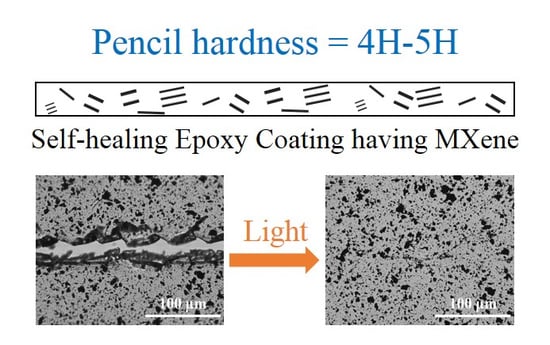
Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. MXene Preparation and Delamination
2.3. Coating Preparation
2.4. Crack Formation
2.5. Characterizations
3. Results and Discussion
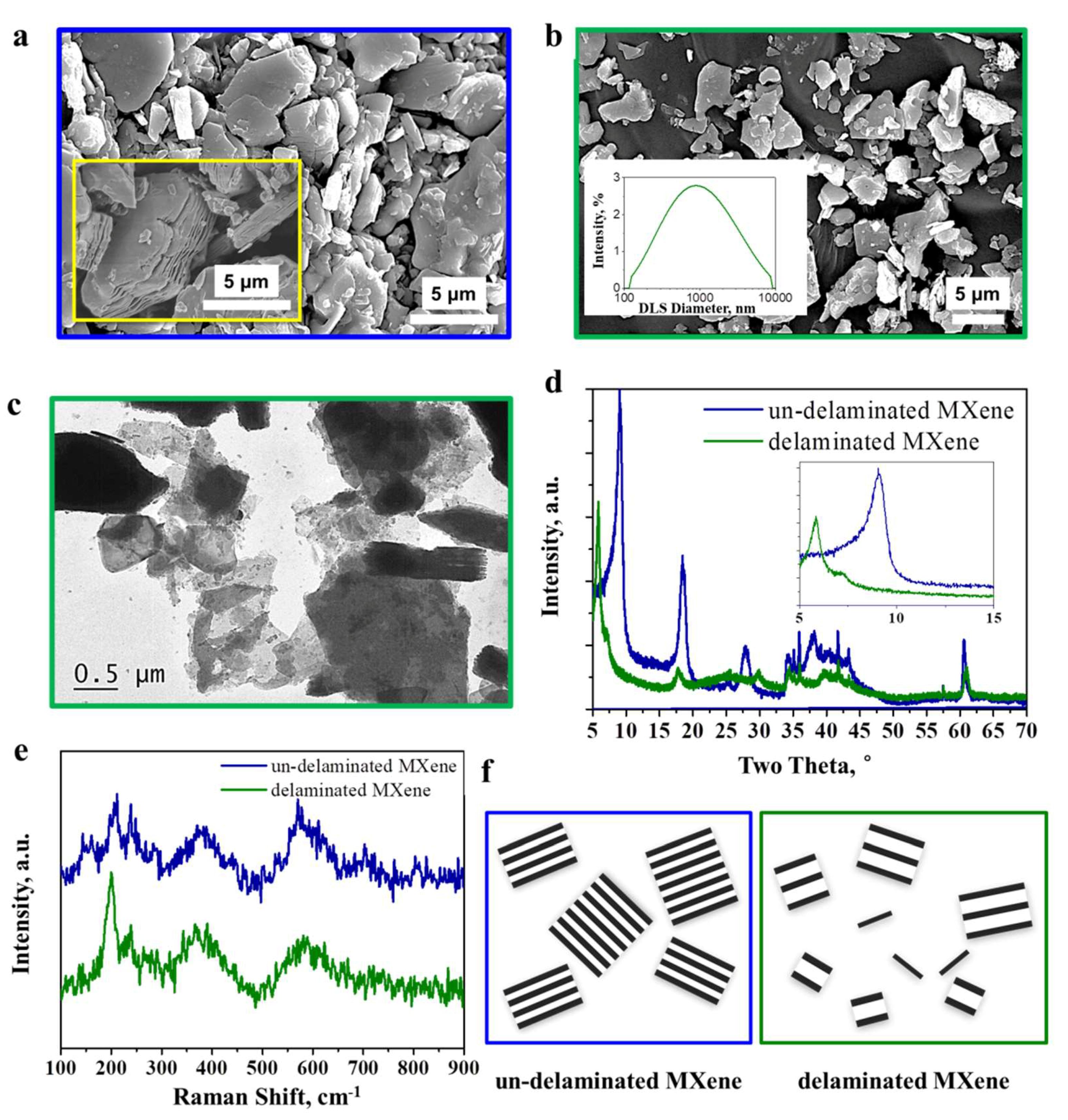
3.1. Structures and Morphologies of Delaminated MXene
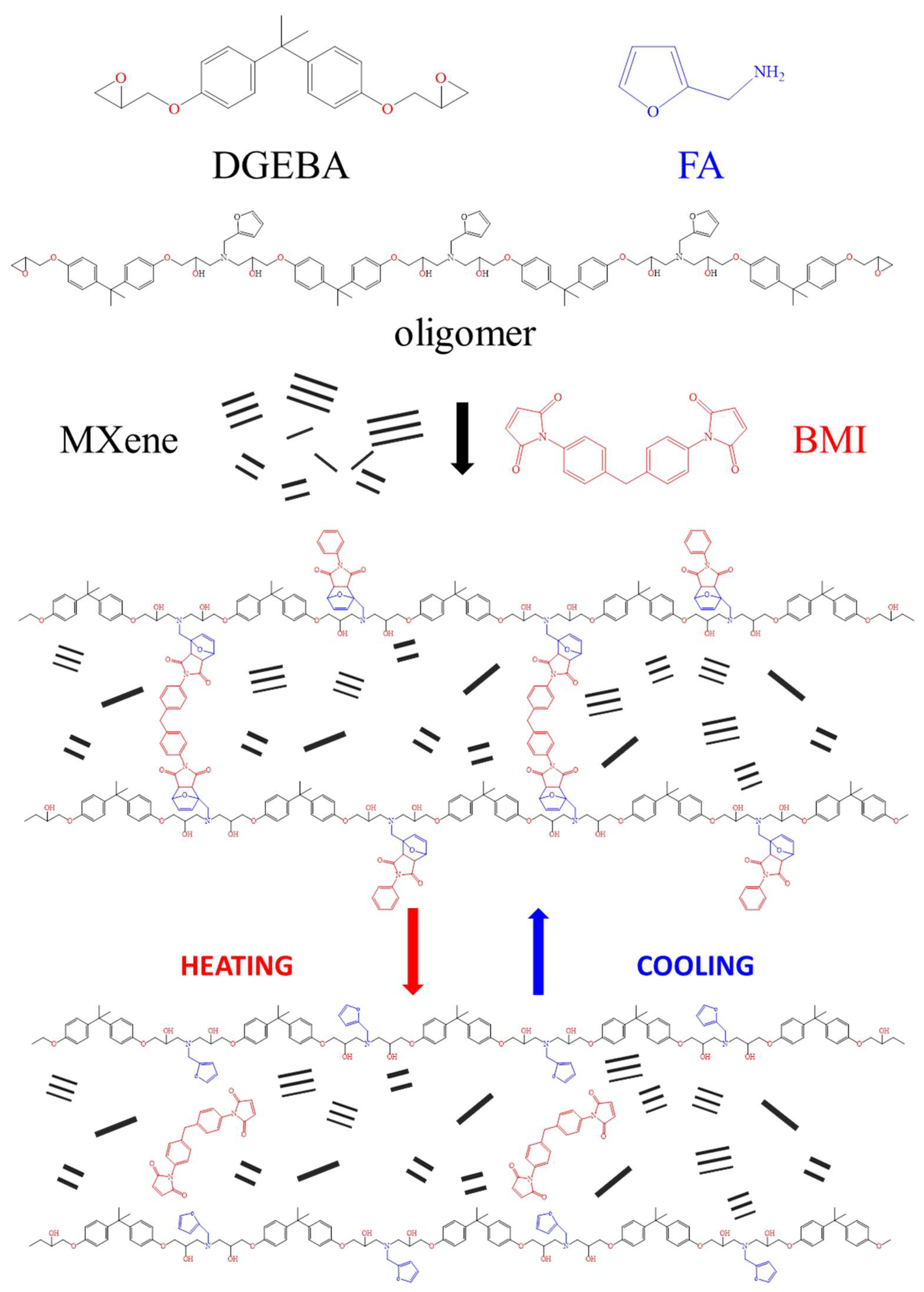
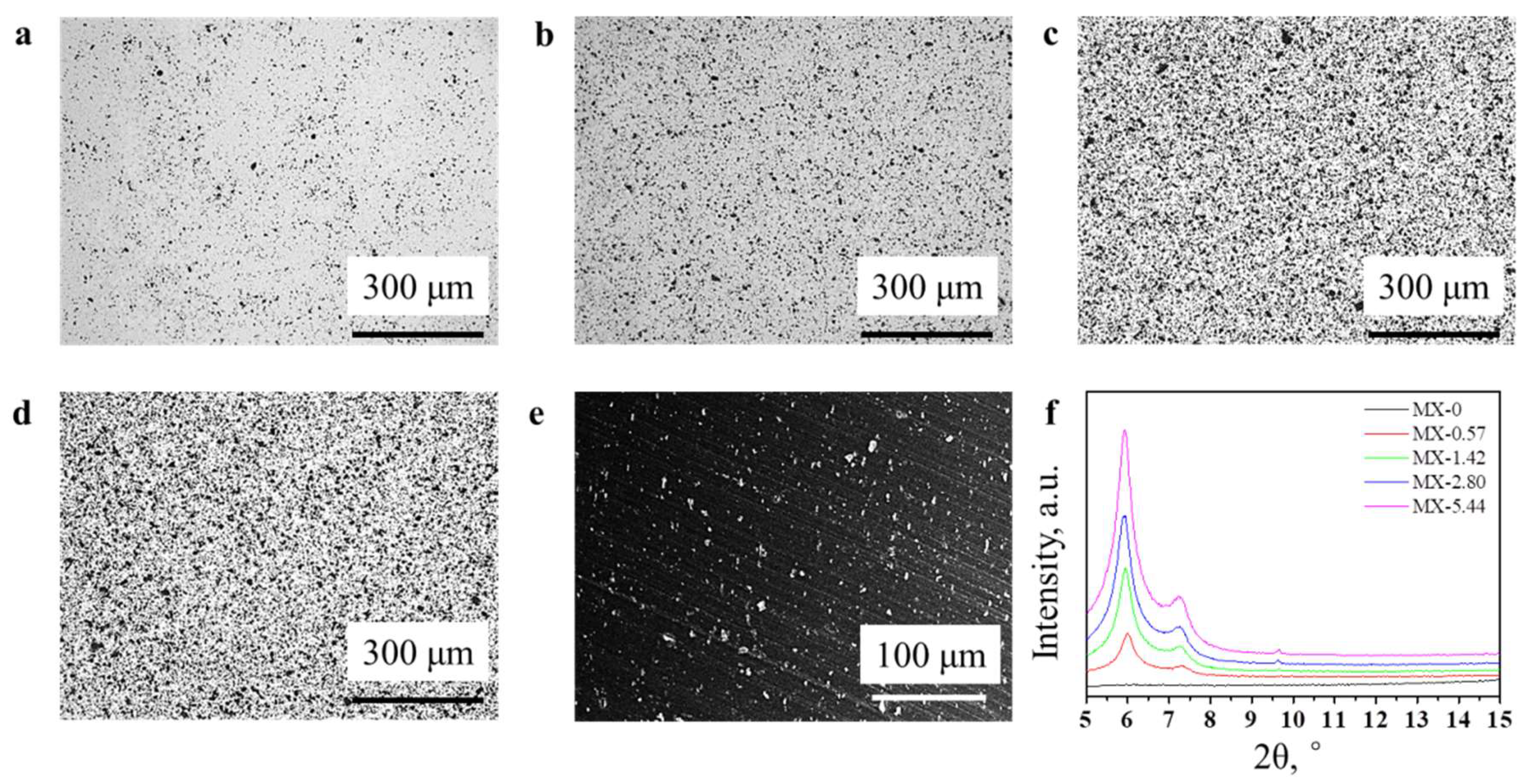
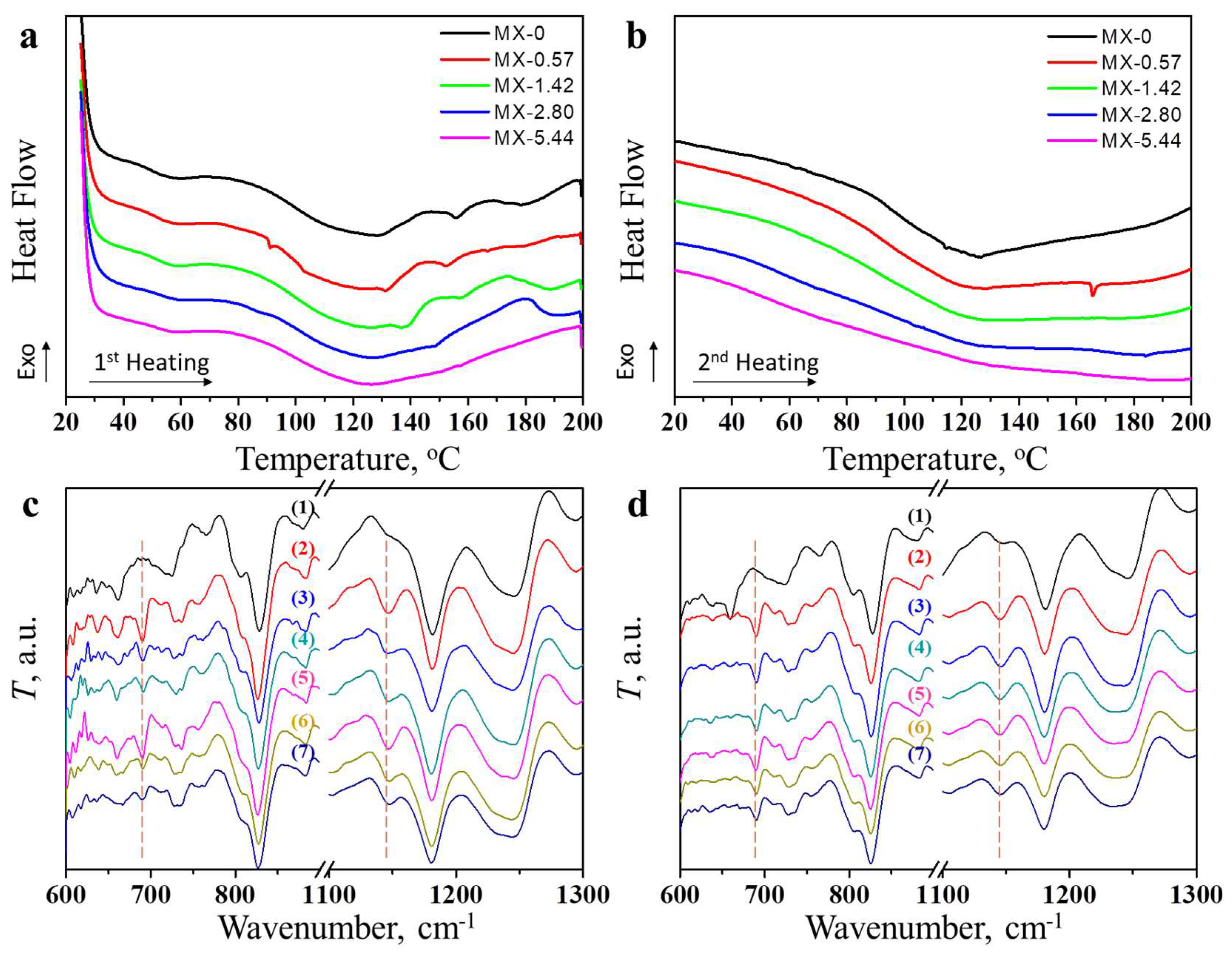
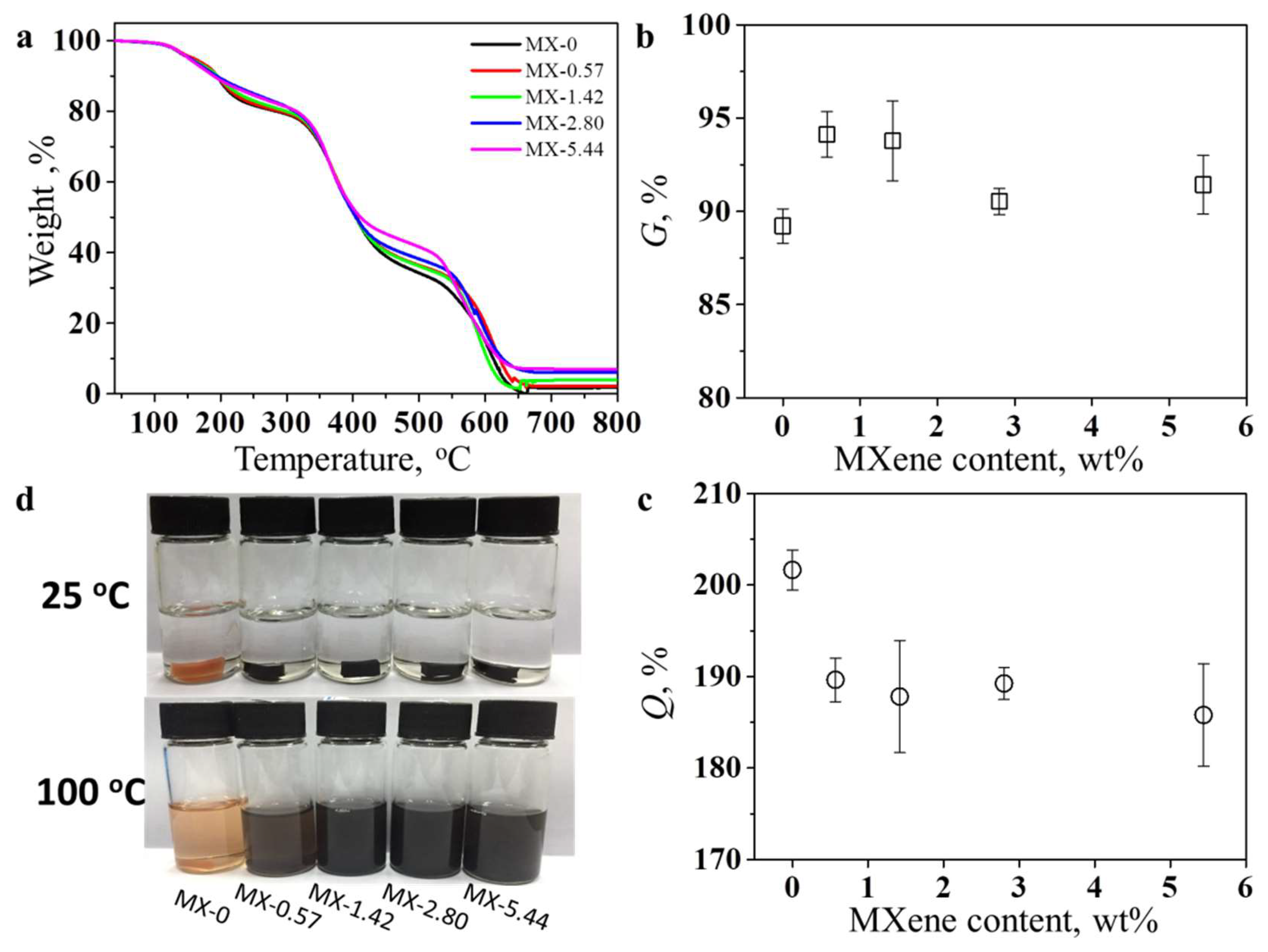
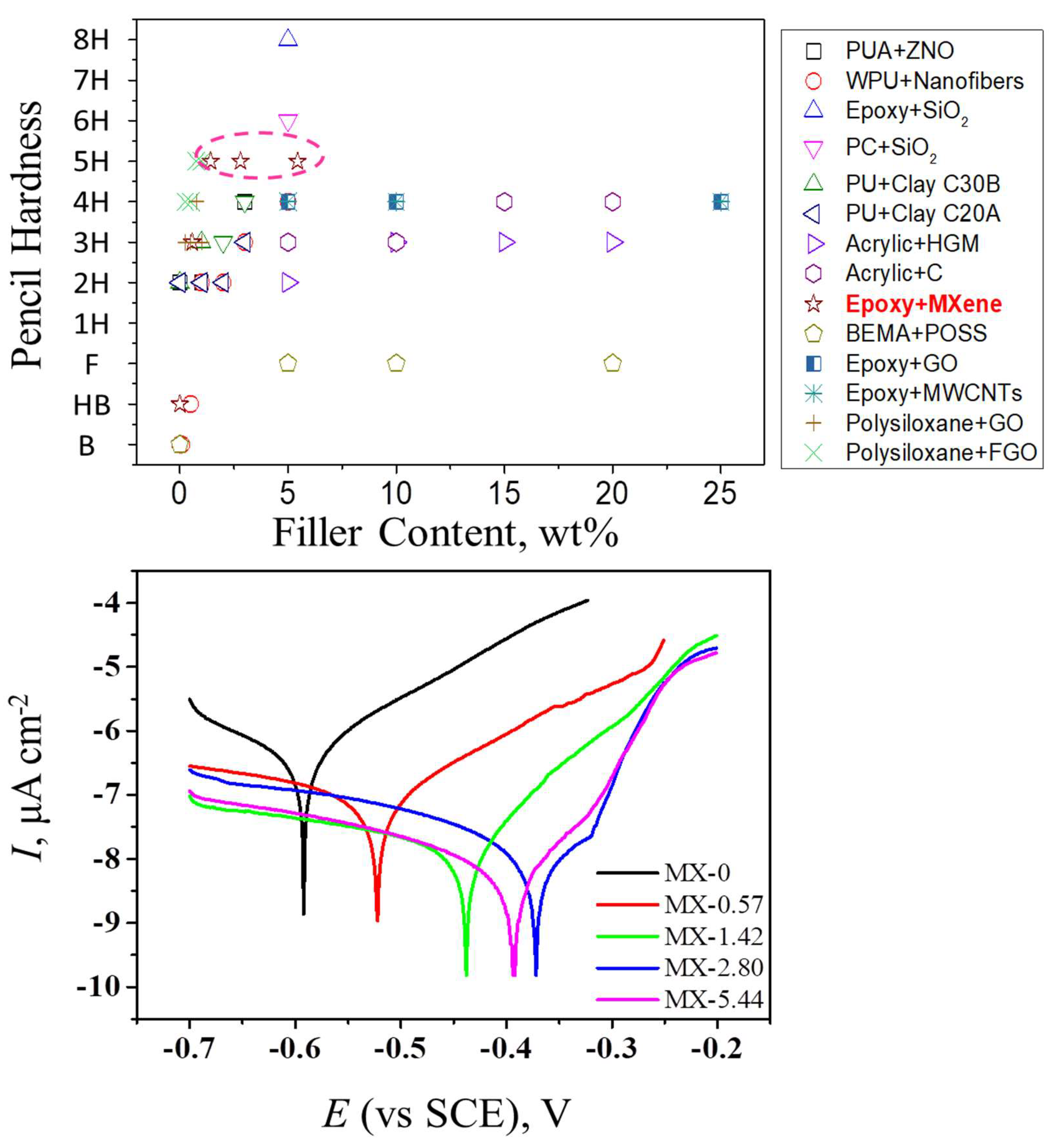
3.2. Structures and Properties of Epoxy Coatings Containing MXene
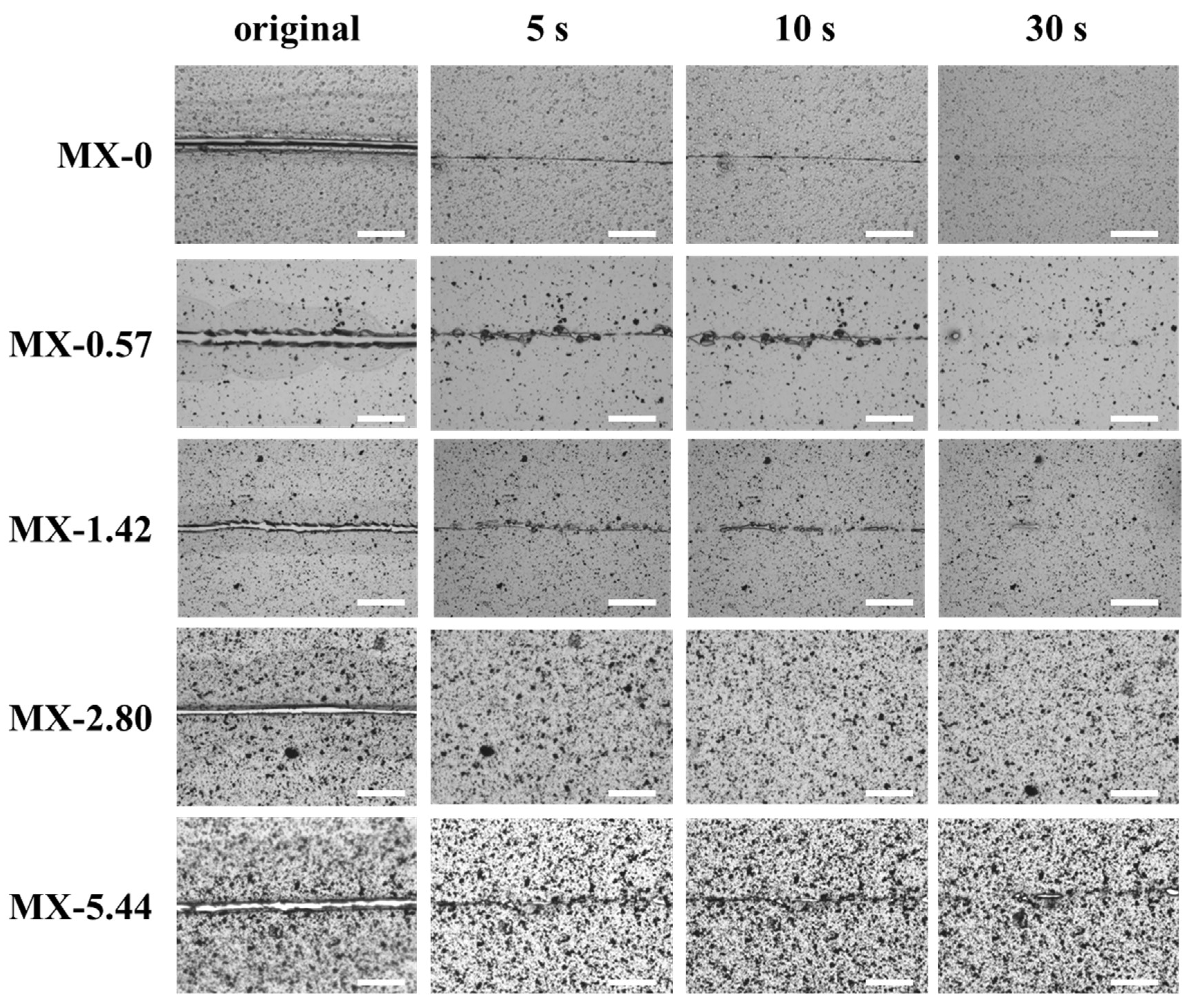
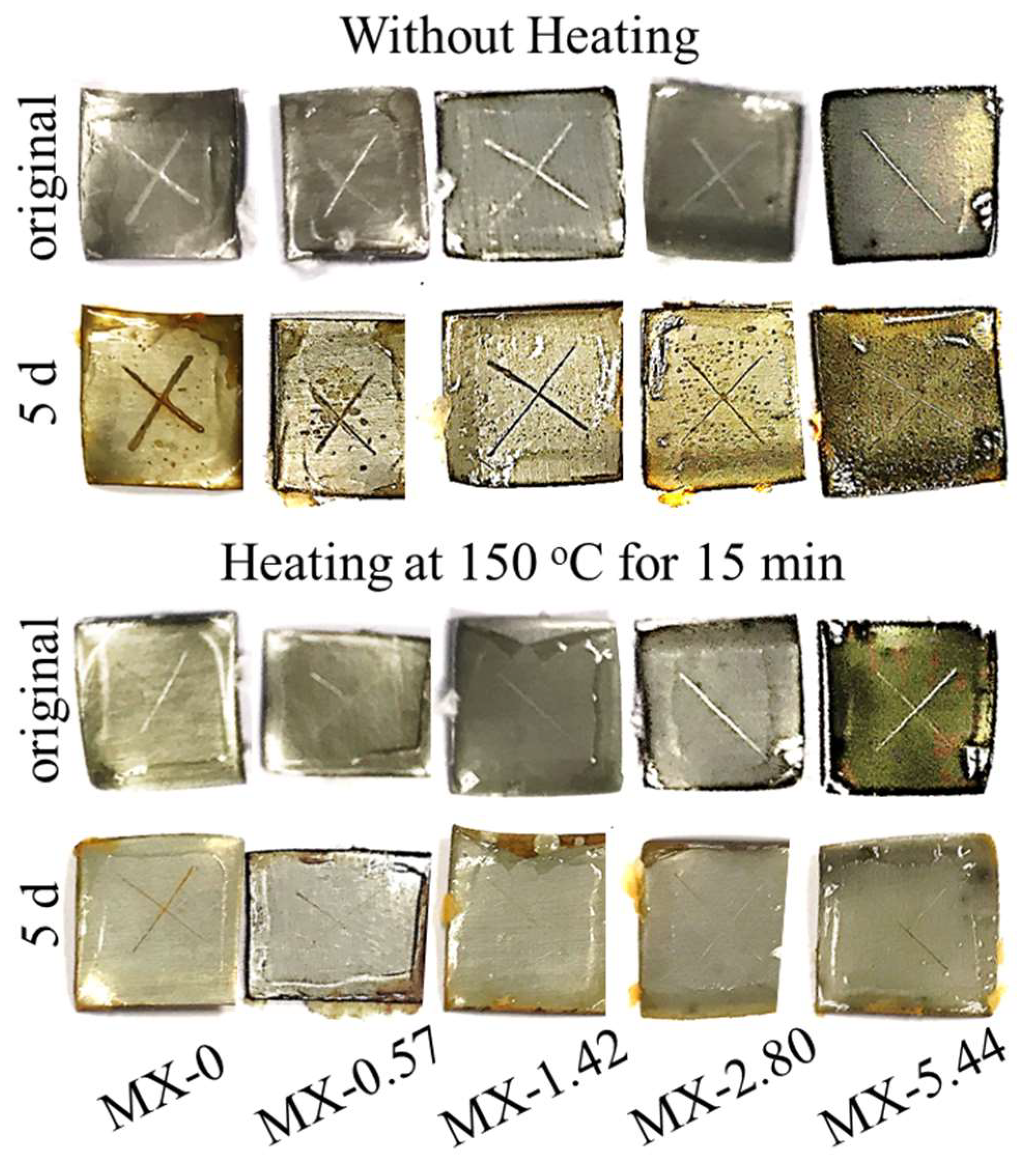
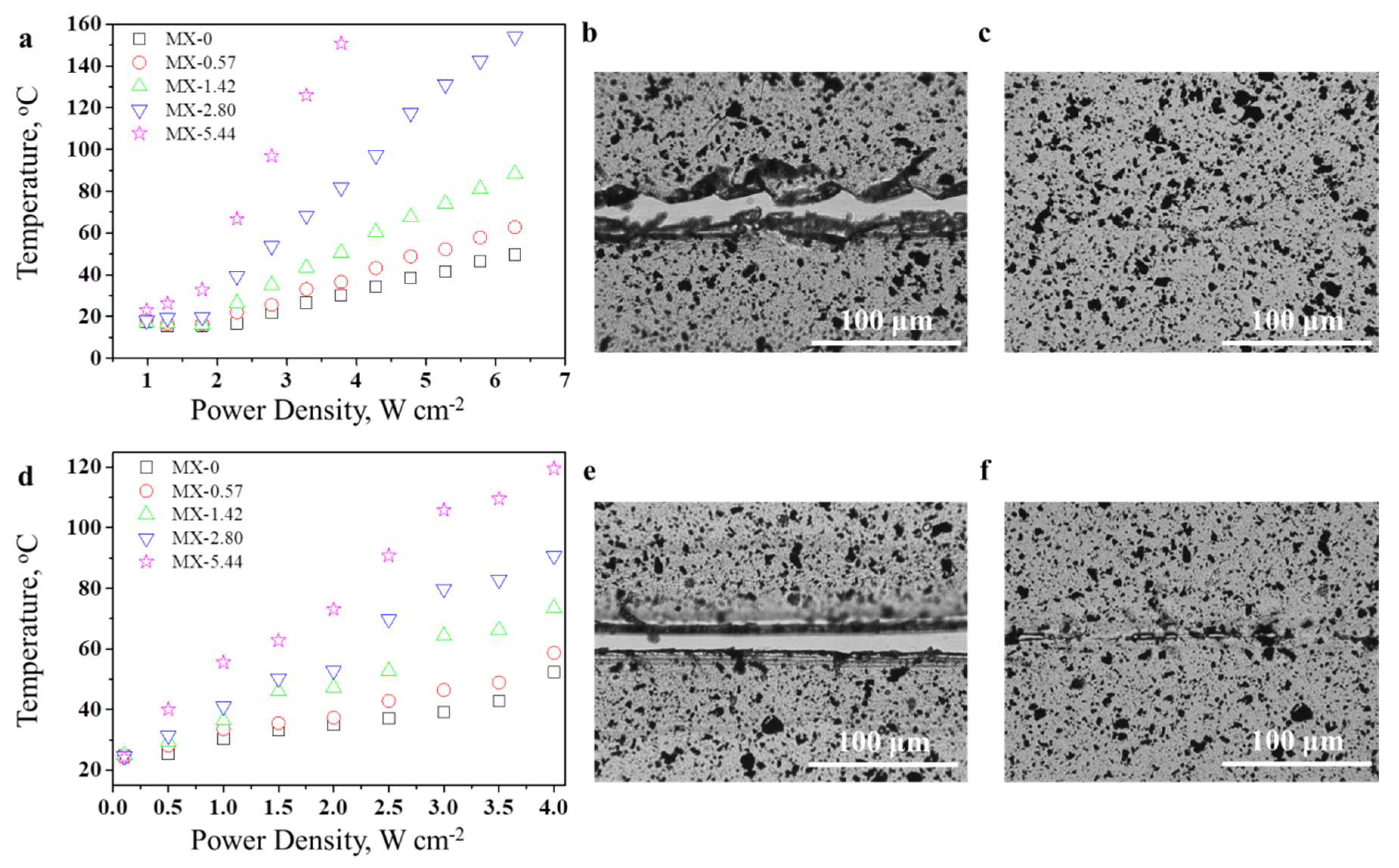
3.3. Thermally-Induced Self-Healing Capability
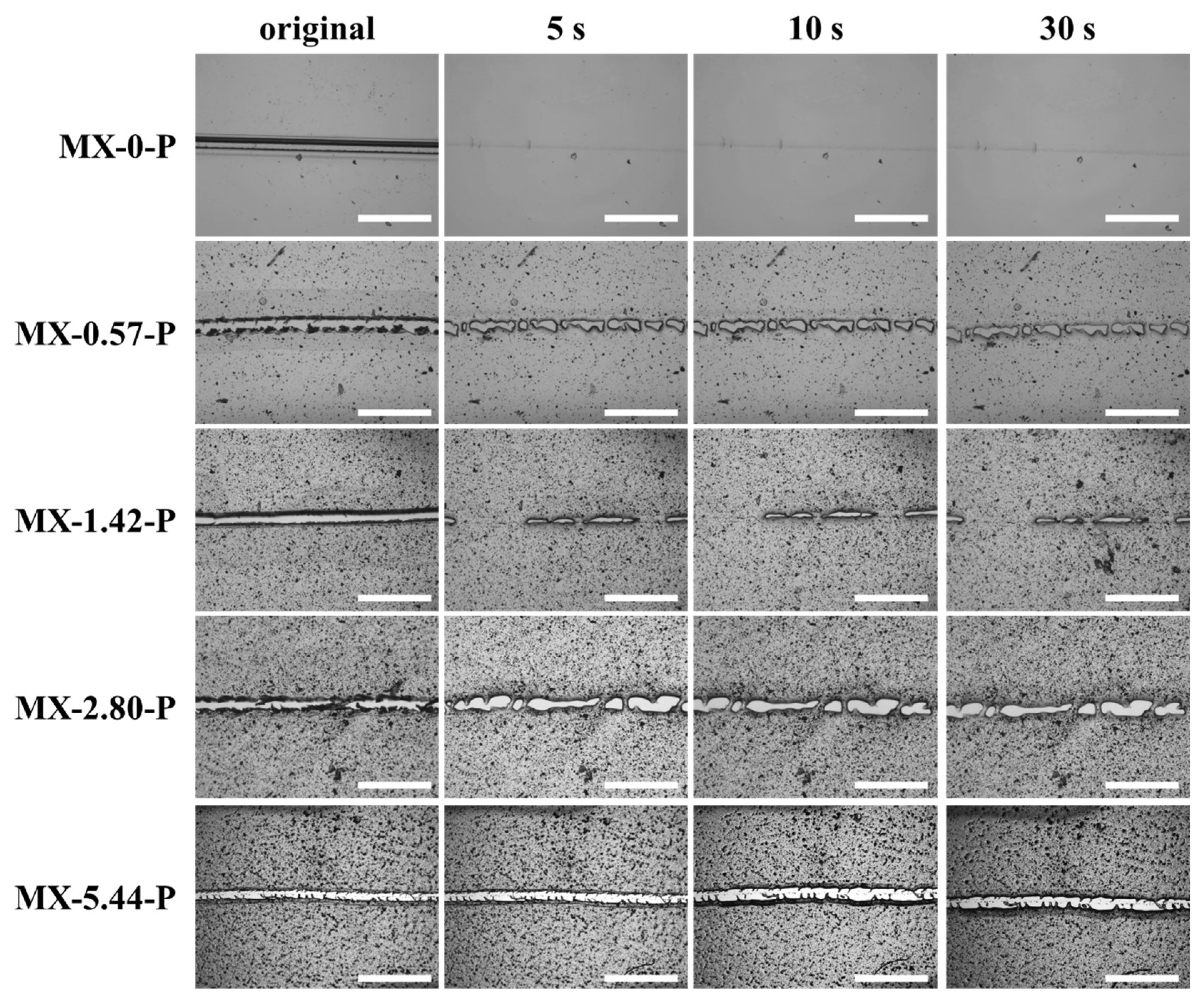
3.4. Light-Induced Self-Healing
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Park, J.H.; Braun, P.V. Coaxial electrospinning of self-healing coatings. Adv. Mater. 2010, 22, 4964–4999. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.; Schmidt, A.M. Progress in the remote-controlled activation of self-healing processes. Smart Mater. Struct. 2016, 25, 084018. [Google Scholar] [CrossRef]
- Samadzadeh, M.; Boura, S.H.; Peikari, M.; Kasiriha, S.M.; Ashrafi, A. A review on self-healing coatings based on micro/nanocapsules. Prog. Org. Coat. 2010, 68, 159–164. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Li, X.; Lin, Z.; Yang, Y.; An, E. Self-healing mechanisms of water triggered smart coating in seawater. J. Mater. Chem. A 2014, 2, 19141–19921. [Google Scholar] [CrossRef]
- Guo, M.; Li, W.; Han, N.; Wang, J.; Su, J.; Li, J.; Zhang, X. Novel dual-component microencapsulated hydrophobic amine and microencapsulated isocyanate used for self-healing anti-corrosion coating. Polymers 2018, 10, 319. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, T.; Cheng, Y.-T. Self-healable graphene polymer composites. J. Mater. Chem. 2010, 20, 3508. [Google Scholar] [CrossRef]
- Oehlenschlaeger, K.K.; Mueller, J.O.; Brandt, J.; Hilf, S.; Lederer, A.; Wilhelm, M.; Graf, R.; Coote, M.L.; Schmidt, F.G.; Barner-Kowollik, C. Adaptable hetero Diels-Alder networks for fast self-healing under mild conditions. Adv. Mater. 2014, 26, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Du, P.; Liu, X.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Synthesis and characterization of linear self-healing polyurethane based on thermally reversible Diels–Alder reaction. RSC Adv. 2013, 3, 15475. [Google Scholar] [CrossRef]
- Wool, R.P.; O’Connor, K.M. A theory crack healing in polymers. J. Appl. Phys. 1981, 52, 5953–5963. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Xiao, F.; Song, J.; Gao, H.; Zan, X.; Xu, R.; Duan, H. Coating graphene paper with 2D-assembly of electrocatalytic nanoparticles: A modular approach toward high-performance flexible electrodes. ACS Nano 2012, 6, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.; Vetter, C.A.; Gelling, V.J. The effect of polymer morphology on the performance of a corrosion inhibiting polypyrrole/aluminum flake composite pigment. Electrochim. Acta 2013, 102, 28–43. [Google Scholar] [CrossRef]
- Cain, A.A.; Nolen, C.R.; Li, Y.-C.; Davis, R.; Grunlan, J.C. Phosphorous-filled nanobrick wall multilayer thin film eliminates polyurethane melt dripping and reduces heat release associated with fire. Polym. Degrad. Stabil. 2013, 98, 2645–2652. [Google Scholar] [CrossRef]
- Ioniţă, M.; Prună, A. Polypyrrole/carbon nanotube composites: Molecular modeling and experimental investigation as anti-corrosive coating. Prog. Org. Coat. 2011, 72, 647–652. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Influence of dielectric nanoparticles addition on electroconductivity and other properties of carbon nanotubes-based acrylic coatings. Prog. Org. Coat. 2016, 92, 66–72. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Kajiyama, S.; Iinuma, H.; Hosono, E.; Oro, S.; Moriguchi, I.; Okubo, M.; Yamada, A. Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nat. Commun. 2015, 6, 6544. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M.; Zhuang, H.L.; Kent, P.R.C. Prediction and characterization of MXene nanosheet anodes for non-lithium-ion batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes promising anode materials for li-ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef] [PubMed]
- Chafidz, A.; Rengga, W.D.P.; Khan, R.; Kaavessina, M.; Almutlaq, A.M.; Almasry, W.A.; Ajbar, A. Polypropylene/multi-wall carbon nanotubes nanocomposites: Nanoindentation, dynamic mechanical, and electrical properties. J. Appl. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Wang, H.; Zhang, J.; Yan, H.; Pan, B.; Zhou, J.; Xie, Y. Ultrathin nanosheets of MAX phases with enhanced thermal and mechanical properties in polymeric compositions: Ti3Si0.75Al0.25C2. Angew. Chem. 2013, 52, 4361–4365. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Deng, Q.; Liu, Z.; Shen, D.; Wang, T.; Huang, Q.; Du, S.; Jiang, N.; Lin, C.-T.; Yu, J. Enhanced thermal properties of poly(vinylidene fluoride) composites with ultrathin nanosheets of MXene. RSC Adv. 2017, 7, 20494–20501. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. De. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Naguib, M.; Saito, T.; Lai, S.; Rager, M.S.; Aytug, T.; Parans Paranthaman, M.; Zhao, M.-Q.; Gogotsi, Y. Ti3C2Tx (MXene)–polyacrylamide nanocomposite films. RSC Adv. 2016, 6, 72069–72073. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chen, J.; Zou, Y.; Xu, Z.; Lu, C. Thermally-induced self-healing behaviors and properties of four epoxy coatings with different network architectures. Polymers 2017, 9, 333. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of postetch annealing gas composition on the structural and electrochemical properties of Ti2CTx MXene electrodes for supercapacitor applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Gogotsi, Y.; Alshareef, H.N. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale 2016, 8, 7580–7587. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yin, X.; Han, M.; Song, C.; Xu, H.; Hou, Z.; Zhang, L.; Cheng, L. Ti3C2 MXenes modified with in situ grown carbon nanotubes for enhanced electromagnetic wave absorption properties. J. Mater. Chem. C 2017, 5, 4068–4074. [Google Scholar] [CrossRef]
- Fu, X.; Qutubuddin, S. Polymer–clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer 2001, 42, 807–813. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J. Appl. Polym. Sci. 2003, 87, 1327–1338. [Google Scholar] [CrossRef]
- Mishra, R.S.; Mishra, A.K.; Raju, K.V.S.N. Synthesis and property study of UV-curable hyperbranched polyurethane acrylate/ZnO hybrid coatings. Eur. Polym. J. 2009, 45, 960–966. [Google Scholar] [CrossRef]
- Hwang, D.K.; Moon, J.H.; Shui, Y.G.; Jung, K.T.; Kim, D.H.; Lee, D.W. Scratch resistant and transparent UV-protective coating on polycarbonate. J. Sol-Gel Sci. Technol. 2003, 26, 783–787. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M. Scratch resistance enhancement of polymer coatings. Macromol. Mater. Eng. 2010, 295, 603–612. [Google Scholar] [CrossRef]
- Sahu, P.K.; Mahanwar, P.A.; Bambole, V.A. Effect of hollow glass microspheres and cenospheres on insulation properties of coatings. Pigm. Resin Technol. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Cheng, D.; Wen, Y.; An, X.; Zhu, X.; Ni, Y. Tempo-oxidized cellulose nanofibers (TOCNs) as a green reinforcement for waterborne polyurethane coating (WPU) on wood. Carbohydr. Polym. 2016, 151, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Kaushik, A.; Ghosh, A.K. Comparative assessment of nano-morphology and properties of spray coated clear polyurethane coatings reinforced with different organoclays. Prog. Org. Coat. 2013, 76, 1046–1056. [Google Scholar] [CrossRef]
- Patil, V.; Dennis, R.V.; Rout, T.K.; Banerjee, S.; Yadav, G.D. Graphene oxide and functionalized multi walled carbon nanotubes as epoxy curing agents: A novel synthetic approach to nanocomposites containing active nanostructured fillers. RSC Adv. 2014, 4, 49264–49272. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Sun, G.; Zhong, Y.; You, B. Evaluation of scratch resistance of functionalized graphene oxide/polysiloxane nanocomposite coatings. Prog. Org. Coat. 2018, 117, 118–129. [Google Scholar] [CrossRef]
- Kowalczyk, K.; Kugler, S.; Spychaj, T. Antistatic polyurethane coats with hybrid carbon nanofillers. Polimery 2014, 59, 650–655. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Influence of synthetic and bio-based amine curing agents on properties of solventless epoxy varnishes and coatings with carbon nanofillers. Prog. Org. Coat. 2017, 109, 83–91. [Google Scholar] [CrossRef]
- Kugler, S.; Kowalczyk, K.; Spychaj, T. Transparent epoxy coatings with improved electrical, barrier and thermal features made of mechanically dispersed carbon nanotubes. Prog. Org. Coat. 2017, 111, 196–201. [Google Scholar] [CrossRef]
| Name a | Oligomer, g b | BMI, g c | MXene, g d | Leveling Agent, g |
|---|---|---|---|---|
| MX-0 | 2.95 | 0.526 | 0 | 0.035 |
| MX-0.57 | 2.95 | 0.526 | 0.02 | 0.035 |
| MX-1.42 | 2.95 | 0.526 | 0.05 | 0.035 |
| MX-2.80 | 2.95 | 0.526 | 0.10 | 0.035 |
| MX-5.44 | 2.95 | 0.526 | 0.20 | 0.035 |
| Properties | Thermo-Degradation Property | Mechanical Property | Electrochemical Corrosion | |||||
|---|---|---|---|---|---|---|---|---|
| Samples | Td,15% | Td,60% | Pencil Hardness | Flexibility | Adhesion | Ecorr | Icorr | Rp |
| °C | °C | N/A | mm | N/A | mV | μA cm−2 | MΩ cm−2 | |
| MX-0 | 216 | 443 | B-HB | <2 | 1 | −590 | 0.4514 | 4.3 |
| MX-0.57 | 220 | 454 | 2H-3H | <2 | 1 | −520 | 0.1030 | 10.4 |
| MX-1.42 | 227 | 452 | 4H-5H | <2 | 1 | −439 | 0.0151 | 76.5 |
| MX-2.80 | 249 | 473 | 4H-5H | <2 | 1 | −371 | 0.0047 | 428.2 |
| MX-5.44 | 244 | 519 | 4H-5H | <2 | 1 | −392 | 0.0382 | 62.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Fang, L.; Chen, T.; Sun, M.; Lu, C.; Xu, Z. Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers. Polymers 2018, 10, 474. https://doi.org/10.3390/polym10050474
Zou Y, Fang L, Chen T, Sun M, Lu C, Xu Z. Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers. Polymers. 2018; 10(5):474. https://doi.org/10.3390/polym10050474
Chicago/Turabian StyleZou, Yuting, Liang Fang, Tianqi Chen, Menglong Sun, Chunhua Lu, and Zhongzi Xu. 2018. "Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers" Polymers 10, no. 5: 474. https://doi.org/10.3390/polym10050474
APA StyleZou, Y., Fang, L., Chen, T., Sun, M., Lu, C., & Xu, Z. (2018). Near-Infrared Light and Solar Light Activated Self-Healing Epoxy Coating having Enhanced Properties Using MXene Flakes as Multifunctional Fillers. Polymers, 10(5), 474. https://doi.org/10.3390/polym10050474