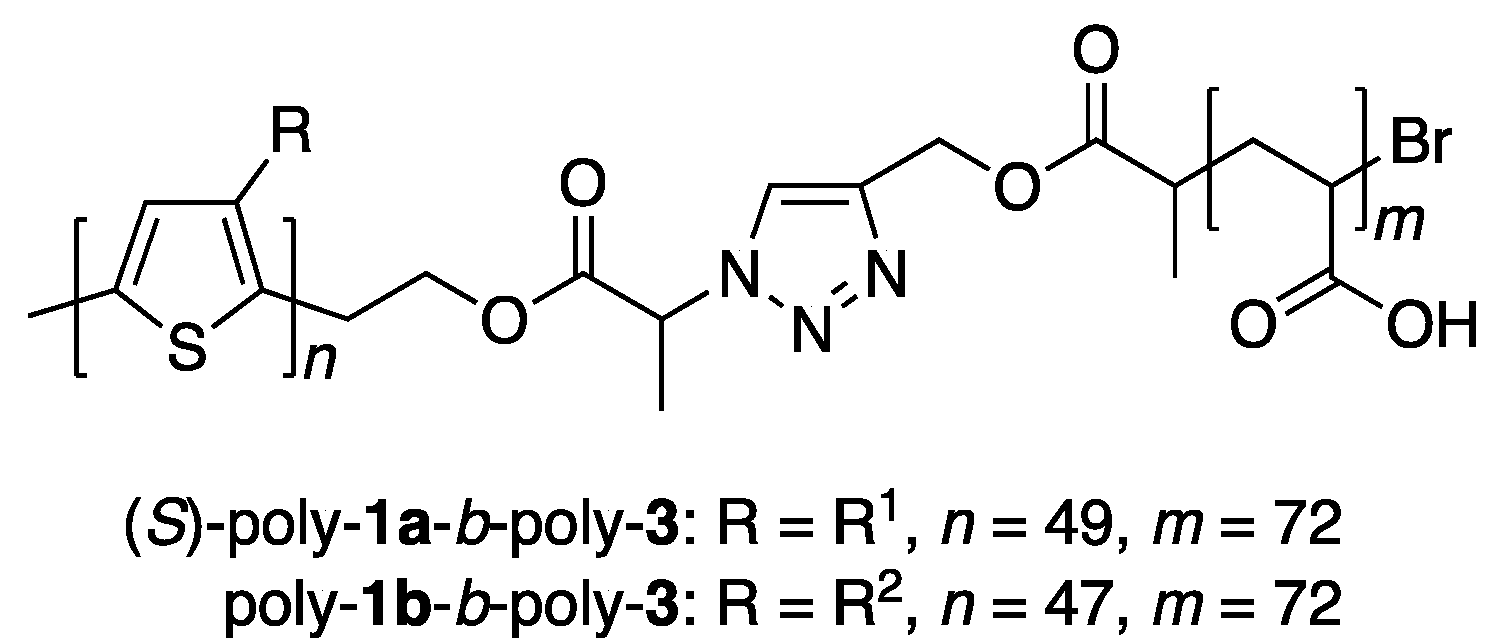
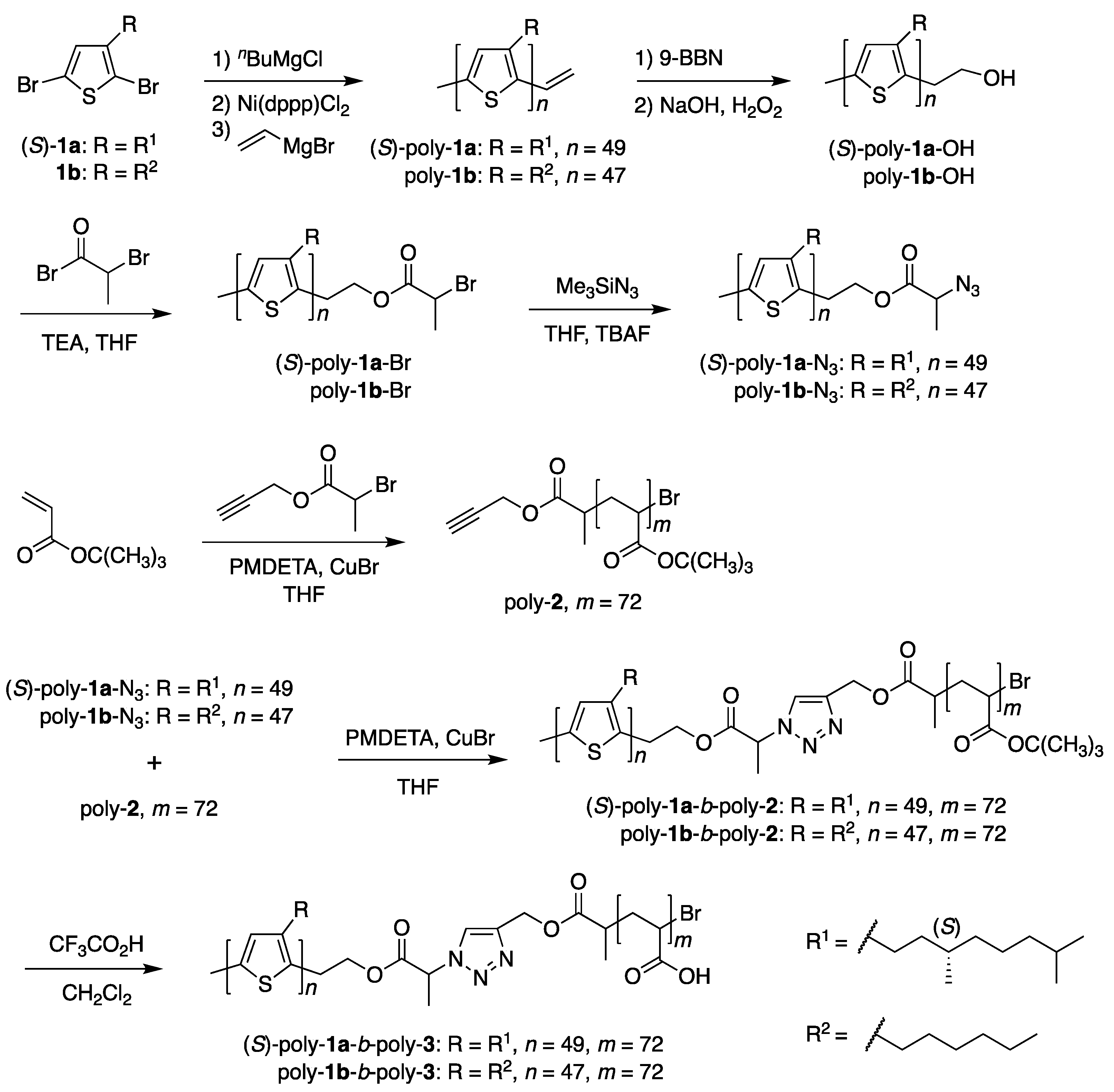
2.3.1. Synthesis of Amphiphilic Block Copolymers
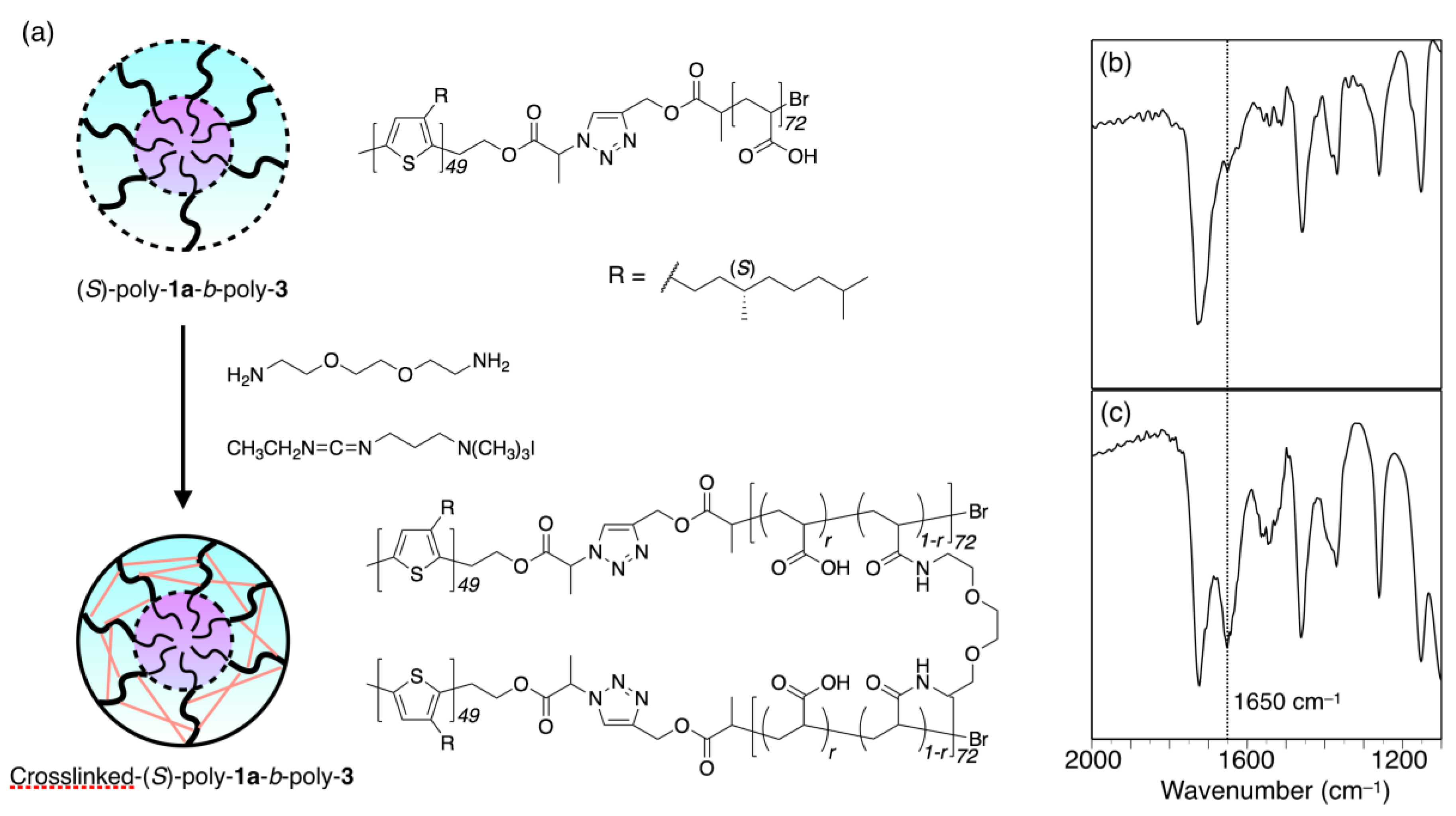
2.3.2. Crosslinking of (S)-Poly-1a-b-poly-3 Micelles
3. Results and Discussion
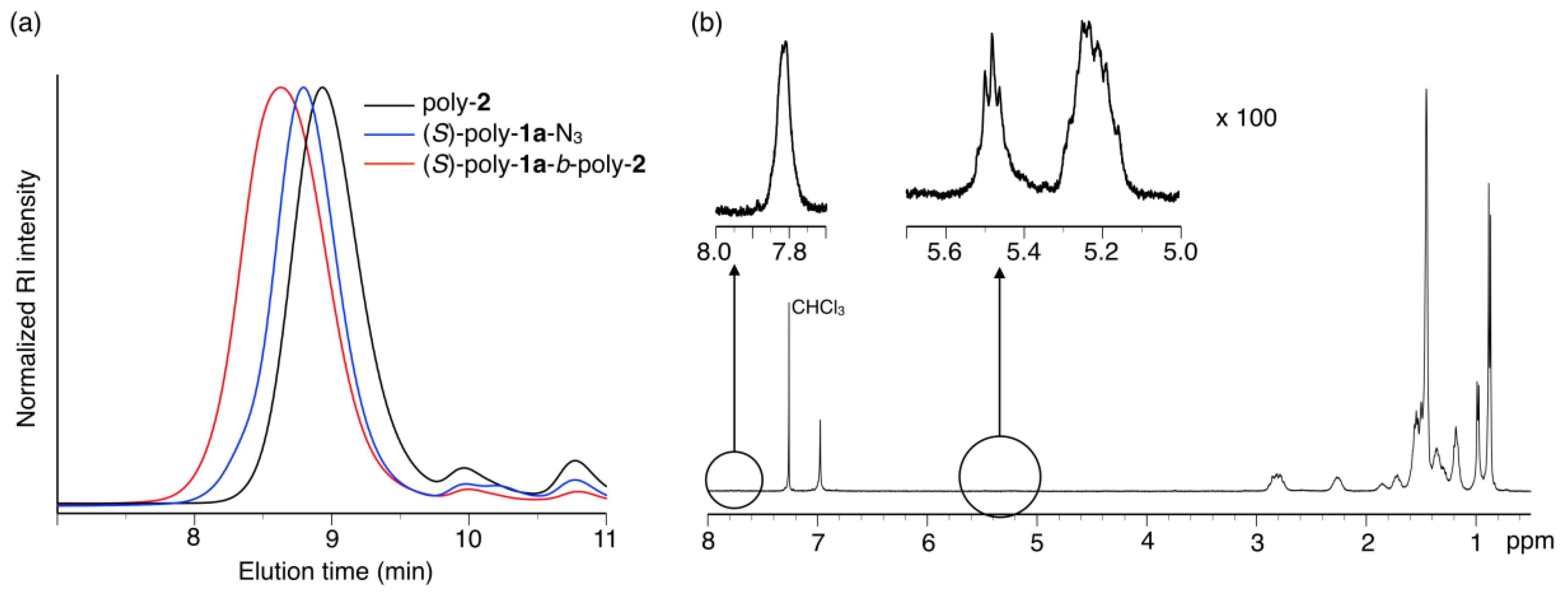
3.1. Synthesis of Amphiphilic Block Copolymers
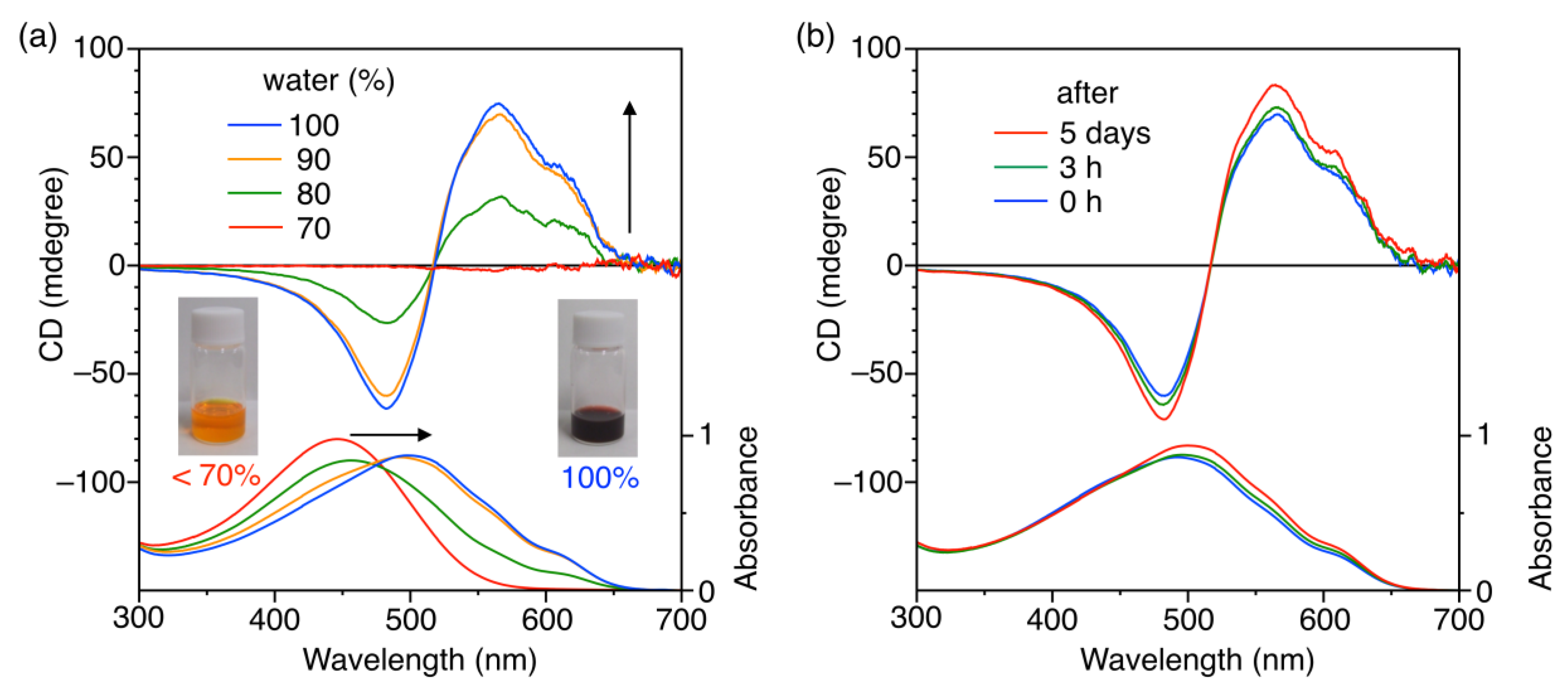
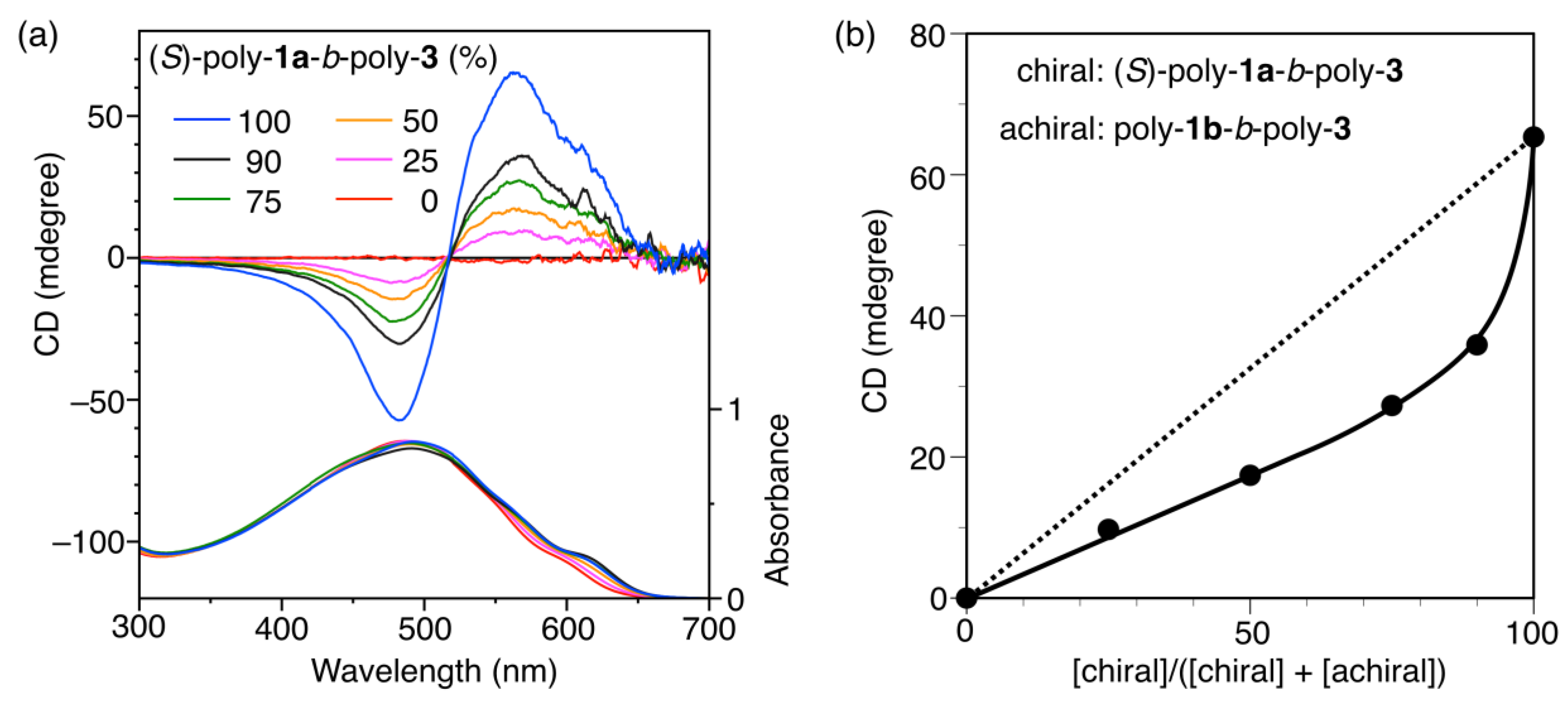
3.2. Chiroptical Properties
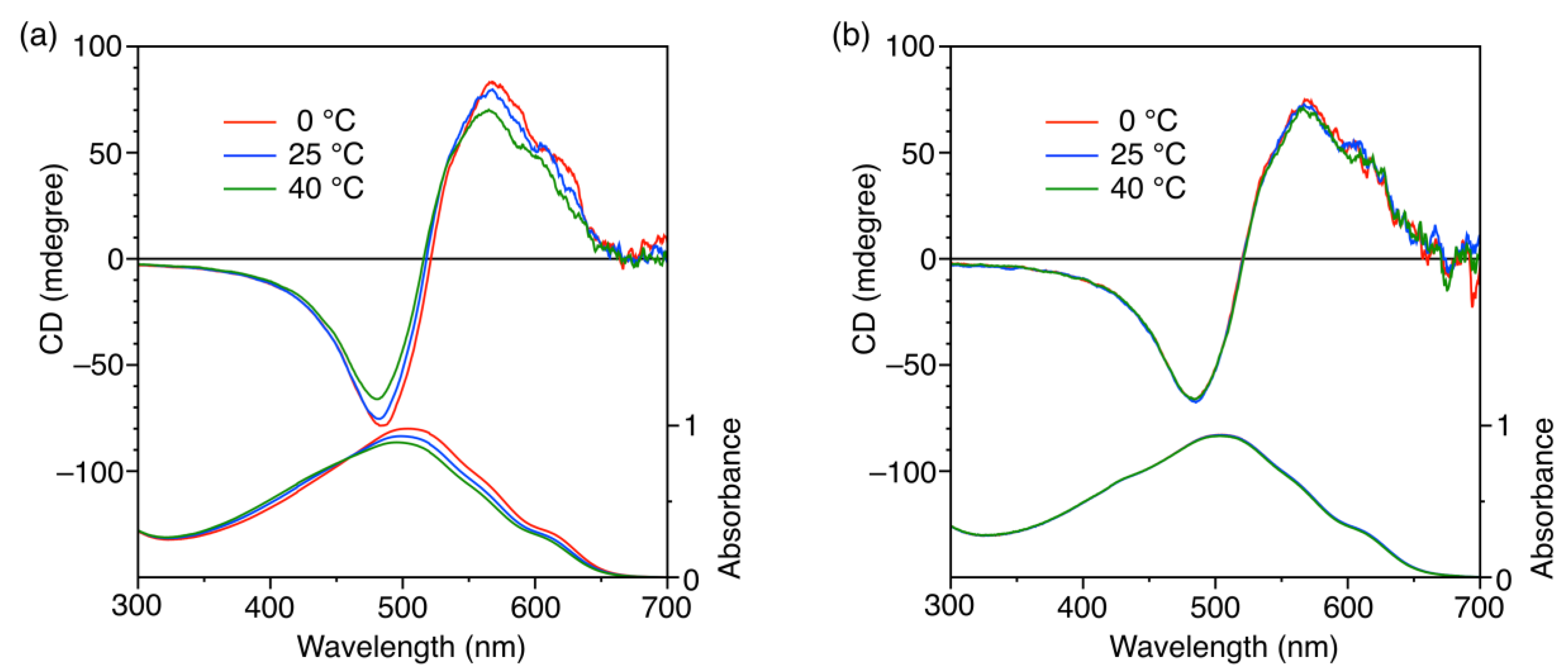
3.3. Micelle Stabilization through Crosslinking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Langeveld-Voss, B.M.W.; Janssen, R.A.J.; Meijer, E.W. On the origin of optical activity in polythiophenes. J. Mol. Struct. 2000, 521, 285–301. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning, A.P.H.J. About supramolecular assemblies of pi-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Yashima, E.; Okamoto, Y. Unusual solvent effects on chiroptical properties of an optically active regioregular polythiophene in solution. Chirality 2000, 12, 396–399. [Google Scholar] [CrossRef]
- Goto, H.; Yashima, E. Electron-induced switching of the supramolecular chirality of optically active polythiophene aggregates. J. Am. Chem. Soc. 2002, 124, 7943–7949. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Okamoto, Y.; Yashima, E. Solvent-induced chiroptical changes in supramolecular assemblies of an optically active, regioregular polythiophene. Macromolecules 2002, 35, 4590–4601. [Google Scholar] [CrossRef]
- Leysen, P.; Teyssandier, J.; De Feyter, S.; Koeckelberghs, G. Controlled Synthesis of a Helical Conjugated Polythiophene. Macromolecules 2018, 51, 3504–3514. [Google Scholar] [CrossRef]
- Liu, Y.L.; Pauloehrl, T.; Presolski, S.I.; Albertazzi, L.; Palmans, A.R.A.; Meijer, E.W. Modular Synthetic Platform for the Construction of Functional Single-Chain Polymeric Nanoparticles: From Aqueous Catalysis to Photosensitization. J. Am. Chem. Soc. 2015, 137, 13096–13105. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Heo, G.S.; Lim, S.M.; Sun, G.R.; Wooley, K.L. Polymeric Nanostructures for Imaging and Therapy. Chem. Rev. 2015, 115, 10967–11011. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, R.K.; Joralemon, M.J.; Wooley, K.L.; Hawker, C.J. Functionalization of micelles and shell cross-linked nanoparticles using click chemistry. Chem. Mater. 2005, 17, 5976–5988. [Google Scholar] [CrossRef]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef] [PubMed]
- Gois, J.R.; Popov, A.V.; Guliashvili, T.; Serra, A.C.; Coelho, J.F.J. Synthesis of functionalized poly(vinyl acetate) mediated by alkyne-terminated RAFT agents. RSC Adv. 2015, 5, 91225–91234. [Google Scholar] [CrossRef]
- Vandeleene, S.; Jivanescu, M.; Stesmans, A.; Cuppens, J.; Van Bael, M.J.; Verbiest, T.; Koeckelberghs, G. Influence of the Supramolecular Organization on the Magnetic Properties of Poly(3-alkylthiophene)s in Their Neutral State. Macromolecules 2011, 44, 4911–4919. [Google Scholar] [CrossRef]
- Hammer, B.A.G.; Bokel, F.A.; Hayward, R.C.; Emrick, T. Cross-Linked Conjugated Polymer Fibrils: Robust Nanowires from Functional Polythiophene Diblock Copolymers. Chem. Mater. 2011, 23, 4250–4256. [Google Scholar] [CrossRef]
- Iovu, M.C.; Jeffries-El, M.; Sheina, E.E.; Cooper, J.R.; McCullough, R.D. Regioregular poly(3-alkylthiophene) conducting block copolymers. Polymer 2005, 46, 8582–8586. [Google Scholar] [CrossRef]
- Verheyen, L.; Leysen, P.; Van den Eede, M.P.; Ceunen, W.; Hardeman, T.; Koeckelberghs, G. Advances in the controlled polymerization of conjugated polymers. Polymer 2017, 108, 521–546. [Google Scholar] [CrossRef]
- Van Camp, W.; Germonpre, V.; Mespouille, L.; Dubois, P.; Goethals, E.J.; Du Prez, F.E. New poly(acrylic acid) containing segmented copolymer structures by combination of “Click” chemistry and atom transfer radical polymerization. React. Funct. Polym. 2007, 67, 1168–1180. [Google Scholar] [CrossRef]
- Craley, C.R.; Zhang, R.; Kowalewski, T.; McCullough, R.D.; Stefan, M.C. Regioregular Poly(3-hexylthiophene) in a Novel Conducting Amphiphilic Block Copolymer. Macromol. Rapid Commun. 2009, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Jeffries-El, M.; Sauve, G.; McCullough, R.D. Facile synthesis of end-functionalized regioregular poly(3-alkylthiophene)s via modified Grignard metathesis reaction. Macromolecules 2005, 38, 10346–10352. [Google Scholar] [CrossRef]
- Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. Catalyst-transfer polycondensation. Mechanism of Ni-catalyzed chain-growth polymerization leading to well-defined poly(3-hexylthiophene). J. Am. Chem. Soc. 2005, 127, 17542–17547. [Google Scholar] [CrossRef] [PubMed]
- Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689–3746. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. Cycloadditions—Definition, Classification, and Characterization. Angew.Chem. Int. Ed. Engl. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Kolb Hartmuth, C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Binder Wolfgang, H.; Sachsenhofer, R. ‘Click’ Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Satyanarayama, T.; Abraham, S.; Kagan, H.B. Nonlinear Effects in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2009, 48, 456–494. [Google Scholar] [CrossRef] [PubMed]
- Langeveld-Voss, B.M.W.; Waterval, R.J.M.; Janssen, R.A.J.; Meijer, E.W. Principles of “majority rules” and “sergeants and soldiers” applied to the aggregation of optically active polythiophenes: Evidence for a multichain phenomenon. Macromolecules 1999, 32, 227–230. [Google Scholar] [CrossRef]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Qi, K.; Khoshdel, E.; Wooley, K.L. Tandem synthesis of core-shell brush copolymers and their transformation to peripherally cross-linked and hollowed nanostructures. J. Am. Chem. Soc. 2006, 128, 6808–6809. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Kowalewski, T.; Remsen, E.E.; Gertzmann, R.; Wooley, K.L. Hydrogel-coated glassy nanospheres: A novel method for the synthesis of shell cross-linked knedels. J. Am. Chem. Soc. 1997, 119, 11653–11659. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirose, D.; Nozaki, S.; Kanoh, S.; Maeda, K. Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties. Polymers 2018, 10, 718. https://doi.org/10.3390/polym10070718
Hirose D, Nozaki S, Kanoh S, Maeda K. Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties. Polymers. 2018; 10(7):718. https://doi.org/10.3390/polym10070718
Chicago/Turabian StyleHirose, Daisuke, Satoru Nozaki, Shigeyoshi Kanoh, and Katsuhiro Maeda. 2018. "Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties" Polymers 10, no. 7: 718. https://doi.org/10.3390/polym10070718
APA StyleHirose, D., Nozaki, S., Kanoh, S., & Maeda, K. (2018). Synthesis of Amphiphilic Block Copolymers Containing Chiral Polythiophene Chains and Their Micelle Formation and Chiroptical Properties. Polymers, 10(7), 718. https://doi.org/10.3390/polym10070718