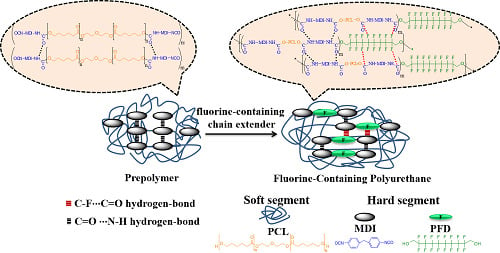
Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PFD/PUs
2.3. Advanced Polymer Chromatography System (APC)
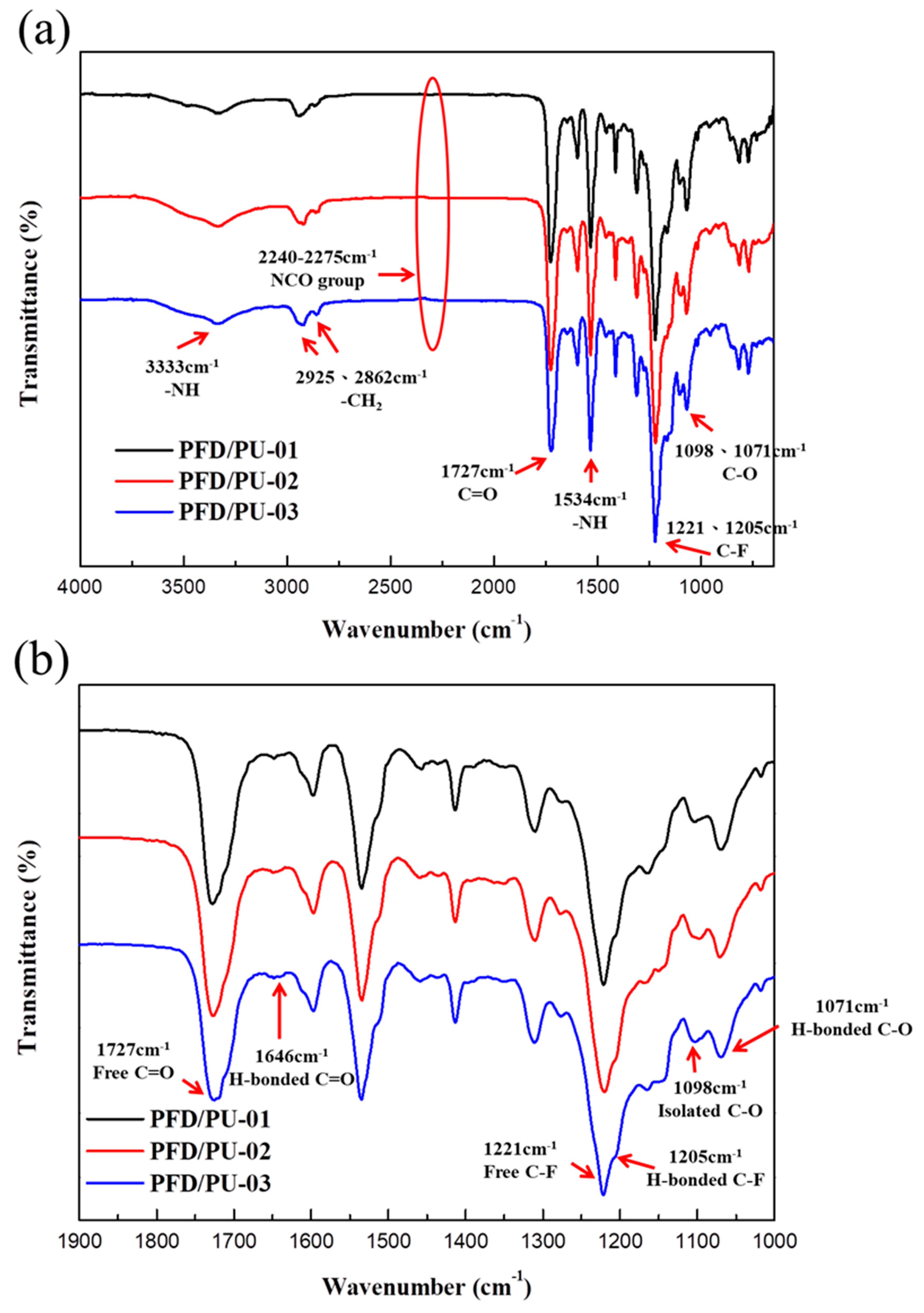
2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5. 1H NMR Spectrometer
2.6. 19F NMR Spectrometer
2.7. X-Ray Photoelectron Spectroscopy (XPS)
2.8. Surface Roughness Analysis
2.9. Thermogravimetric Analysis (TGA)
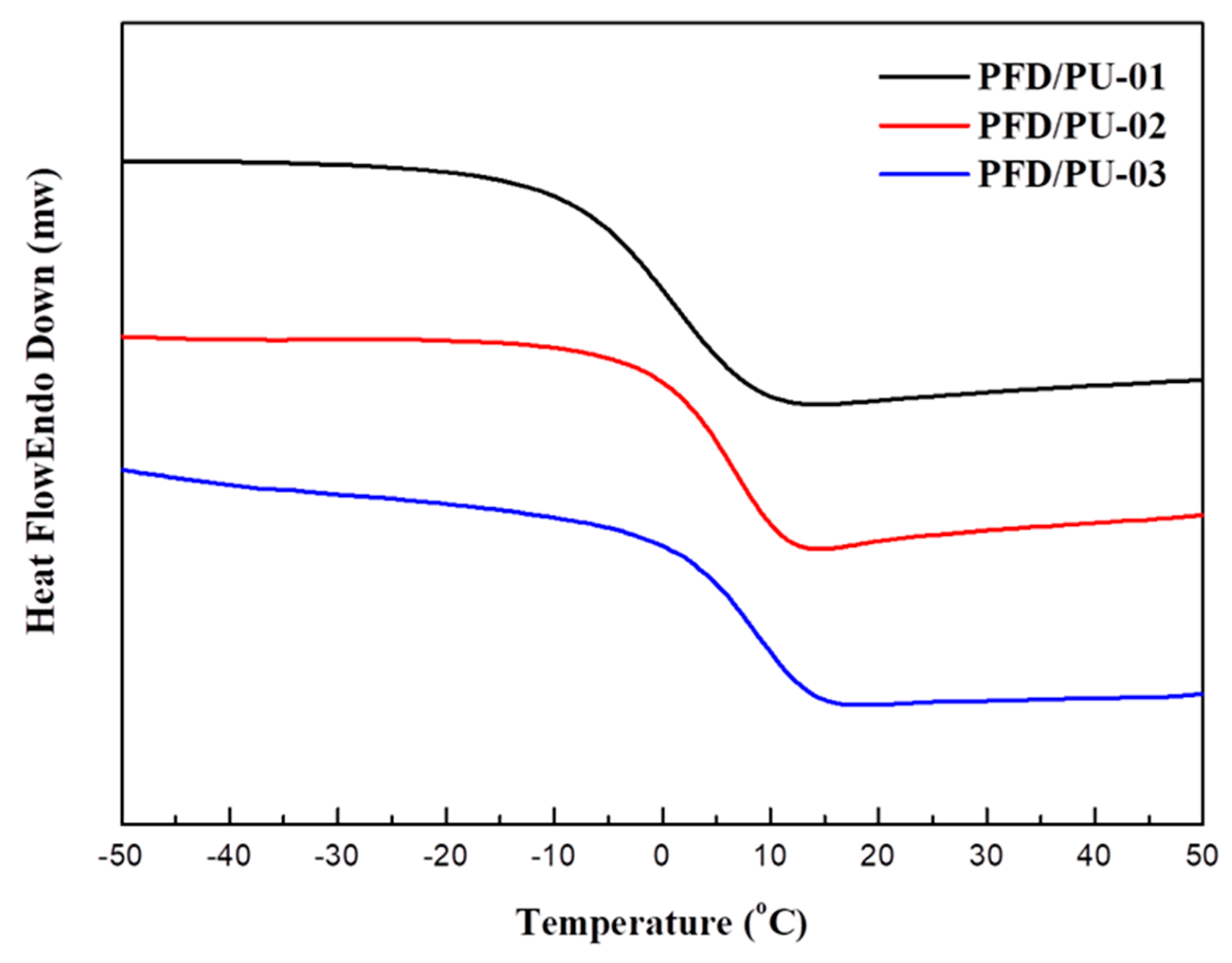
2.10. Differential Scanning Calorimetry (DSC)
2.11. Dynamic Mechanical Analysis (DMA)
2.12. Stress–Strain Testing
3. Results and Discussion
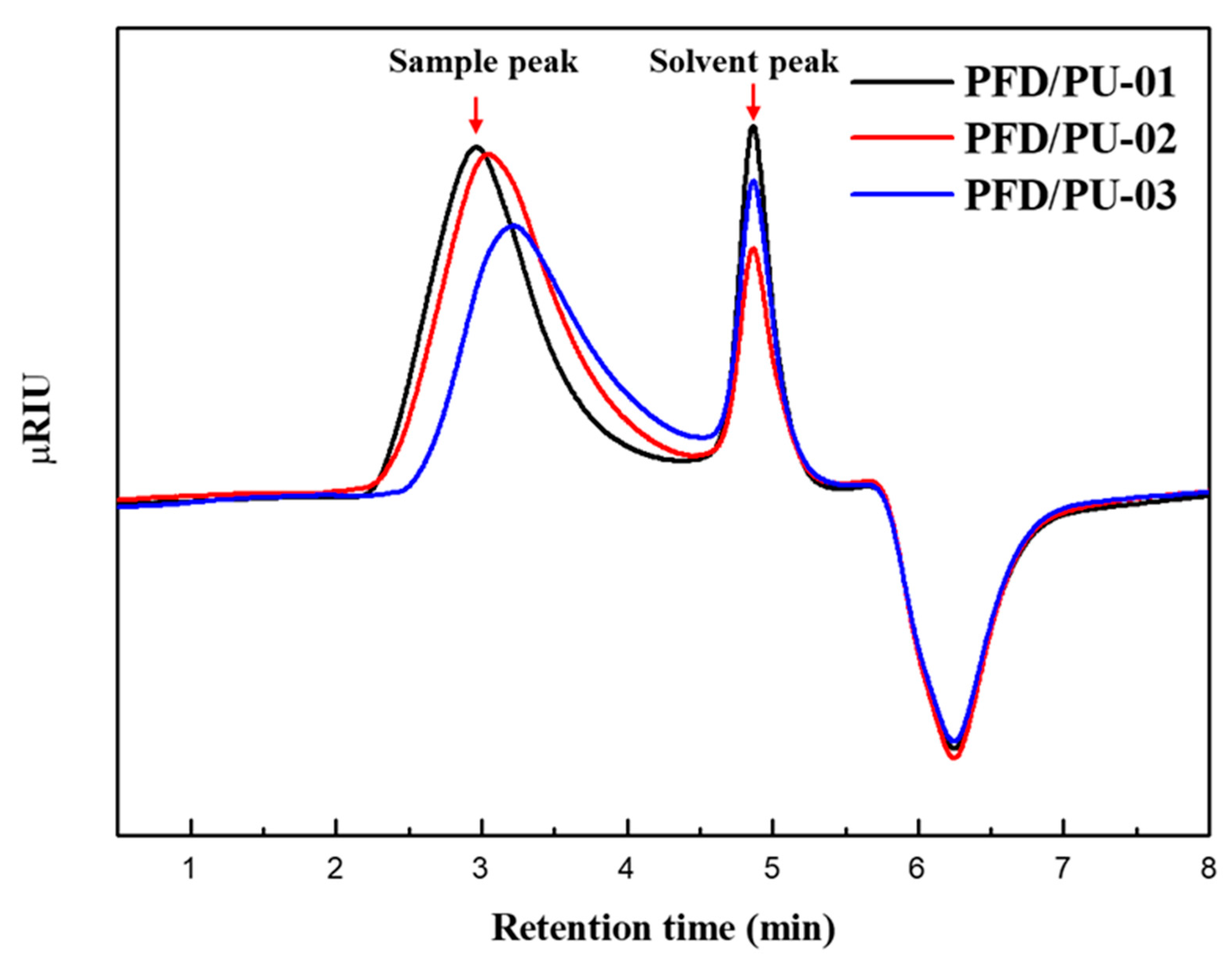
3.1. Gel Permeation Chromatography Analysis
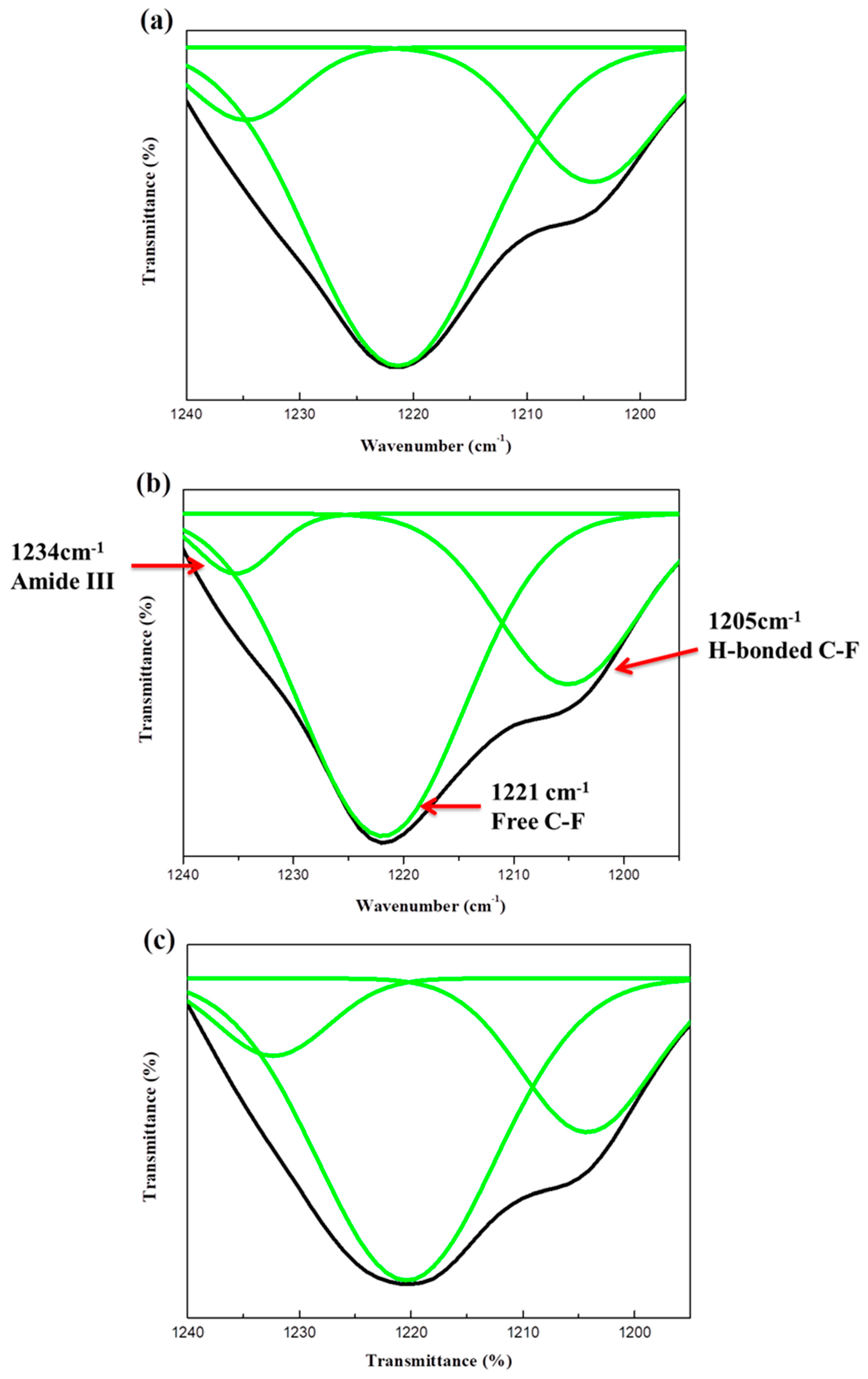
3.2. FTIR
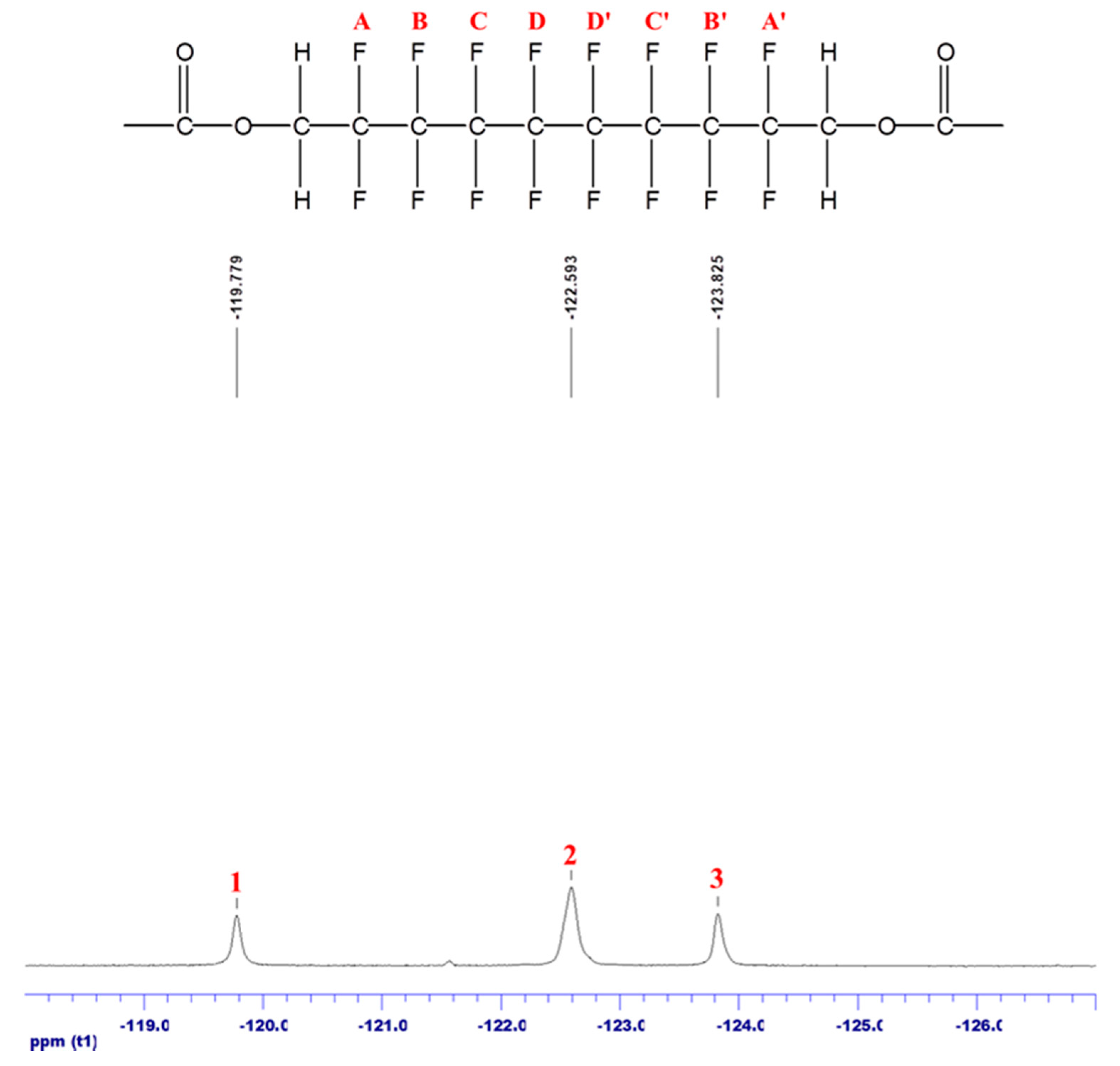
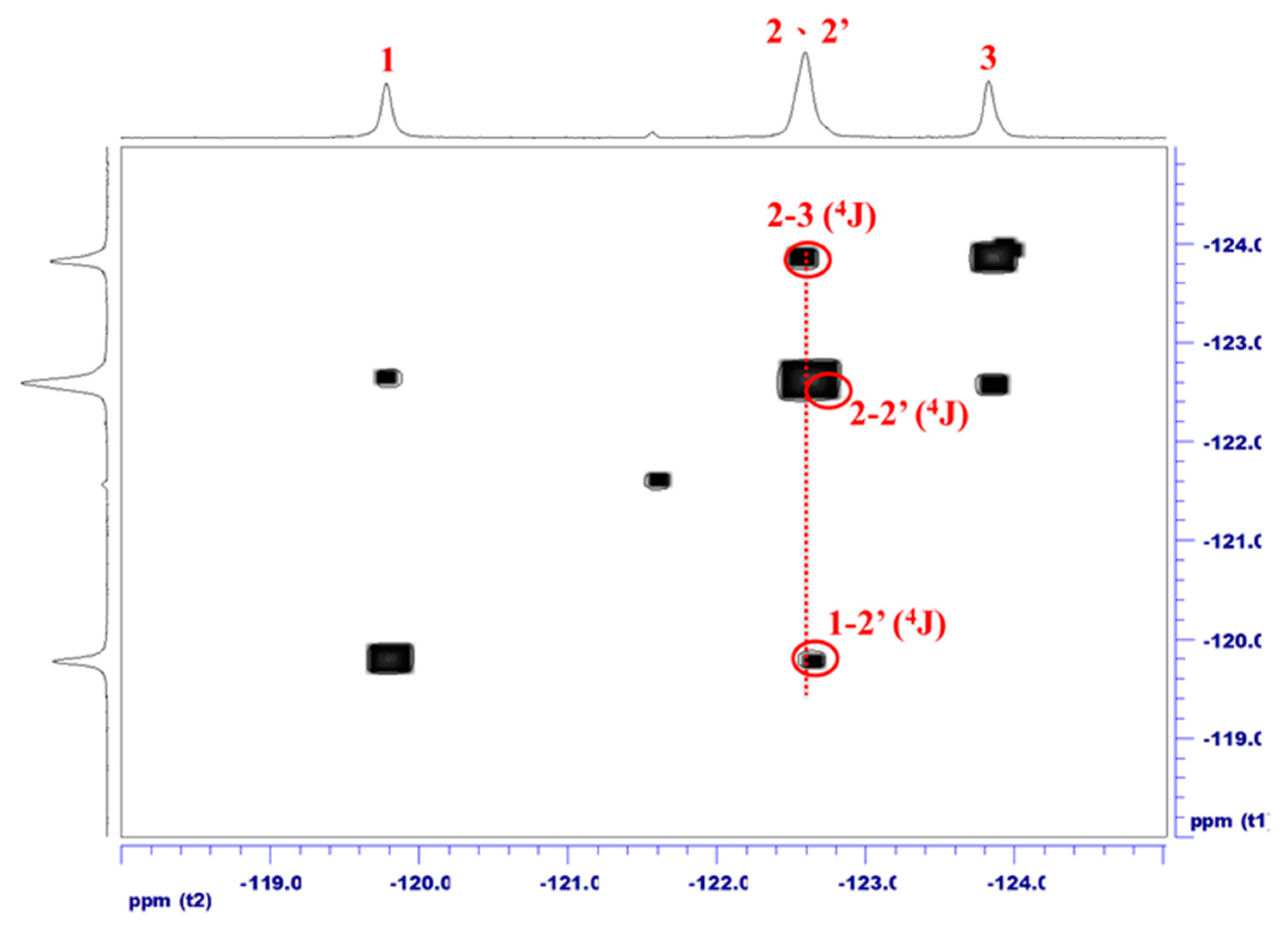
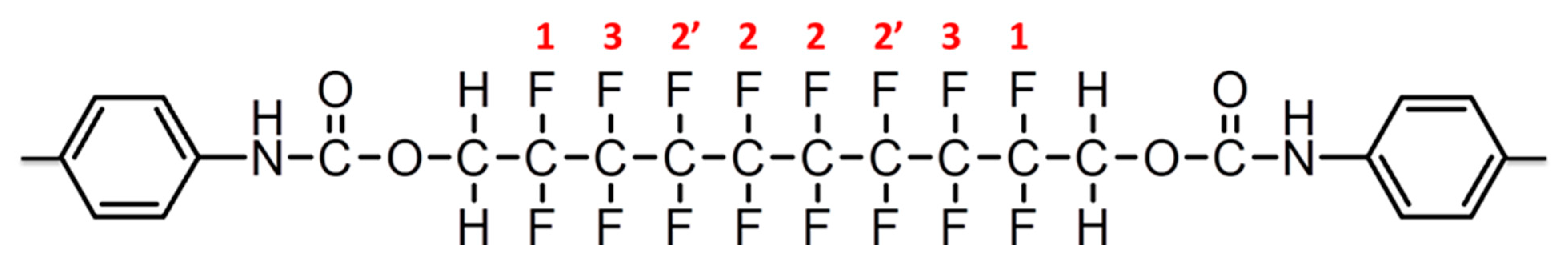
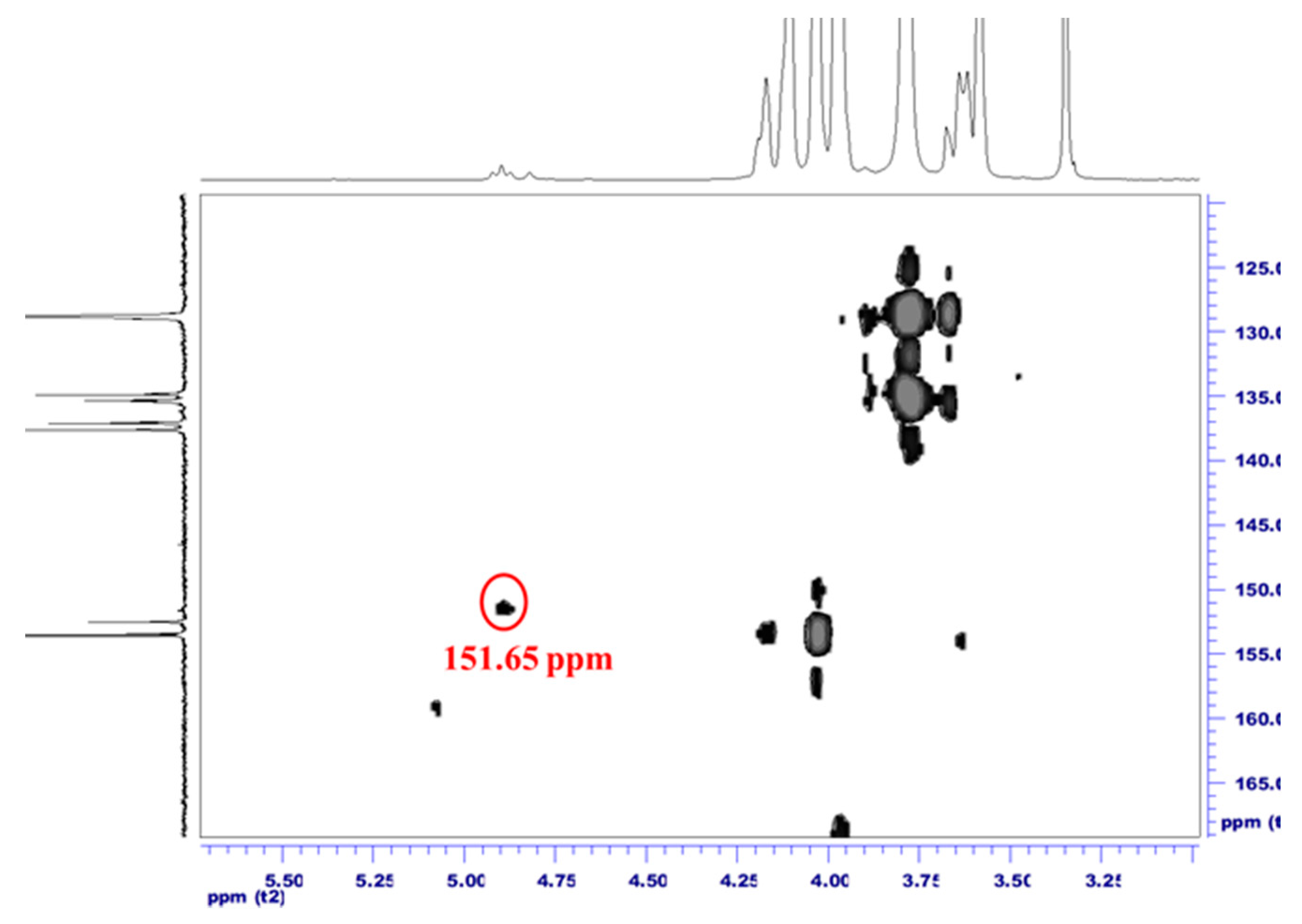
3.3. Fluorine-19 NMR
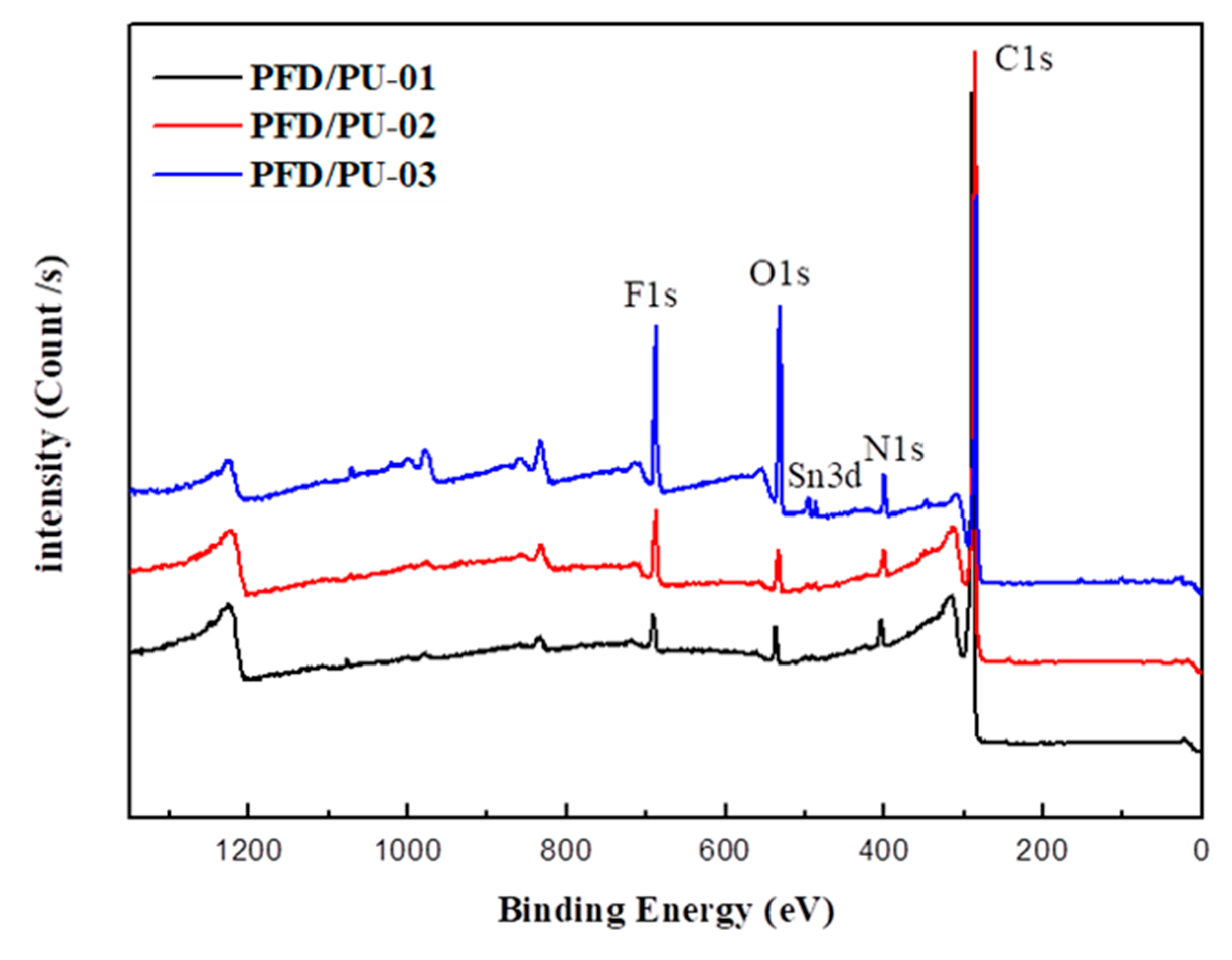
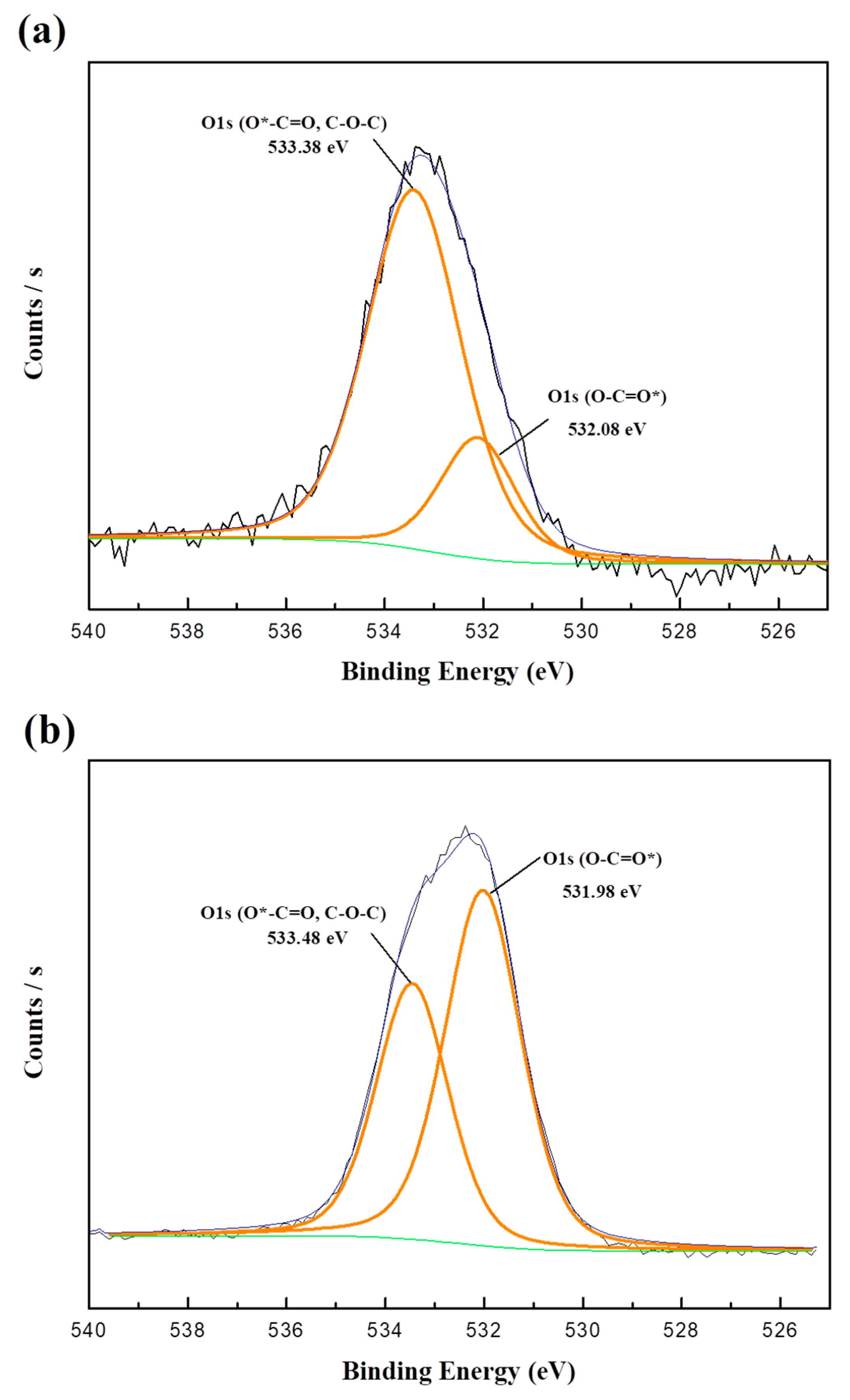
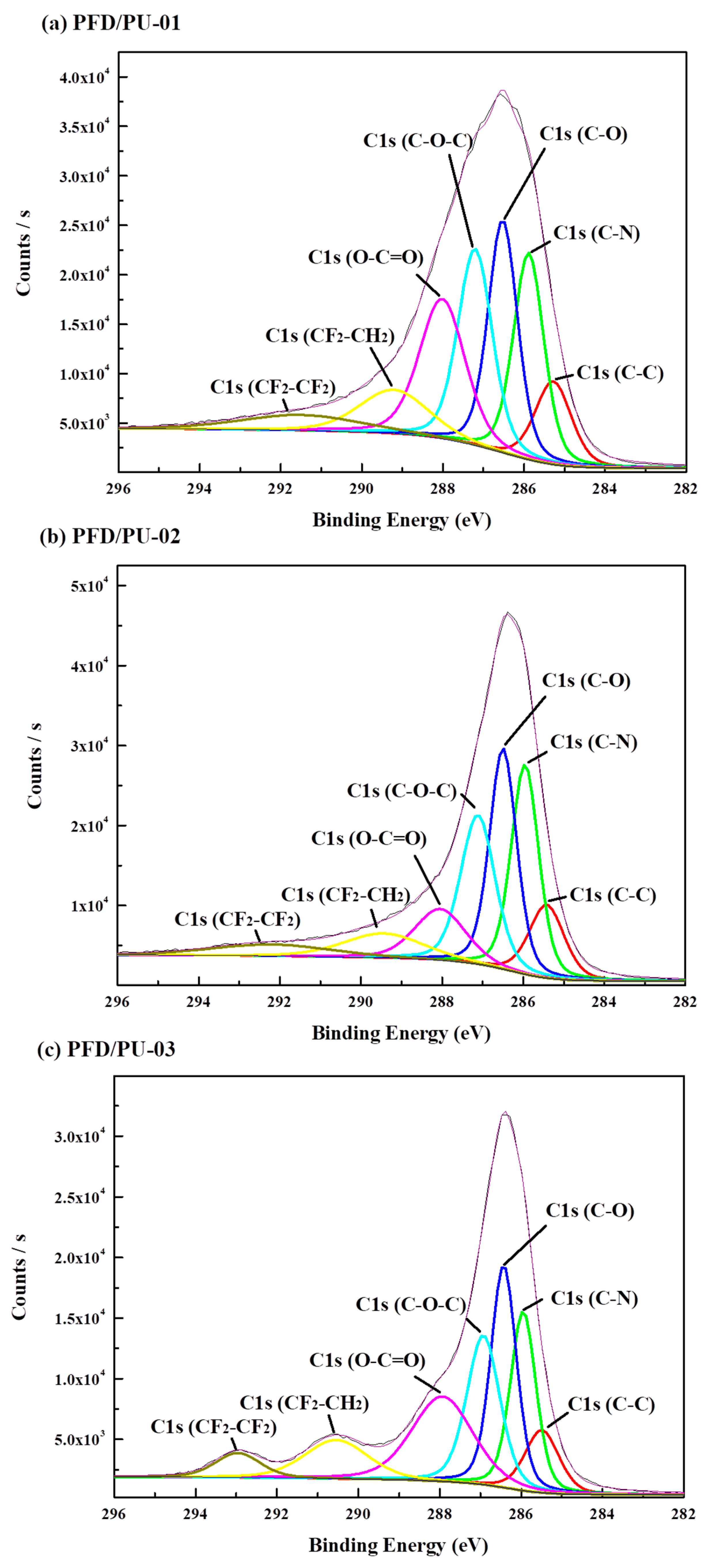
3.4. X-Ray Photoelectron Spectroscopy
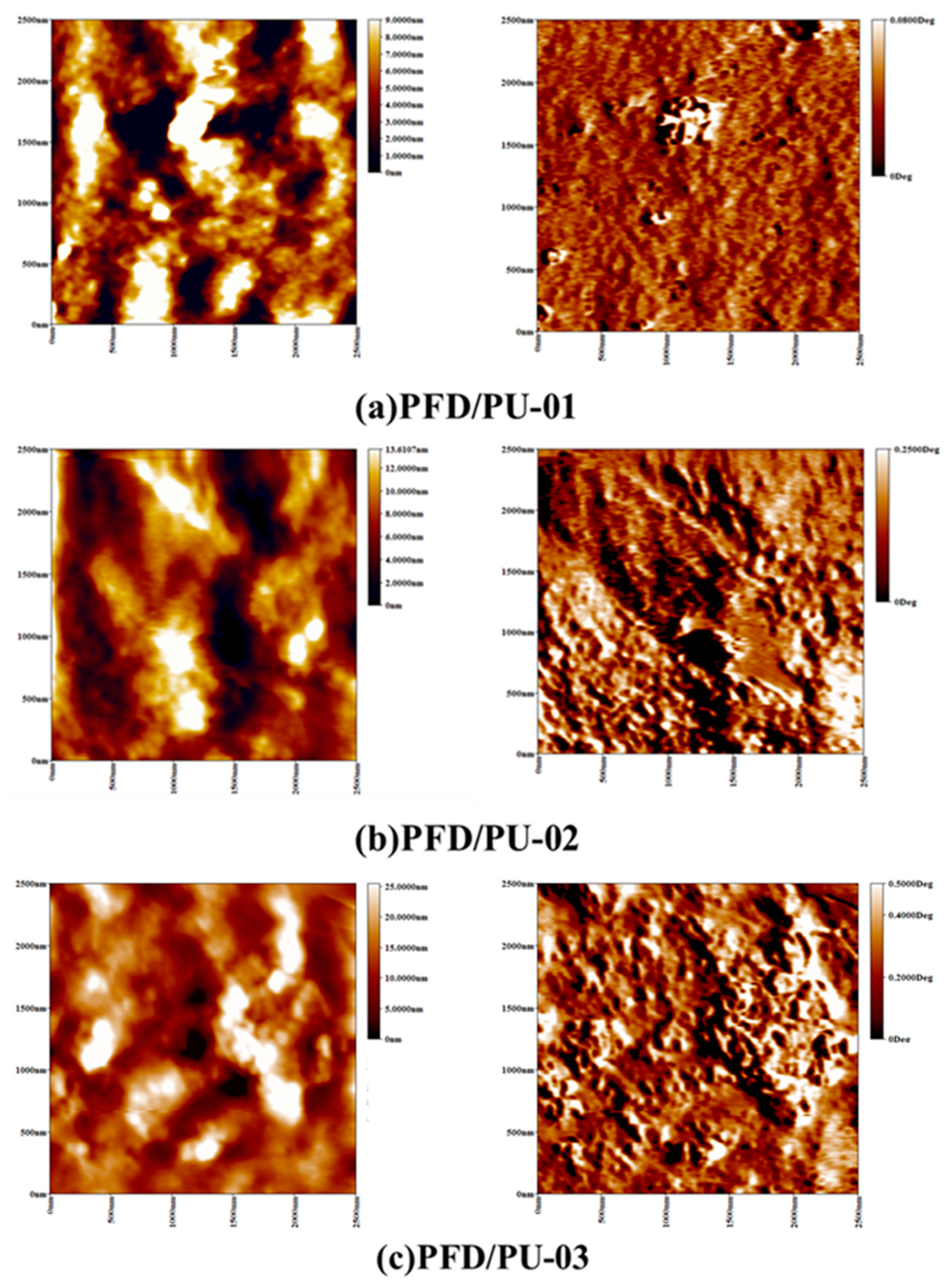
3.5. Surface Roughness Analysis
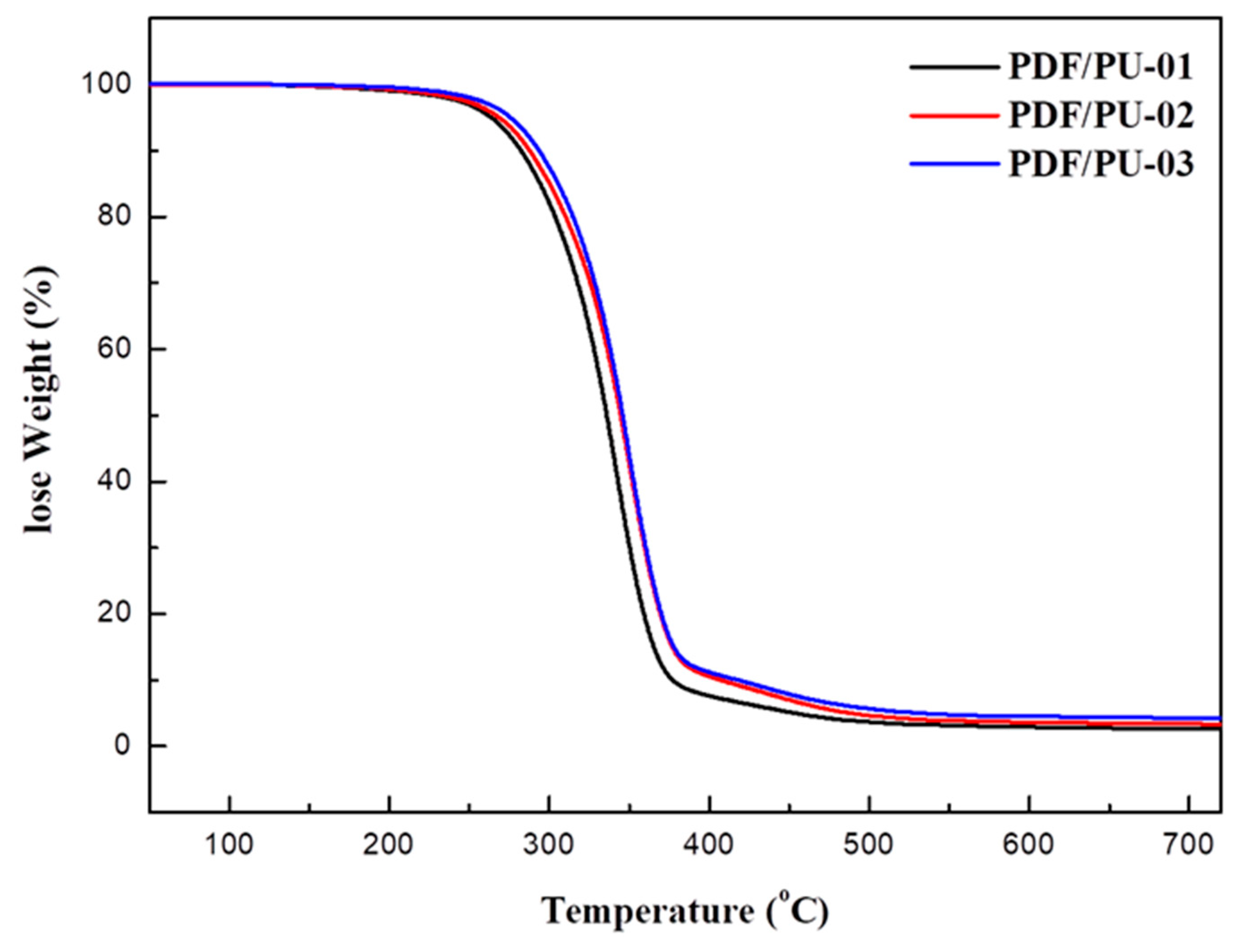
3.6. Thermal Properties
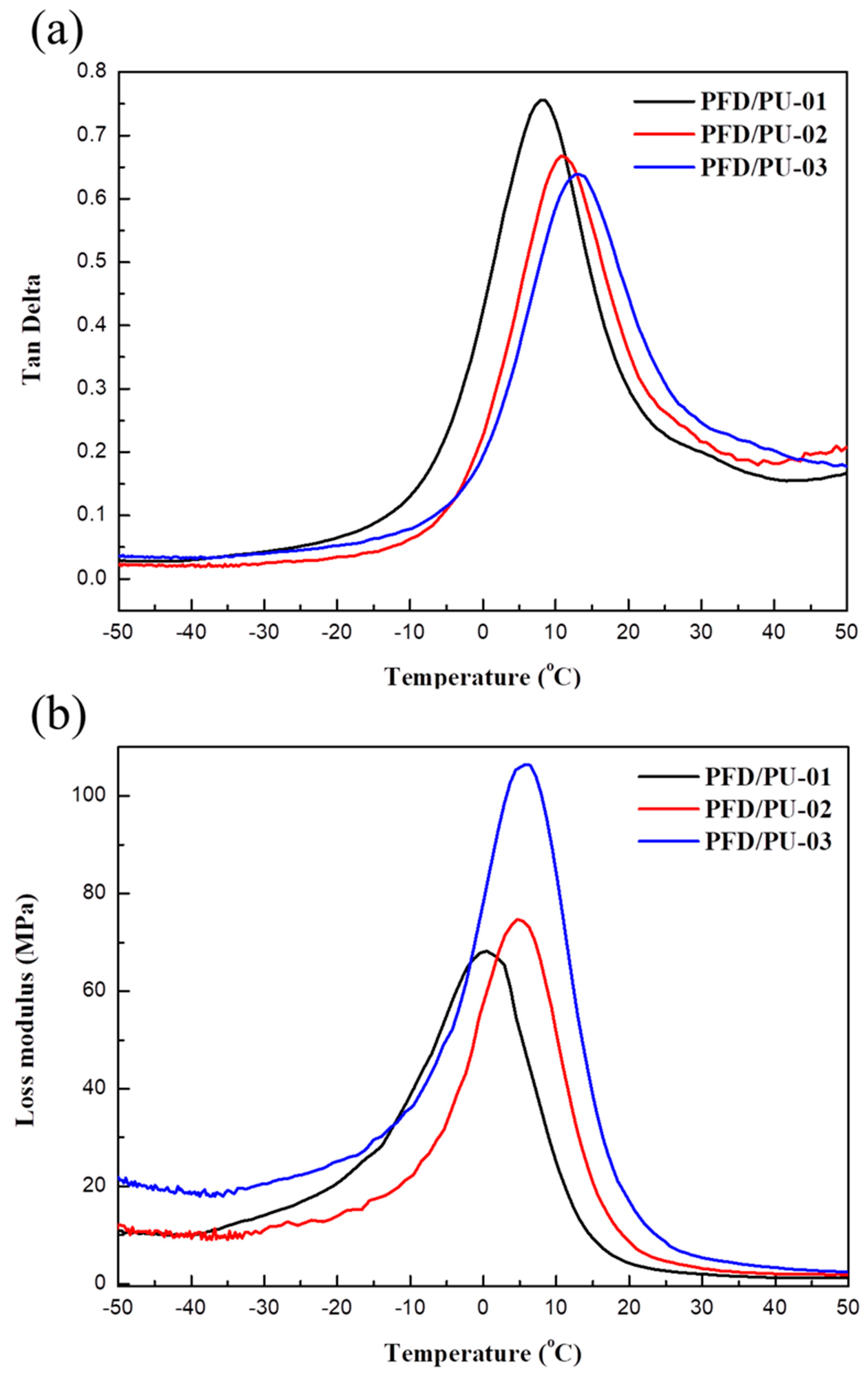
3.7. Dynamic Mechanical Analysis
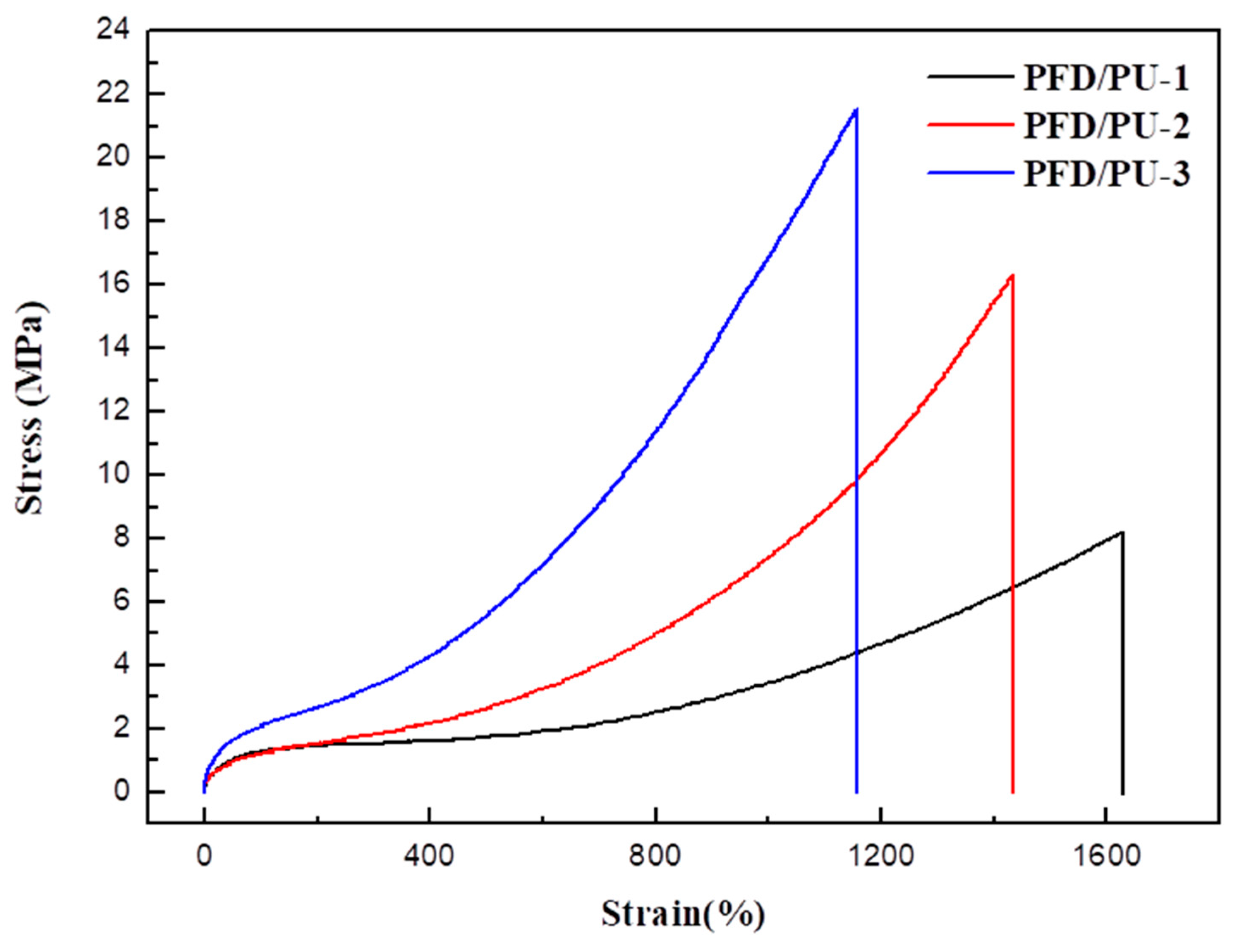
3.8. Tensile Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alagi, P.; Choi, Y.J.; Hong, S.C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60. [Google Scholar] [CrossRef]
- Schneider, N.S.; Sung, C.S.P.; Matton, R.W.; Illinger, J.L. Thermal Transition Behavior of Polyurethanes Based on Toluene Diisocyanate. Macromolecules 1975, 8, 62–67. [Google Scholar] [CrossRef]
- Paik Sung, C.S.; Schneider, N.S. Structure-property relationships of polyurethanes based on toluene di-isocyanate. J. Mater. Sci. 1978, 13, 1689–1699. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, R.; Xiao, Q.; Guo, H.; Wang, Z.; Li, X.; Chen, J.; Zhu, J. Asynchronous fracture of hierarchical microstructures in hard domain of thermoplastic polyurethane elastomer: Effect of chain extender. Polymer 2018, 138, 242–254. [Google Scholar] [CrossRef]
- Oprea, S. Effect of Composition and Hard-segment Content on Thermo-mechanical Properties of Cross-linked Polyurethane Copolymers. High Perform. Polym. 2009, 21, 353–370. [Google Scholar] [CrossRef]
- Li, G.; Li, E.; Wang, C.; Niu, Y. Effect of the blocked ratio on properties of natural fiber–waterborne blocked polyurethane composites. J. Compos. Mater. 2015, 49, 1929–1936. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.A. Lazzeri, Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Javni, I.; Petrović, Z.S.; Guo, A.; Fuller, R. Thermal stability of polyurethanes based on vegetable oils. J. Appl. Polym. Sci. 2000, 77, 1723–1734. [Google Scholar] [CrossRef]
- Chuang, F.S.; Tsen, W.C.; Shu, Y.C. The effect of different siloxane chain-extenders on the thermal degradation and stability of segmented polyurethanes. Polym. Degrad. Stab. 2004, 84, 69–77. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, H.T.; Tsou, C.H.; Gu, J.H.; Suen, M.C.; Chen, J.K. In Situ Polymerization and Characteristics of Biodegradable Waterborne Thermally-Treated Attapulgite Nanorods and Polyurethane Composites. J. Inorg. Organomet. Polym. Mater. 2017, 27, 244–256. [Google Scholar] [CrossRef]
- Tayfun, U.; Kanbur, Y.; Abacı, U.; Güney, H.Y.; Bayramlı, E. Mechanical, electrical, and melt flow properties of polyurethane elastomer/surface-modified carbon nanotube composites. J. Compos. Mater. 2017, 51, 1987–1996. [Google Scholar] [CrossRef]
- Verma, G.; Kaushik, A.; Ghosh, A.K. Preparation, characterization and properties of organoclay reinforced polyurethane nanocomposite coatings. J. Plast. Film Sheet. 2013, 29, 56–77. [Google Scholar] [CrossRef]
- Jiang, X.; Gu, J.; Shen, Y.; Wang, S.; Tian, X. New fluorinated siloxane-imide block copolymer membranes for application in organophilic pervaporation. Desalination 2011, 265, 74–80. [Google Scholar] [CrossRef]
- Chen, J.H.; Hu, D.D.; Li, Y.D.; Meng, F.; Zhu, J.; Zeng, J.B. Castor oil derived poly (urethane urea) networks with reprocessibility and enhanced mechanical properties. Polymer 2018, 143, 79–86. [Google Scholar] [CrossRef]
- Baradie, B.; Shoichet, M.S. Novel fluoro-terpolymers for coatings applications. Macromolecules 2005, 38, 5560–5568. [Google Scholar] [CrossRef]
- Lee, S.R.; Kim, M.R.; Jo, E.H.; Yoon, K.B. Synthesis of very low birefringence polymers using fluorinated macromers for polymeric waveguides. High Perform. Polym. 2016, 28, 131–139. [Google Scholar] [CrossRef]
- Saritha, B.; Thiruvasagam, P. Synthesis of diol monomers and organosoluble fluorinated polyimides with low dielectric: Study of structure–property relationship and applications. High Perform. Polym. 2015, 27, 842–851. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.P.; Zong, A.X.; He, X.Y.; Xu, J.Y.; Huang, M.S. Synthesis of newly fluorinated thermoplastic polyurethane elastomers and their blood compatibility. Fiber. Polym. 2015, 16, 231–238. [Google Scholar] [CrossRef]
- Liu, T.; Ye, L. Synthesis and properties of fluorinated thermoplastic polyurethane elastomer. J. Fluorine Chem. 2010, 131, 36–41. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Peng, X. Synthesis and characterization of novel fluorinated thermoplastic polyurethane with high transmittance and superior physical properties. Pure Appl. Chem. 2017, 54, 516–523. [Google Scholar] [CrossRef]
- Wu, C.L.; Chiu, S.H.; Lee, H.T.; Suen, M.C. Synthesis and properties of biodegradable polycaprolactone/polyurethanes using fluoro chain extenders. Polym. Adv. Technol. 2016, 27, 665–676. [Google Scholar] [CrossRef]
- Su, S.K.; Gu, J.H.; Lee, H.T.; Wu, C.L.; Hwang, J.J.; Suen, M.C. Synthesis and properties of novel biodegradable polyurethanes containing fluorinated aliphatic side chains. J. Polym. Res. 2017, 24, 142. [Google Scholar] [CrossRef]
- Dieterich, D. Aqueous emulsions, dispersions and solutions of polyurethanes; synthesis and properties. Prog. Org. Coat. 1981, 9, 281–340. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, F.; Chen, S.; Yang, H.; Ge, Z.; Chen, S. Development of shape memory polyurethane based on polyethylene glycol and liquefied 4, 4′-diphenylmethane diisocyanate using a bulk method for biomedical applications. Polym. Int. 2015, 64, 477–485. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, X.; Wang, H.; Liu, Y.; Du, Z. Surface and mechanical properties of waterborne polyurethane films reinforced by hydroxyl-terminated poly (fluoroalkyl methacrylates). Polymer 2017, 133, 68–77. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Li, L.; Liu, Y.; Li, Y.; Dong, Q. Influences of fluorine on microphase separation in fluorinated polyurethanes. Polymer 2016, 98, 311–319. [Google Scholar] [CrossRef]
- Kysilka, O.; Rybáčková, M.; Skalický, M.; Kvíčalová, M.; Cvačka, J.; Kvíčala, J. HFPO Trimer-Based Alkyl Triflate, a Novel Building Block for Fluorous Chemistry. Preparation, Reactions and 19F gCOSY Analysis. Collect. Czech. Chem. Commun. 2008, 73, 1799–1813. [Google Scholar] [CrossRef]
- Tang, Q.; Gao, K. Structure analysis of polyether-based thermoplastic polyurethane elastomers by FTIR, 1H NMR and 13C NMR. Int. J. Polym. Anal. Charact. 2017, 22, 569–574. [Google Scholar] [CrossRef]
- Kerr, S.; Naumkin, F.Y. Noncovalently bound complexes of polar molecules: Dipole-inside-of-dipole vs. dipole–dipole systems. New J. Chem. 2017, 41, 13576–13584. [Google Scholar] [CrossRef]
- Laoharojanaphand, P.; Lin, T.J.; Stoffer, J.O. Glow discharge polymerization of reactive functional silanes on poly(methyl methacrylate). J. Appl. Polym. Sci. 1990, 40, 369–384. [Google Scholar] [CrossRef]
- Li, N.; Zeng, F.; Wang, Y.; Qu, D.; Hu, W.; Luan, Y.; Dong, S.; Zhang, J.; Bai, Y. Fluorinated polyurethane based on liquid fluorine elastomer (LFH) synthesis via two-step method: The critical value of thermal resistance and mechanical properties. RSC Adv. 2017, 7, 30970–30978. [Google Scholar] [CrossRef]
- Sauer, B.B.; Mclean, R.S.; Thomas, R.R. Tapping mode AFM studies of nano-phases on fluorine-containing polyester coatings and octadecyltrichlorosilane monolayers. Langmuir 1998, 14, 3045–3051. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, W.; Hao, L.; Wang, S.; Pei, M.; Wang, X. Synthesis of novel cationic fluoroalkyl-terminated hyperbranched polyurethane latex and morphology, physical properties of its latex film. Prog. Org. Coat. 2018, 121, 209–217. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Hu, Y.; Lu, B.; Cai, T. Synthesis and Characterization of Novel Fluorinated Polyurethane Elastomer Based on 1,4-Bis (4-amino-2-trifluoformethyloxyphenyl) benzene. Asian J. Chem. 2012, 24, 3976–3980. [Google Scholar]
- Tsou, C.H.; Lee, H.T.; Tsai, H.A.; Cheng, H.J.; Suen, M.C. Synthesis and properties of biodegradable polycaprolactone/polyurethanes by using 2, 6-pyridinedimethanol as a chain extender. Polym. Degrad. Stabil. 2013, 98, 643–650. [Google Scholar] [CrossRef]


| Designation | MDI (moles) | PCL (moles) | PFD (moles) | Theoretical Hard Segment (wt%) | Theoretical Soft Segment (wt%) |
|---|---|---|---|---|---|
| PFD/PU-01 | 4 | 3.50 | 0.50 | 39.91 | 60.09 |
| PFD/PU-02 | 4 | 3.25 | 0.75 | 43.89 | 56.11 |
| PFD/PU-03 | 4 | 3.00 | 1.00 | 47.92 | 52.08 |
| Sample | Retention Time of the Peak (min) | |||
|---|---|---|---|---|
| PFD/PU-01 | 2.97 | 3.90 | 6.68 | 1.7 |
| PFD/PU-02 | 3.05 | 3.08 | 5.46 | 1.8 |
| PFD/PU-03 | 3.22 | 2.31 | 3.79 | 1.6 |
| Content | Binding Energy (eV) | Atomic Ratio (%) | |
|---|---|---|---|
| PFD/PU-01 | C1s | 289.18 | 92.19 |
| O1s | 536.83 | 2.56 | |
| N1s | 403.56 | 2.94 | |
| F1s | 690.86 | 2.31 | |
| PFD/PU-02 | C1s | 286.08 | 89.36 |
| O1s | 533.85 | 3.00 | |
| N1s | 400.42 | 3.23 | |
| F1s | 688.04 | 4.41 | |
| PFD/PU-03 | C1s | 284.54 | 71.45 |
| O1s | 532.23 | 14.9 | |
| N1s | 399.76 | 4.18 | |
| F1s | 688.22 | 9.47 |
| Designation | TGA | DSC | |
|---|---|---|---|
| Tonset (°C) | Residue at 700 °C (%) | Tg (°C) | |
| PFD/PU-01 | 299.2 | 2.7 | 3.7 |
| PFD/PU-02 | 305.9 | 4.1 | 5.6 |
| PFD/PU-03 | 308.6 | 4.2 | 10.3 |
| Sample | Tgd from Tan δ (°C) | Tan δmax | Tgd from E” (°C) |
|---|---|---|---|
| PFD/PU-01 | 7.9 | 0.756 | 0.1 |
| PFD/PU-02 | 10.8 | 0.668 | 3.6 |
| PFD/PU-03 | 13.3 | 0.638 | 5.4 |
| Sample | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PFD/PU-01 | 8.0 ± 0.2 | 0.5 | 1614 ± 34 |
| PFD/PU-02 | 16.1 ± 0.7 | 1.4 | 1427 ± 15 |
| PFD/PU-03 | 21.0 ± 1.3 | 2.1 | 1148 ± 57 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-W.; Lee, H.-T.; Tsai, H.-A.; Suen, M.-C.; Chiu, C.-W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers 2018, 10, 1292. https://doi.org/10.3390/polym10111292
Li J-W, Lee H-T, Tsai H-A, Suen M-C, Chiu C-W. Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers. 2018; 10(11):1292. https://doi.org/10.3390/polym10111292
Chicago/Turabian StyleLi, Jia-Wun, Hsun-Tsing Lee, Hui-An Tsai, Maw-Cherng Suen, and Chih-Wei Chiu. 2018. "Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders" Polymers 10, no. 11: 1292. https://doi.org/10.3390/polym10111292
APA StyleLi, J.-W., Lee, H.-T., Tsai, H.-A., Suen, M.-C., & Chiu, C.-W. (2018). Synthesis and Properties of Novel Polyurethanes Containing Long-Segment Fluorinated Chain Extenders. Polymers, 10(11), 1292. https://doi.org/10.3390/polym10111292