Molecular Design of Soluble Biopolyimide with High Rigidity
Abstract
1. Introduction
2. Experimental
2.1. Materials
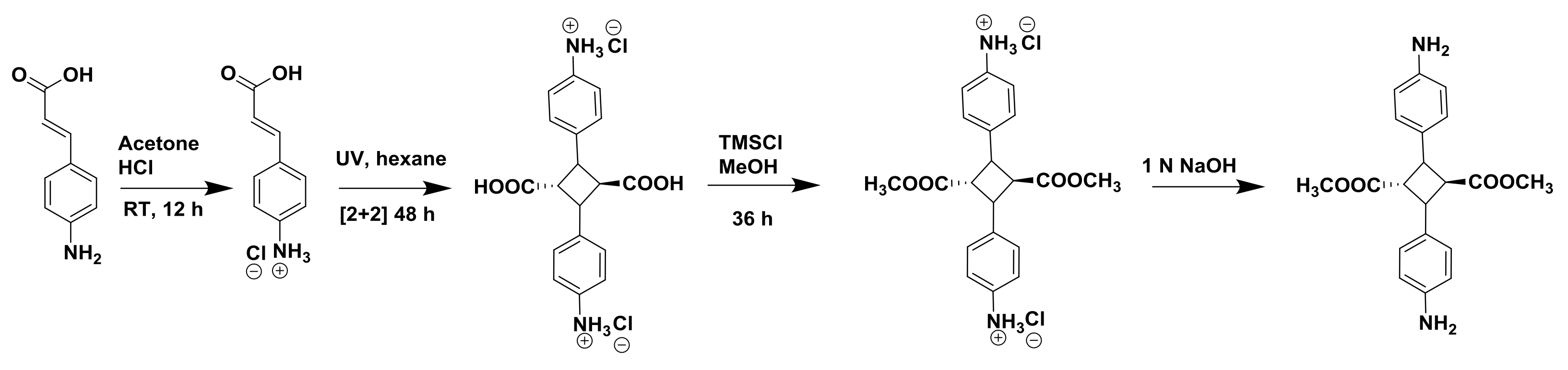
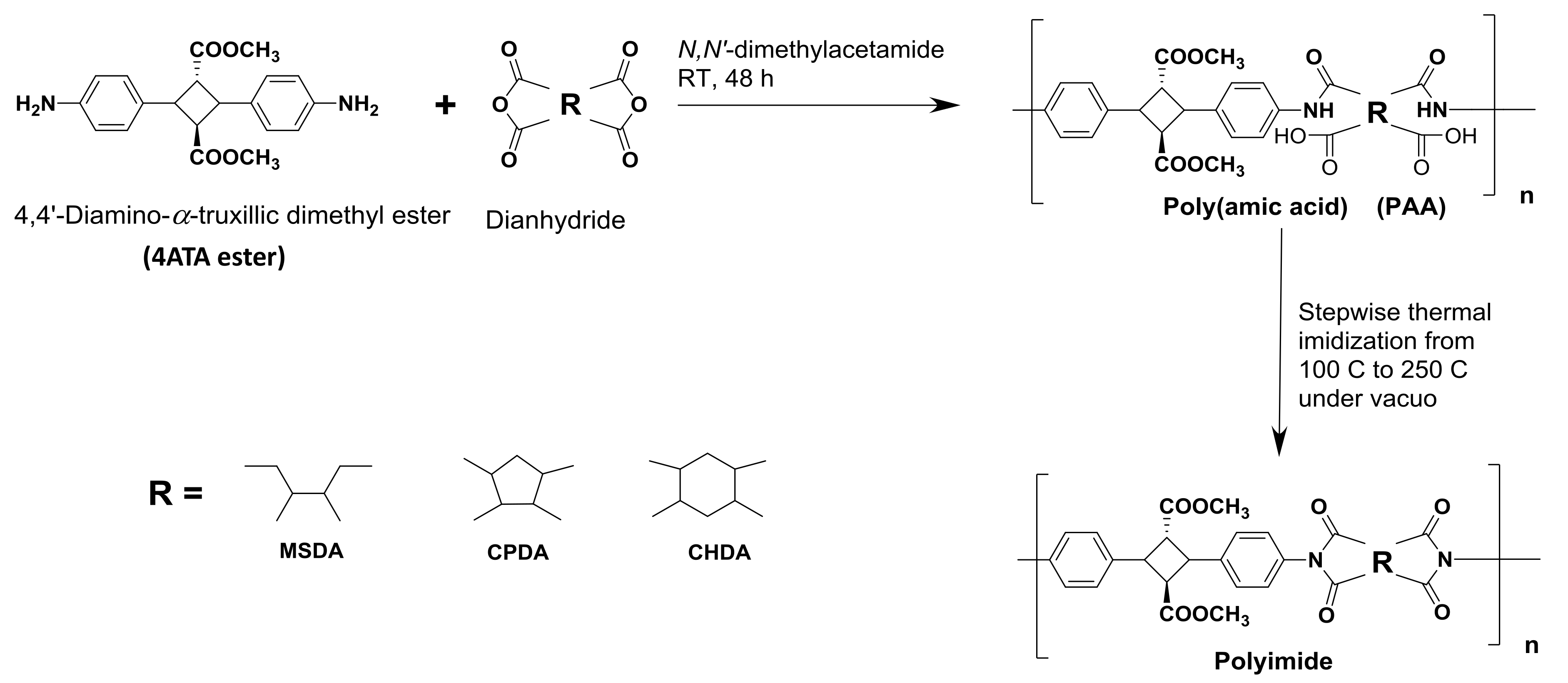
2.2. Syntheses
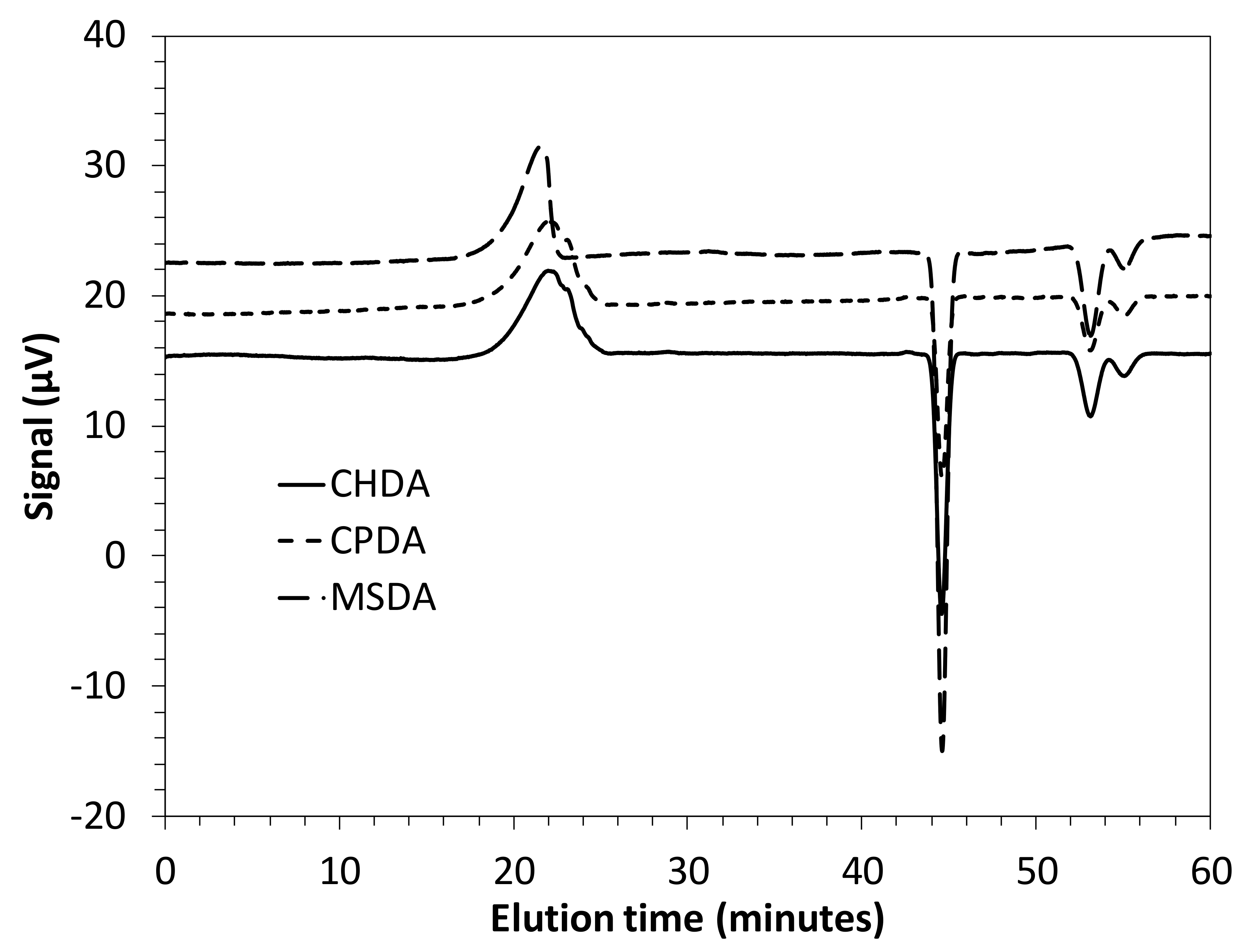
2.3. Characterization
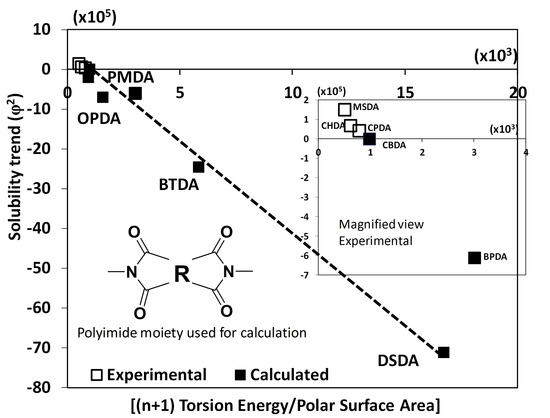
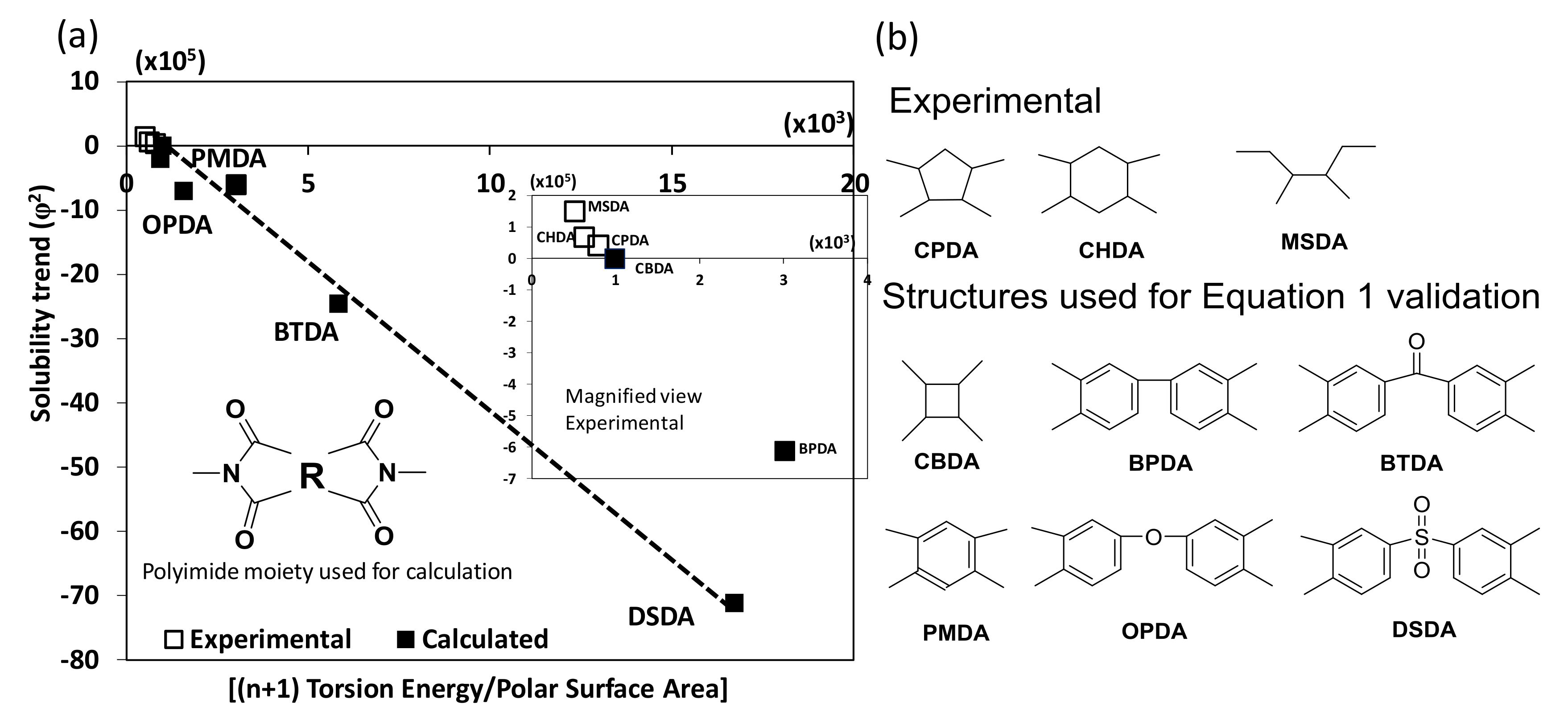
3. Results and Discussion
Biopolyimide Synthesis and Structural Characteristics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, K.L. Polyimides: Synthesis, Characterization and Applications; John Wiley Sons: New York, NY, USA, 1985; Volume 1. [Google Scholar]
- Cassidy, P.E. Thermally Stable Polymers: Synthesis and Properties; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Kricheldorf, H.R.; Linzer, V. Liquid crystalline polyimides: 18. Thermotropic polyimides based on biphenyl-3,3′,4,4′-tetracarboxylic anhydride. Polymer 1995, 36, 1893–1902. [Google Scholar] [CrossRef]
- Marek, M.; Doskocilova, D.; Schmidt, P.; Schneider, B.; Kriz, J.; Labsky, J.; Puffer, R. New soluble polyimides prepared from 4,4′-(alkylenediyldioxy)dianilines. Polymer 1994, 35, 4881–4888. [Google Scholar] [CrossRef]
- Acevedo, M.; Harris, F.W. Polyimides derived from 2-methyl-2-propyl-1,3-bis(4-aminophenoxy)propane and 2,2-dimethyl-1,3-bis(4-aminophenoxy)propane. Polymer 1994, 35, 4456–4461. [Google Scholar] [CrossRef]
- Giesa, R.; Keller, U.; Eiselt, P.; Schmidt, H.W. Synthesis and thermal properties of aryl-substituted rod-like polyimides. J. Polym. Sci. A 1993, 31, 141–151. [Google Scholar] [CrossRef]
- Becker, K.H.; Schmidt, H.W. Para-linked aromatic poly(amic ethyl esters): Precursors to rodlike aromatic polyimides. 1. Synthesis and imidization study. Macromolecules 1992, 25, 6784–6790. [Google Scholar] [CrossRef]
- Chung, I.S.; Kim, S.Y. Soluble Polyimides from Unsymmetrical Diamine with Trifluoromethyl Pendent Group. Macromolecules 2000, 33, 3190–3193. [Google Scholar] [CrossRef]
- Takekoshi, T. Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Wei, Q.; Hirota, K.; Tajima, K.; Hashimoto, K. Design and Synthesis of TiO2Nanorod Assemblies and Their Application for Photovoltaic Devices. Chem. Mater. 2006, 18, 5080–5087. [Google Scholar] [CrossRef]
- Kang, H.A.; Chung, I.S.; Kakimoto, M.; Kim, S.Y. Synthesis and Characterization of Polyimides from Unsymmetrical Diamine with Cyano Groups. Polym. J. 2001, 33, 284–289. [Google Scholar] [CrossRef][Green Version]
- Huang, S.J.; Hoyt, A.E. The synthesis of soluble polyimides. Trends Polym. Sci. 1995, 3, 262–271. [Google Scholar]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Cui, Z.; Lee, J.; Lee, Y.M.; Guiver, M.D. Intrinsically Microporous Soluble Polyimides Incorporating Tröger’s Base for Membrane Gas Separation. Macromolecules 2014, 47, 3254–3262. [Google Scholar] [CrossRef]
- Yagci, H.; Mathias, L. Synthesis and characterization of aromatic polyamides and polyimides from trimethyl- and di-t-butylhydroquinone-based ether-linked diamines. J. Polym. 1998, 39, 3779–3786. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Yang, C.P.; Chen, S.H. Synthesis and properties of ortho-linked aromatic polyimides based on 1,2-bis(4-aminophenoxy)-4-tert-butylbenzene. J. Polym. Sci. A 2000, 38, 1551–1559. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y. Synthesis and Properties of Polyimides Derived from 1,4-Bis(4-aminophenoxy)-2-tert-butylbenzene. Polym. J. 1996, 28, 970–975. [Google Scholar] [CrossRef][Green Version]
- Langsam, M.; Burgoyne, W.F. Effects of diamine monomer structure on the gas permeability of polyimides. I. Bridged diamines. J. Polym. Sci. A 1993, 31, 909–921. [Google Scholar] [CrossRef]
- Huang, W.; Yan, D.; Lu, Q.; Tao, P. Preparation of aromatic polyimides highly soluble in conventional solvents. J. Polym. Sci. A 2002, 40, 229–234. [Google Scholar] [CrossRef]
- Liaw, D.J.; Liaw, B.Y.; Li, L.J.; Sillion, B.; Mercier, R.; Thiria, R.; Sekiguchi, H. Synthesis and Characterization of New Soluble Polyimides from 3,3′,4,4′-Benzhydrol Tetracarboxylic Dianhydride and Various Diamines. Chem. Mater. 1998, 10, 734–739. [Google Scholar] [CrossRef]
- Li, F.; Ge, J.J.; Honigfort, P.S.; Fang, S.; Chen, J.C.; Harris, F.W.; Cheng, S.Z.D. Dianhydride architectural effects on the relaxation behaviors and thermal and optical properties of organo-soluble aromatic polyimide films. Polymer 1999, 40, 4987–5002. [Google Scholar] [CrossRef]
- Suvannasara, P.; Tateyama, S.; Miyasato, A.; Matsumura, K.; Shimoda, T.; Ito, T.; Yamagata, Y.; Fujita, T.; Takaya, N.; Kaneko, T. Biobased Polyimides from 4-Aminocinnamic Acid Photodimer. Macromolecules 2014, 47, 1586–1593. [Google Scholar] [CrossRef]
- Tateyama, S.; Masuo, S.; Suvannasara, P.; Oka, Y.; Miyasato, A.; Yasaki, K.; Teerawatananond, T.; Muangsin, N.; Zhou, S.; Kawasaki, Y.; et al. Ultrastrong, Transparent Polytruxillamides Derived from Microbial Photodimers. Macromolecules 2016, 49, 3336–3342. [Google Scholar] [CrossRef]
- Anslyn, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Herndon, VA, USA, 2006; ISBN 978-1-891389-31-3. [Google Scholar]
- Bicerano, J. Prediction of Polymer Properties; Mercel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Scatchard, G. Equilibria in Non-electrolyte Solutions in Relation to the Vapor Pressures and Densities of the Components. Chem. Rev. 1931, 8, 321–333. [Google Scholar] [CrossRef]
- Hildebrand, J.H. Solubility. J. Am. Chem. Soc. 1916, 38, 1452–1473. [Google Scholar] [CrossRef]
- Hildebrand, J.H. Solubility III Relative values of internal pressures and their practical applications. J. Am. Chem. Soc. 1919, 41, 1067–1080. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Prausnitz, J.M.; Scott, R.L. Regular and Related Solutions: The Solubility of Gases, Liquids, and Solids; Reinhold: New York, NY, USA, 1970. [Google Scholar]
- Chen, X.; Yuan, C.; Wong, C.K.Y.; Zhang, G. Molecular modeling of temperature dependence of solubility parameters for amorphous polymers. J. Mol. Model. 2012, 18, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, N.; Chamjangali, M.A.; Amin, A.H. Calculation of Hildebrand Solubility Parameters of Some Polymers Using QSPR Methods Based on LS-SVM Technique and Theoretical Molecular Descriptors. Chin. J. Polym. Sci. 2014, 32, 587–594. [Google Scholar] [CrossRef]
- Li, L.; Totton, T.; Frenkel, D. Computational methodology for solubility prediction: Application to the sparingly soluble solutes. J. Chem. Phys. 2017, 146, 214110–214115. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; McCabe, G. Introduction to the Practice of Statistics; W. H. Freeman and Co.: London, UK, 2003. [Google Scholar]
| Polyimide | CHDA | MSDA | CPDA |
|---|---|---|---|
| Dianhydride | |||
| Td10 a (°C) | 397 | 375 | 391 |
| Tg b (°C) | 259 | 273 | 294 |
| T c (%) | 51 | 57 | ND |
| Mn d (g/mol) | 4.8 × 105 | 7.8 × 105 | 5.9 × 105 |
| Mw/Mn d | 1.4 | 1.4 | 1.3 |
| Solubility Test | |||
| Hexane | - | - | - |
| Toluene | - | - | - |
| Dichloromethane | - | - | - |
| Chloroform | - | - | - |
| Diethylether | - | - | - |
| Water | - | - | - |
| Methanol | - | - | - |
| Ethanol | - | - | - |
| Acetone | - | - | - |
| Acetonitrile | - | - | - |
| Ethylacetate | - | - | - |
| Tetrahydrofuran | ± | ± | ± |
| N-Methyl-2-pyrrolidone | + | + | + |
| Dimethyl sulfoxide | + | + | + |
| N,N-dimethylformamide | + | + | + |
| N,N-dimethylacetamide | + | + | + |
| Conc. H2SO4 | + | + | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwivedi, S.; Kaneko, T. Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers 2018, 10, 368. https://doi.org/10.3390/polym10040368
Dwivedi S, Kaneko T. Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers. 2018; 10(4):368. https://doi.org/10.3390/polym10040368
Chicago/Turabian StyleDwivedi, Sumant, and Tatsuo Kaneko. 2018. "Molecular Design of Soluble Biopolyimide with High Rigidity" Polymers 10, no. 4: 368. https://doi.org/10.3390/polym10040368
APA StyleDwivedi, S., & Kaneko, T. (2018). Molecular Design of Soluble Biopolyimide with High Rigidity. Polymers, 10(4), 368. https://doi.org/10.3390/polym10040368