A Novel Fabrication of Spherical Fe50Ni50 Alloy Powders via in-Situ De-Wetting of Liquid Solid Interface
Abstract
1. Introduction
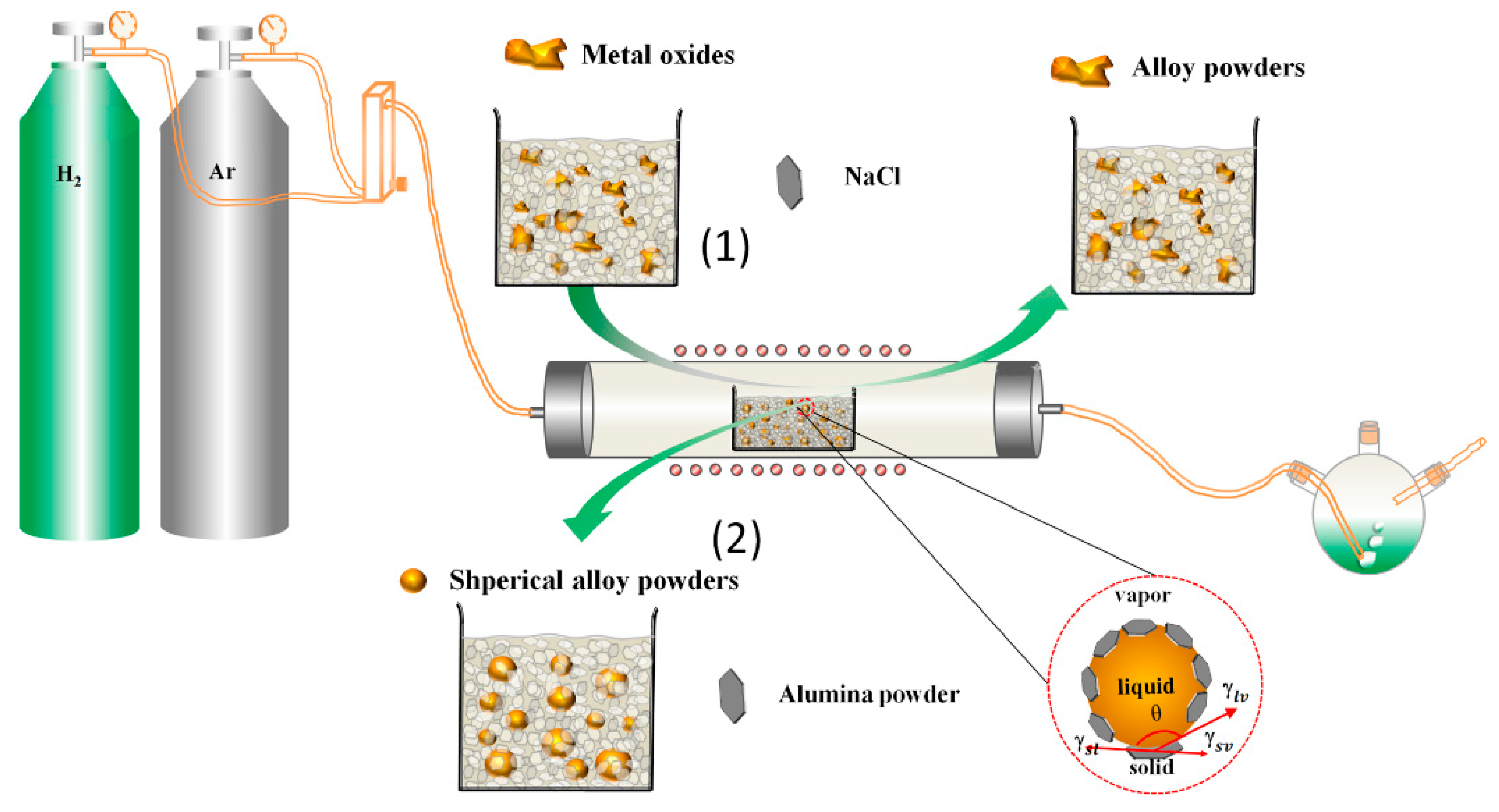
2. Experiment Procedure
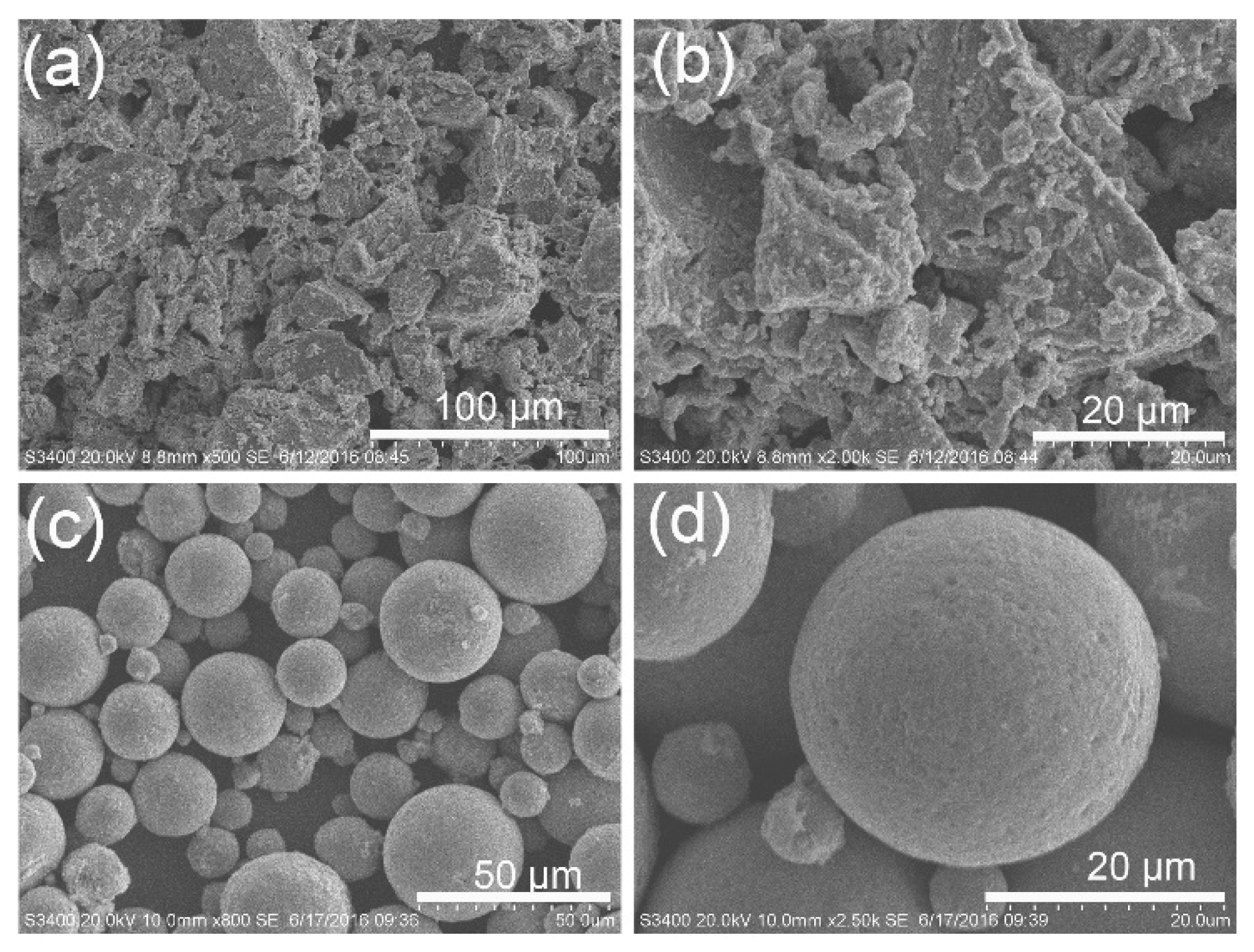
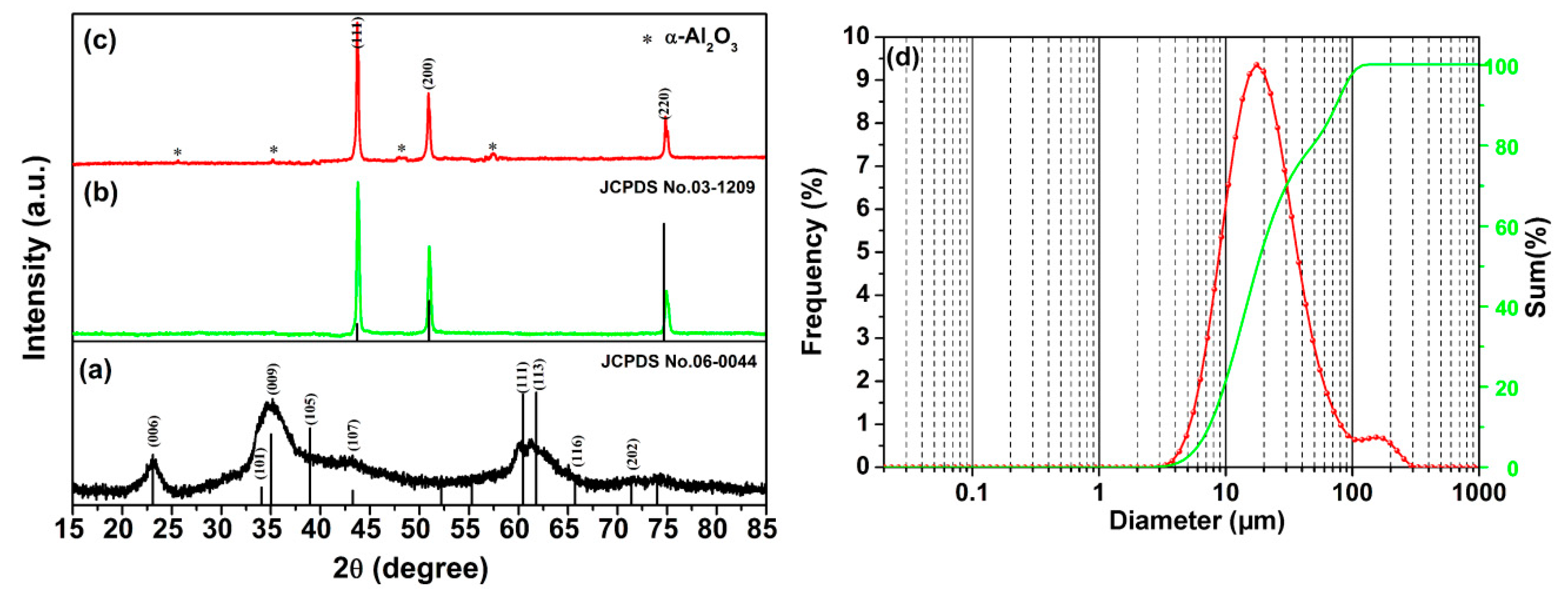
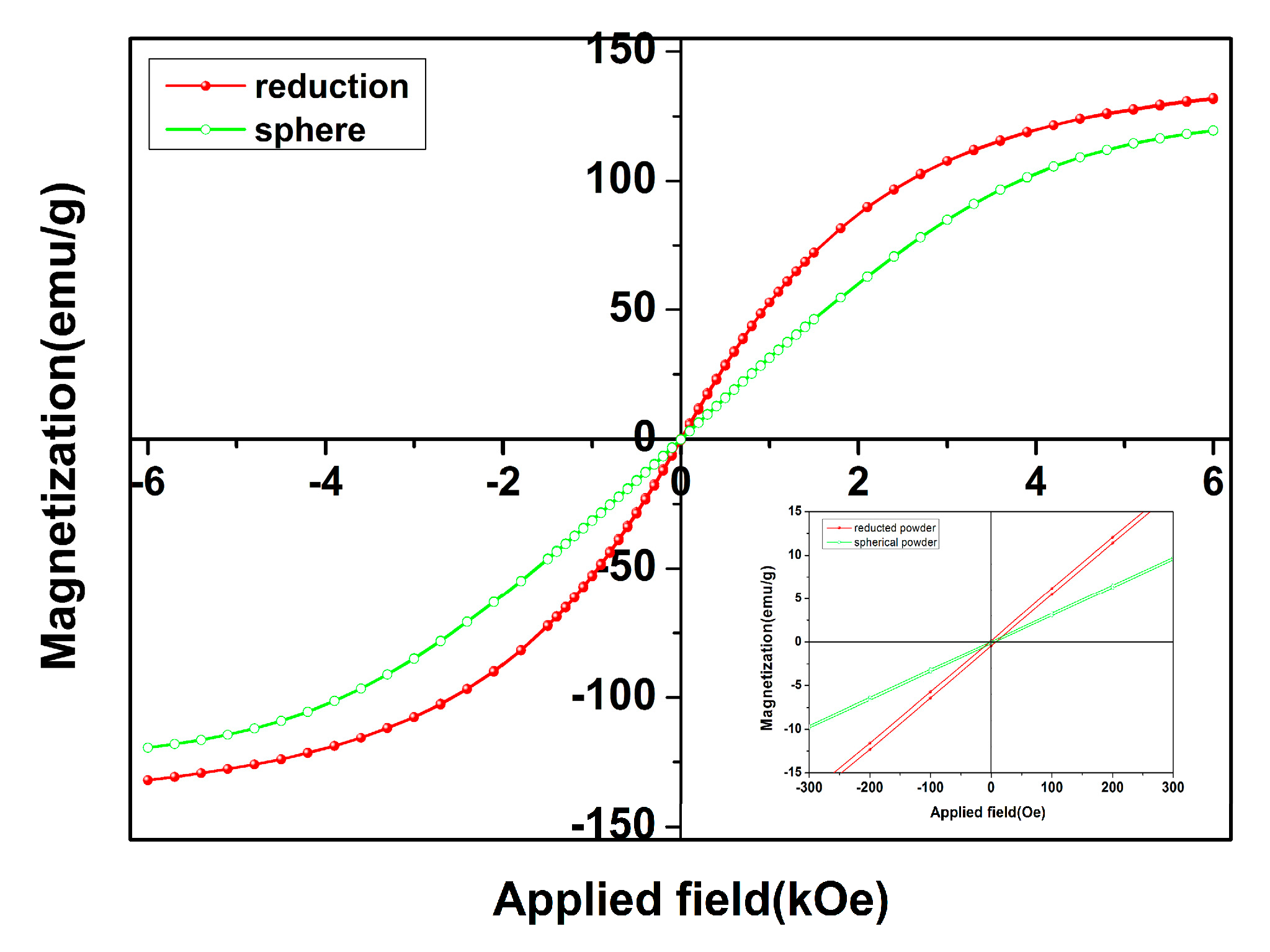
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meng, X.; Shen, X. Preparation of FeCo-, FeNi- and NiCo-alloy coated cenosphere composites by heterogeneous precipitation. Particuology 2012, 10, 334–338. [Google Scholar] [CrossRef]
- Godec, M.; Mandrino, D.; Sustarsic, B.; Jenko, M. Surface and electrical studies of Fe-Si-B powders for soft magnetic applications. Surf. Interface Anal. 2002, 34, 346–351. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, T. Preparation, characterization and microwave absorbing properties of FeNi alloy prepared by gas atomization method. J. Alloy. Compd. 2012, 513, 455–459. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, C.; Xia, A.; Zhang, J.; Zhu, G. Structural and magnetic properties of nanocrystalline nickel-rich Fe–Ni alloy powders prepared via hydrazine reduction. J. Magn. Magn. Mater. 2013, 336, 14–19. [Google Scholar] [CrossRef]
- Dong, S.; Song, B.; Zhang, X.; Deng, C.; Fenineche, N.; Hansz, B.; Liao, H.; Coddet, C. Fabrication of FeSiB magnetic coatings with improved saturation magnetization by plasma spray and dry-ice blasting. J. Alloy. Compd. 2014, 584, 254–260. [Google Scholar] [CrossRef]
- Fan, X.A.; Wang, J.; Wu, Z.; Li, G. Core–shell structured FeSiAl/SiO2 particles and Fe3Si/Al2O3 soft magnetic composite cores with tunable insulating layer thicknesses. Mater. Sci. Eng. B 2015, 201, 79–86. [Google Scholar] [CrossRef]
- Azizi, A.; Sadrnezhaad, S.K. Synthesis of Fe–Ni nano-particles by low-temperature hydrogen reduction of mechanically alloyed Ni-ferrite. J Alloy. Compd. 2009, 485, 484–487. [Google Scholar] [CrossRef]
- Shen, X.Q.; Jing, M.X.; Li, W.X.; Li, D.H. Fabrication of Fe-, Ni- and FeNi-coated Al2O3 core-shell microspheres by heterogeneous precipitation. Powder Technol. 2005, 160, 229–233. [Google Scholar] [CrossRef]
- Mirhoseini, F.; Bateni, A.; Firoozi, S. Gas phase synthesis of Ni3Fe nanoparticles by magnesium reduction of metal chlorides. Powder Technol. 2012, 228, 158–162. [Google Scholar] [CrossRef]
- Azizi, A. Worthwhile effects of salt-matrix reduction on shape, morphology and magnetic properties of FeNi nanoparticles. Mater. Sci. Eng. B 2011, 176, 1517–1520. [Google Scholar] [CrossRef]
- Lei, C.; Huang, H.; Huang, Y.; Cheng, Z.; Tang, S.; Du, Y. In-situ de-wetting assisted fabrication of spherical Cu-Sn alloy powder via the reduction of mixture metallic oxides. Powder Technol. 2016, 301, 356–359. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, T. Enhancement of electromagnetic and microwave absorbing properties of gas atomized Fe-50wt%Ni alloy by shape modification. J. Magn. Magn. Mater. 2012, 324, 2528–2533. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Harper, H.; Smith, D.J.; Hu, H.; Jiang, Q.; Solomon, V.C.; Radousky, H.B. Hollow metallic microspheres produced by spark erosion. Appl. Phys. Lett. 2004, 85, 940. [Google Scholar] [CrossRef]
- Habenicht, A.; Olapinski, M.; Burmeister, F.; Leiderer, P.; Boneberg, J. Jumping nanodroplets. Science 2005, 309, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Giermann, A.L.; Thompson, C.V. Solid-state dewetting for ordered arrays of crystallographically oriented metal particles. Appl. Phys. Lett. 2005, 86, 121903. [Google Scholar] [CrossRef]
- Pournaderi, S.; Mahdavi, S.; Akhlaghi, F. Fabrication of Al/Al2O3 composites by in-situ powder metallurgy (IPM). Powder Technol. 2012, 229, 276–284. [Google Scholar] [CrossRef]
- Humenik, M., Jr.; Kingery, W.D. Metal-ceramic interactions: III, surface tension and wettability of metal-ceramic systems. J. Am. Ceram. Soc.-Humenik Kingery 1954, 37, 18–23. [Google Scholar] [CrossRef]
- Batyrev, I.G.; Kleinman, L. In-plane relaxation of Cu(111) and Al(111)/α−Al2O3(0001) interfaces. Phys. Rev. B 2001, 64. [Google Scholar] [CrossRef]
- Zhou, X.B.; de Hosson, J.T.M. Reactive wetting of liquid metals on ceramic substrates. Acta Mater. 1996, 44, 421–426. [Google Scholar] [CrossRef]
- Oshanin, G.; de Coninck, J.; Cazabat, A.M.; Moreau, M. Dewetting, partial wetting, and spreading of a two-dimensional monolayer on solid surface. Phys. Rev. E 1998, 58, R20. [Google Scholar] [CrossRef]
- Silva, H.; Nielsen, M.G.; Fiordaliso, E.M.; Damsgaard, C.D.; Gundlach, C.; Kasama, T.; Chorkendorff, I.B.; Chakraborty, D. Synthesis and characterization of Fe–Ni/ɣ-Al2O3 egg-shell catalyst for H2 generation by ammonia decomposition. Appl. Catal. A Gen. 2015, 505, 548–556. [Google Scholar] [CrossRef]
- Chen, M.; Gao, M.; Dang, F.; Wang, N.; Zhang, B.; Pan, S. Tunable negative permittivity and permeability in FeNiMo/Al2O3 composites prepared by hot-pressing sintering. Ceram. Int. 2016, 42, 6444–6449. [Google Scholar] [CrossRef]
- Passerone, A.; Muolo, M.L.; Passerone, D. Wetting of group IV diborides by liquid metals. J. Mater. Sci. 2006, 41, 5088–5098. [Google Scholar] [CrossRef]
- Yasinskaya, G. The wetting of refractory carbides, borides, and nitrides by molten metals. Sov. Powder Metall. Metal Ceram. 1966, 5, 557–559. [Google Scholar] [CrossRef]
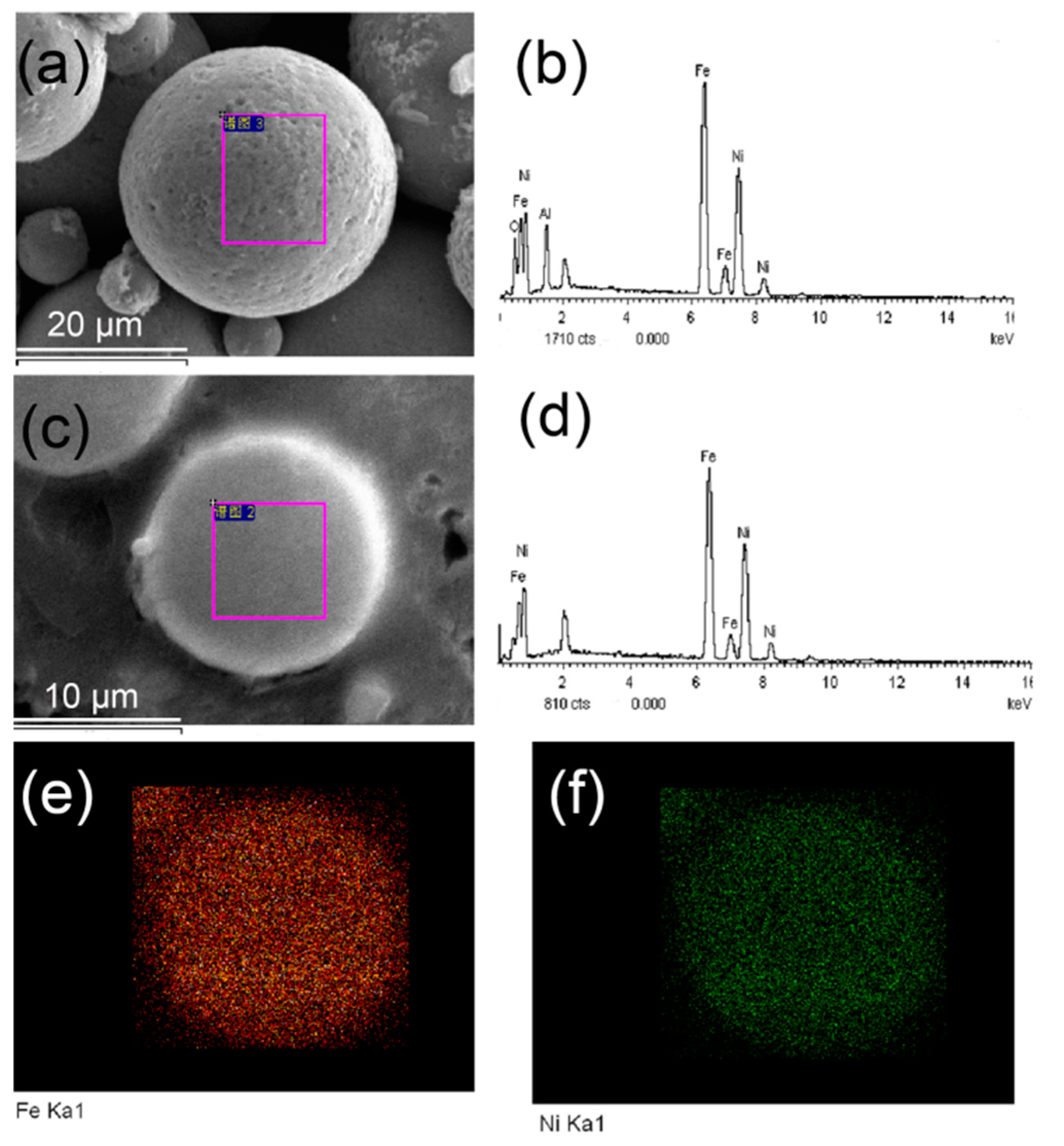
| Element | Surface of Particle | Cross-Section of Particle | ||
|---|---|---|---|---|
| at.% | wt.% | at.% | wt.% | |
| Fe | 34.78 | 43.06 | 50.56 | 49.31 |
| Ni | 32.96 | 42.90 | 49.44 | 50.69 |
| O | 21.59 | 7.66 | - | - |
| Al | 10.67 | 6.39 | - | - |
| Sample | Reduced with Salt Matrixat 973 K | Spheroidizing with Alumina at 1823 K |
|---|---|---|
| Ms (emu/g) | 131.6 | 119.4 |
| Mr (emu/g) | 0.14 | 0.05 |
| Hc (Oe) | 4.83 | 3.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Lei, C.; Zhong, W. A Novel Fabrication of Spherical Fe50Ni50 Alloy Powders via in-Situ De-Wetting of Liquid Solid Interface. Crystals 2019, 9, 325. https://doi.org/10.3390/cryst9070325
Song X, Lei C, Zhong W. A Novel Fabrication of Spherical Fe50Ni50 Alloy Powders via in-Situ De-Wetting of Liquid Solid Interface. Crystals. 2019; 9(7):325. https://doi.org/10.3390/cryst9070325
Chicago/Turabian StyleSong, Xueyin, Chenglong Lei, and Wei Zhong. 2019. "A Novel Fabrication of Spherical Fe50Ni50 Alloy Powders via in-Situ De-Wetting of Liquid Solid Interface" Crystals 9, no. 7: 325. https://doi.org/10.3390/cryst9070325
APA StyleSong, X., Lei, C., & Zhong, W. (2019). A Novel Fabrication of Spherical Fe50Ni50 Alloy Powders via in-Situ De-Wetting of Liquid Solid Interface. Crystals, 9(7), 325. https://doi.org/10.3390/cryst9070325




