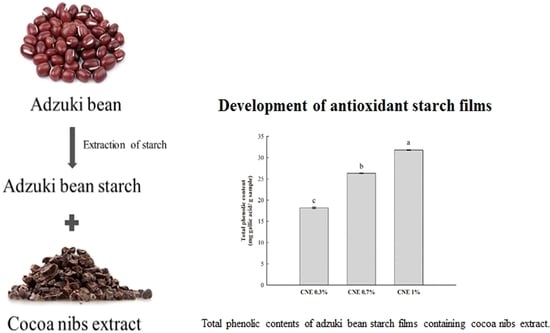
Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of ABS
2.3. Extraction of CNE
2.4. Amylose Content of ABS
2.5. CNE Analysis
2.6. Preparation of ABS Films Containing CNE
2.7. Physical Properties
2.8. Color Measurement and Ultraviolet (UV)-Visible Light Transmittance
2.9. Total Phenolic Content (TPC)
2.10. Antioxidant Activity
2.11. Attenuated Total Reflectance-Fourier Transformation Infrared (ATR-FTIR) Spectroscopy
2.12. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.13. Biodegradation Test
2.14. Statistical Analysis
3. Results and Discussion
3.1. Starch Yield and Amylose Content
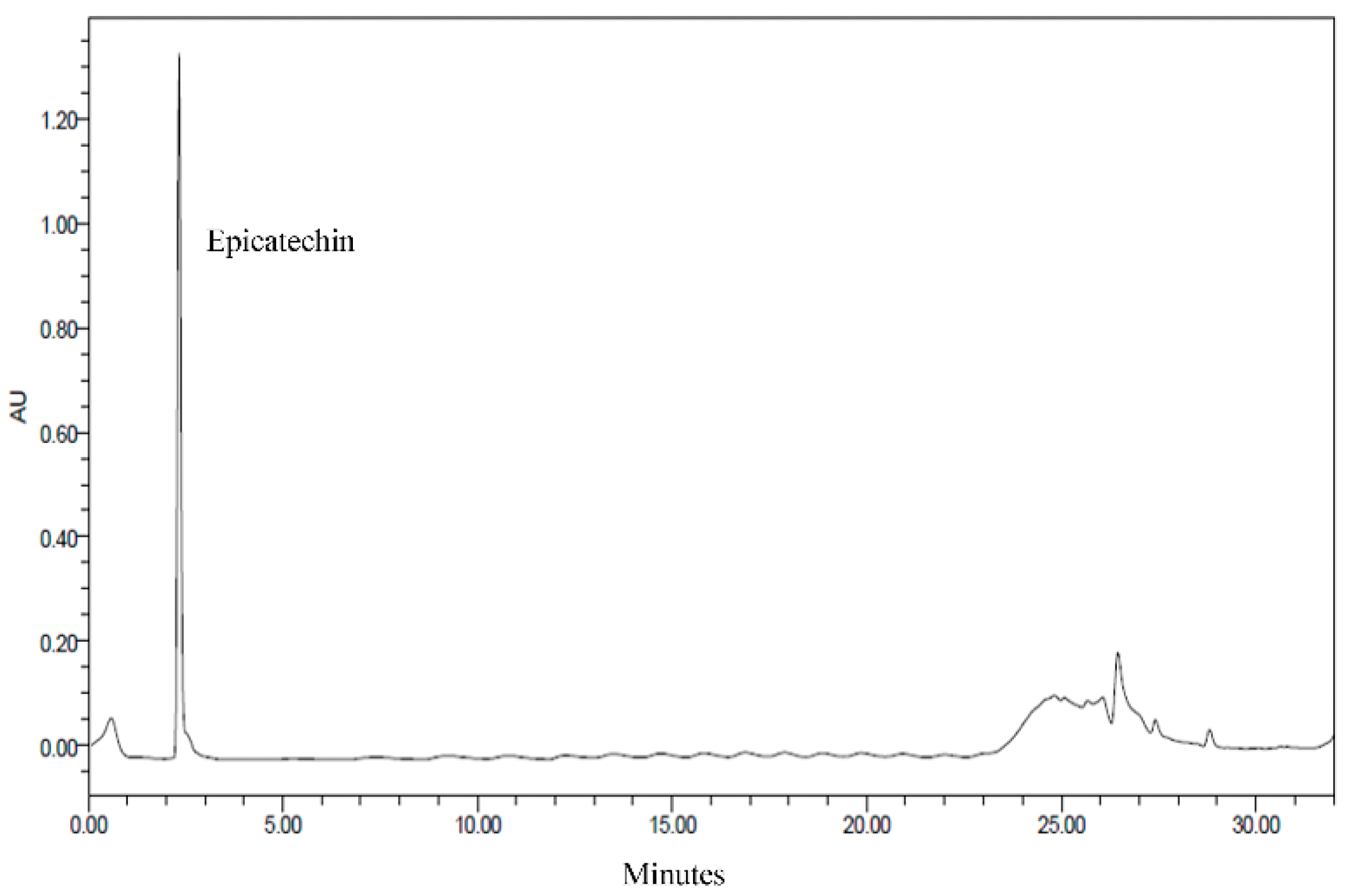
3.2. HPLC Analysis of CNE
3.3. Physical Properties of ABS Films Containing CNE
3.4. Color Measurement
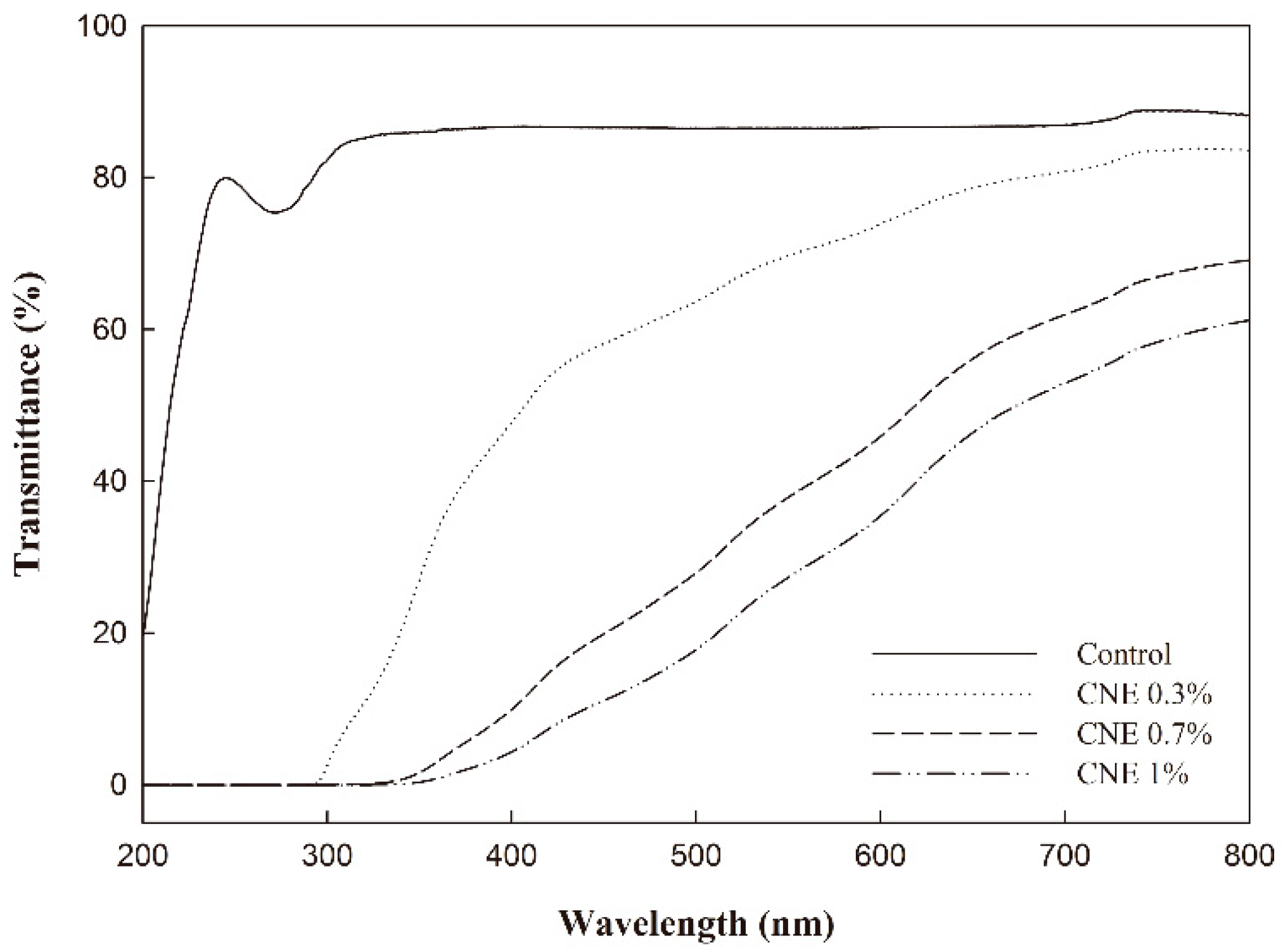
3.5. UV-Visible Light Transmittance
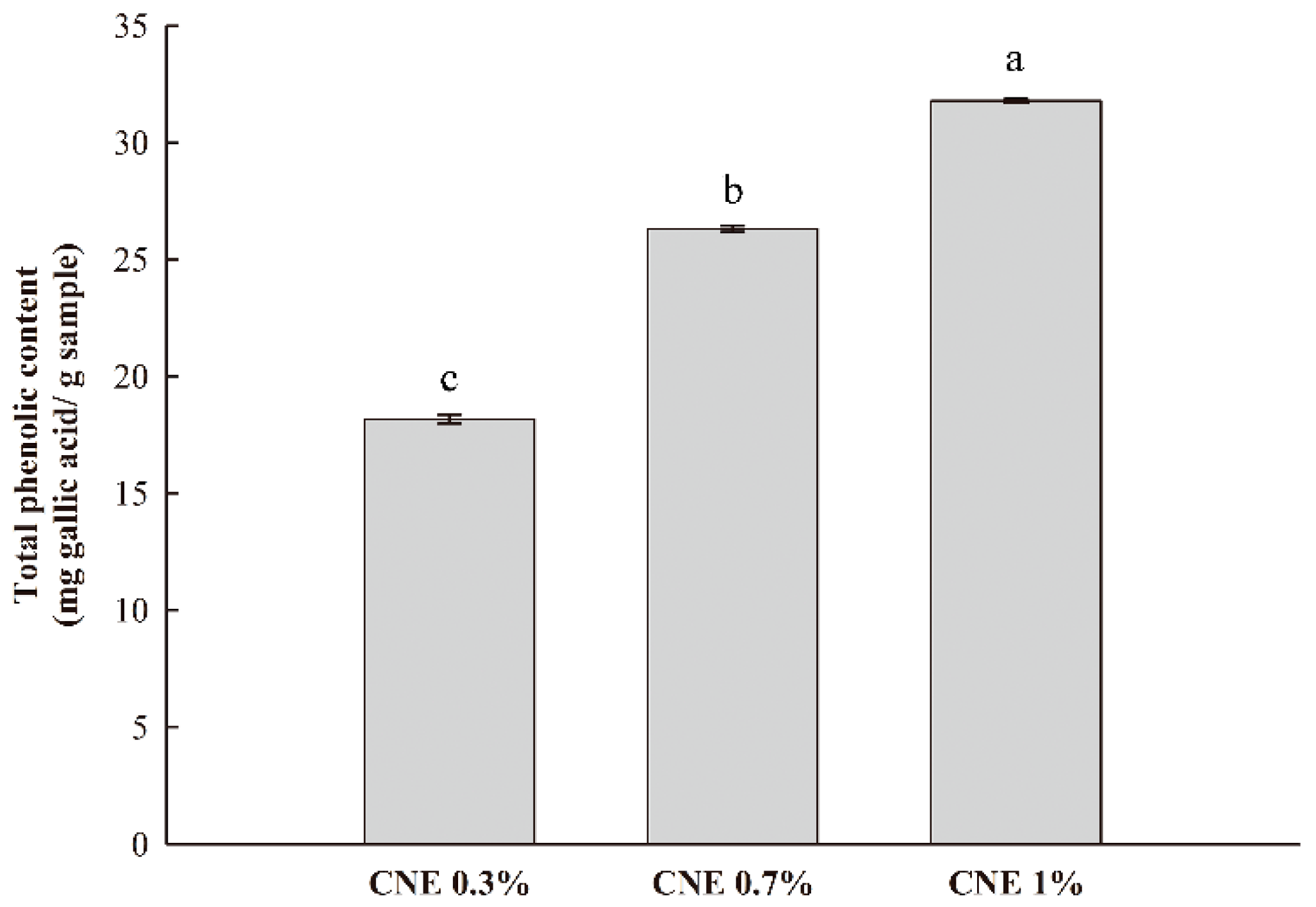
3.6. TPC Analysis
3.7. Antioxidant Activity
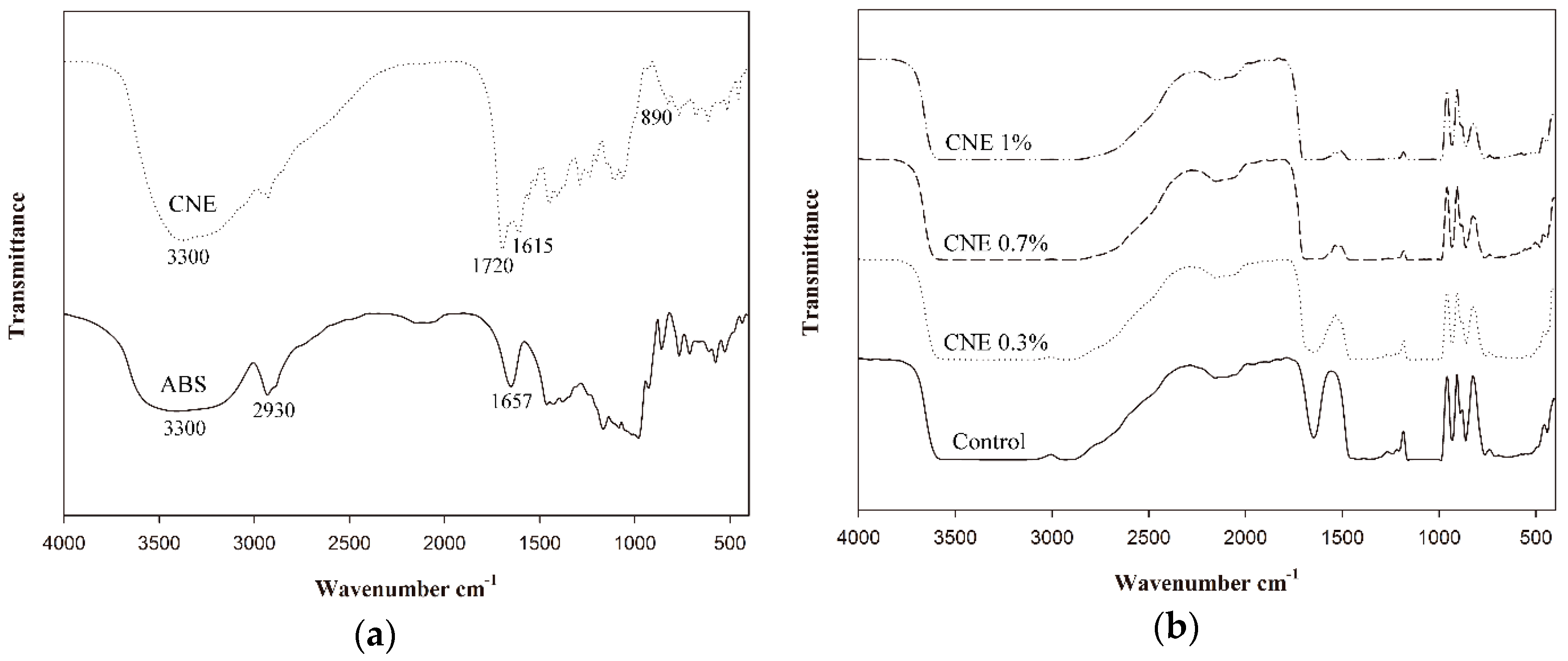
3.8. ATR-FTIR Analysis
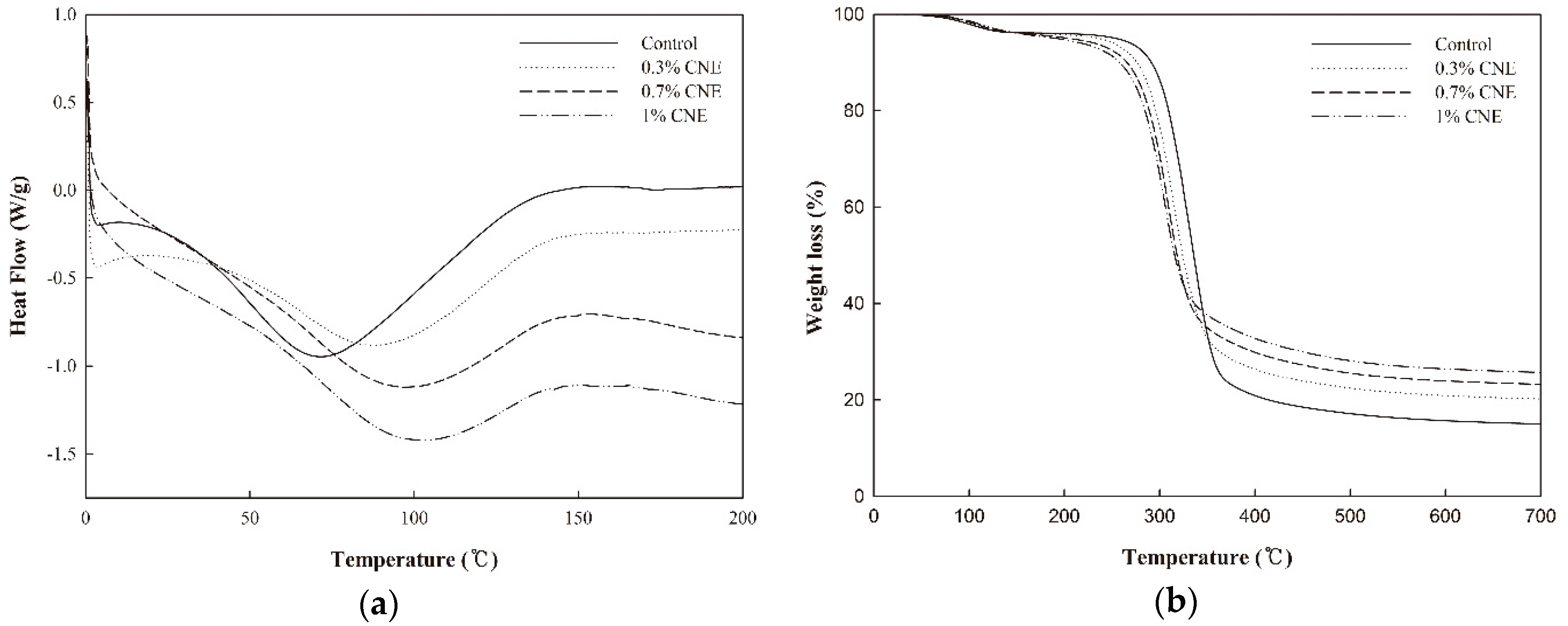
3.9. DSC
3.10. TGA
3.11. Biodegradability Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.K.; Luan, F.; Xu, B. Morphology, crystallinity, pasting, thermal and quality characteristics of starches from adzuki bean (Vigna angularis L.) and edible kudzu (Pueraria thomsonii Benth). Int. J. Biol. Macromol. 2017, 105, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Sherasia, P.L.; Garg, M.R.; Bhanderi, B.M. Pulses and Their by-Products as Animal Feed; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018. [Google Scholar]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Tran, T.N.; Athanassiou, A.; Basit, A.; Bayer, I.S. Starch-based bio-elastomers functionalized with red beetroot natural antioxidant. Food Chem. 2017, 216, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of chitosan–starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Lee, J.H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Pasamar, M.Á.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Obtention and characterization of phenolic extracts from different cocoa sources. J. Agric. Food Chem. 2008, 56, 9621–9627. [Google Scholar] [CrossRef] [PubMed]
- Mazor Jolić, S.; Radojčić Redovniković, I.; Marković, K.; Ivanec Šipušić, Đ.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)–A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cik, T.T.; Lii, C.Y.; Lai, P.; Chen, H.H. Effect of amylose content on structure, texture and α-amylase reactivity of cooked rice. LWT-Food Sci. Technol. 2013, 54, 224–228. [Google Scholar] [CrossRef]
- Yang, S.Y.; Cao, L.; Kim, H.; Beak, S.E.; Song, K.B. Utilization of foxtail millet starch film incorporated with clove leaf oil for the packaging of Queso Blanco cheese as a model food. Starch-Stärke 2018, 70, 1700171. [Google Scholar] [CrossRef]
- Cao, T.L.; Yang, S.Y.; Song, K.B. Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018, 19, 131. [Google Scholar]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; Rios, A.D.O. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhu, J.; Zhang, S.; Li, X.; Zhang, B.; Pu, H.; Li, L.; Wang, Q. Hierarchical structure and thermal behavior of hydrophobic starch-based films with different amylose contents. Carbohydr. Polym. 2018, 181, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberán, F.A.; Cienfuegos-Jovellanos, E.; Marín, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerdá, B.; Zafrilla, P.; Morillas, J.; Ibarra, A.; Pasamar, M.A. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Physico-mechanical properties of starch-based films containing naturally derived antimicrobial agents. Packag. Technol. Sci. 2014, 27, 149–159. [Google Scholar] [CrossRef]
- Bof, M.J.; Jiménez, A.; Locaso, D.E.; Garcia, M.A.; Chiralt, A. Grapefruit seed extract and lemon essential oil as active agents in corn starch–chitosan blend films. Food Bioprocess Tech. 2016, 9, 2033–2045. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Nouri, L.; Nafchi, A.M. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int. J. Biol. Macromol. 2014, 66, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wang, B.J.; Weng, Y.M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT-Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Cerruti, P.; Santagata, G.; d’Ayala, G.G.; Ambrogi, V.; Carfagna, C.; Malinconico, M.; Persico, P. Effect of a natural polyphenolic extract on the properties of a biodegradable starch-based polymer. Polym. Degrad. Stab. 2011, 96, 839–846. [Google Scholar] [CrossRef]
- Xiong, H.; Tang, S.; Tang, H.; Zou, P. The structure and properties of a starch-based biodegradable film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef] [PubMed]
| CNE (%) | Thickness (mm) | Tensile Strength (MPa) | Elongation at Break (%) | Water Vapor Permeability (10−9 g /m s Pa) |
|---|---|---|---|---|
| 0 | 0.053 ± 0.002 d | 30.59 ± 2.21 a | 10.11 ± 2.30 d | 2.11 ± 0.12 b |
| 0.3 | 0.058 ± 0.001 c | 27.74 ± 0.98 b | 13.81 ± 0.90 c | 2.42 ± 0.27 ab |
| 0.7 | 0.062 ± 0.001 b | 25.50 ± 1.12 c | 24.51 ± 2.51 b | 2.55 ± 0.30 ab |
| 1 | 0.066 ± 0.003 a | 18.58 ± 1.60 d | 27.80 ± 2.46 a | 2.81 ± 0.49 a |
| CNE (%) | L * | a * | b * | ∆E | Opacity (A/mm) |
|---|---|---|---|---|---|
| 0 | 95.93 ± 0.50 a | −0.22 ± 0.03 d | 2.31 ± 0.26 d | - | 1.34 ± 0.10 d |
| 0.3 | 88.69 ± 0.13 b | 1.94 ± 0.07 c | 14.48 ± 0.12 c | 14.32 ± 0.06 c | 2.02 ± 0.11 c |
| 0.7 | 75.99 ± 0.18 c | 6.94 ± 0.08 b | 30.92 ± 0.26 b | 35.66 ± 0.28 b | 4.84 ± 0.03 b |
| 1 | 69.49 ± 0.86 d | 10.97 ± 0.50 a | 36.62 ± 0.45 a | 44.91 ± 0.87 a | 5.63 ± 0.08 a |
| CNE (%) | ABTS Radical Scavenging (%) | DPPH Radical Scavenging (%) |
|---|---|---|
| 0 | - | - |
| 0.3 | 97.70 ± 0.86 b | 66.13 ± 0.21 c |
| 0.7 | 99.72 ± 0.24 a | 94.22 ± 0.40 b |
| 1 | 100.00 ± 0.00 a | 94.88 ± 0.05 a |
| CNE (%) | Tm (°C) | ΔH (J/g) |
|---|---|---|
| 0 | 71.97 | 199.51 |
| 0.3 | 87.69 | 129.52 |
| 0.7 | 96.81 | 146.50 |
| 1 | 102.29 | 110.48 |
| CNE (%) | Weight Loss (%) | ||||
|---|---|---|---|---|---|
| 4 d | 7 d | 14 d | 21 d | 28 d | |
| 0 | 22.58 ± 0.23 Ec | 42.27 ± 1.93 Dc | 58.12 ± 1.56 Cc | 75.52 ± 2.05 Bc | 98.28 ± 0.52 Ab |
| 0.3 | 26.75 ± 1.09 Eb | 51.32 ± 2.11 Db | 67.84 ± 2.50 Cb | 93.38 ± 0.66 Bb | 99.26 ± 0.35 Aa |
| 0.7 | 28.41 ± 1.67 Eb | 52.14 ± 2.50 Dab | 74.25 ± 2.66 Ca | 96.07 ± 1.09 Ba | 99.57 ± 0.24 Aa |
| 1 | 34.41 ± 0.59 Ea | 56.05 ± 1.98 Da | 75.89 ± 1.28 Ca | 97.45 ± 0.63 Ba | 99.76 ± 0.24 Aa |
| CNE (%) | Water Solubility (%) | Moisture Content (%) |
|---|---|---|
| 0 | 32.38 ± 1.09 d | 7.25 ± 0.33 b |
| 0.3 | 35.25 ± 0.41 c | 7.90 ± 0.80 ab |
| 0.7 | 39.77 ± 0.52 b | 8.37 ± 0.31 a |
| 1 | 44.30 ± 0.54 a | 8.43 ± 0.34 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Baek, S.-K.; Go, E.; Song, K.B. Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract. Polymers 2018, 10, 1210. https://doi.org/10.3390/polym10111210
Kim S, Baek S-K, Go E, Song KB. Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract. Polymers. 2018; 10(11):1210. https://doi.org/10.3390/polym10111210
Chicago/Turabian StyleKim, Sujin, Su-Kyoung Baek, Eunjeong Go, and Kyung Bin Song. 2018. "Application of Adzuki Bean Starch in Antioxidant Films Containing Cocoa Nibs Extract" Polymers 10, no. 11: 1210. https://doi.org/10.3390/polym10111210