Synthesis, Characterization, and Fluorescence Properties of Two New Heterocyclic Compounds Containing 1,5-Dioxaspiro Group
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
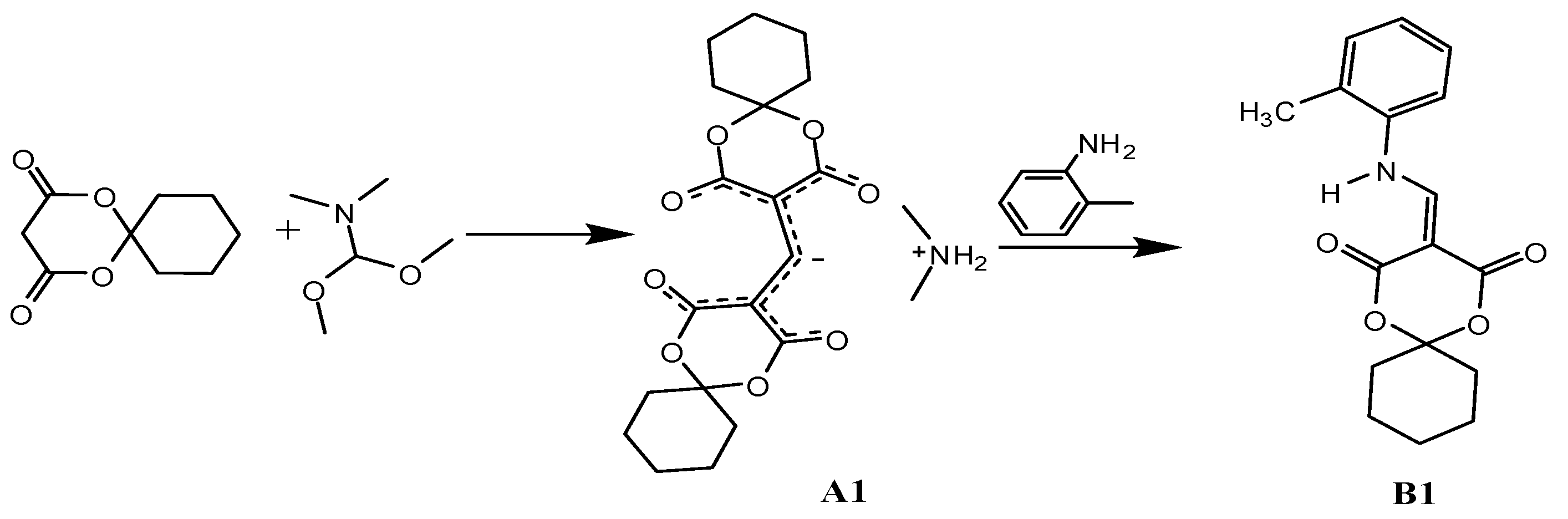
2.2. Synthetic Procedures of A1 and B1
2.3. Crystal Data and Structure Refinement
3. Results and Discussion
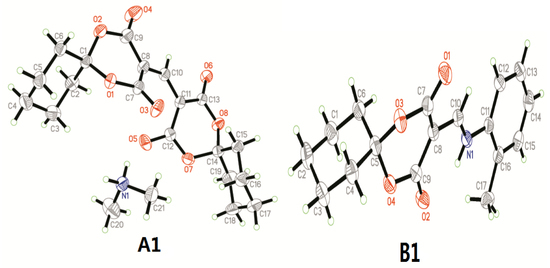
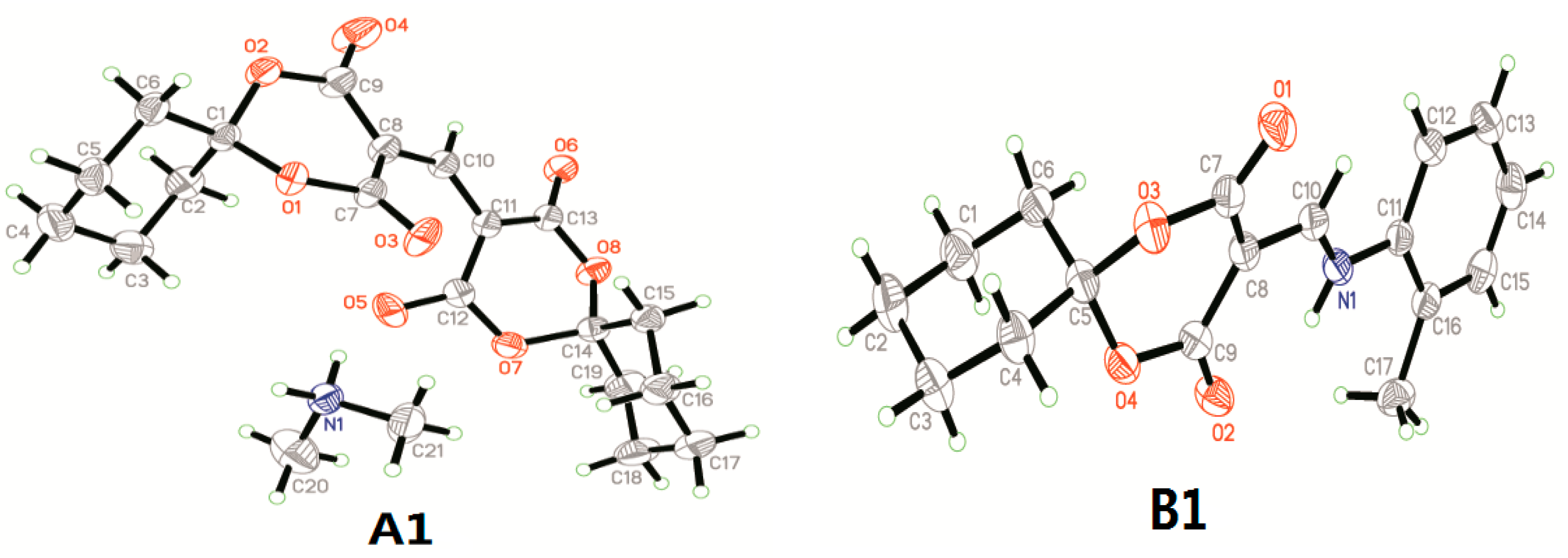
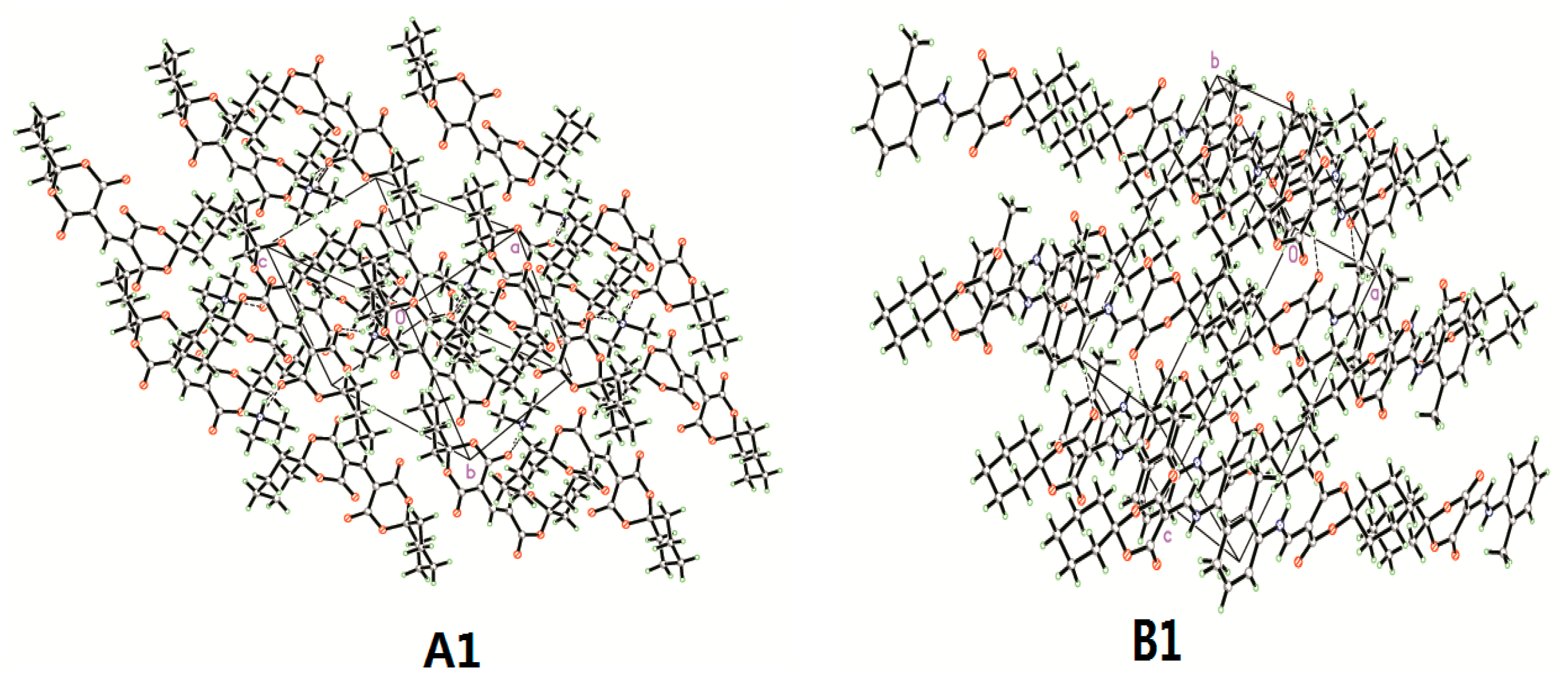
3.1. Crystal Structure Determination of A1 and B1
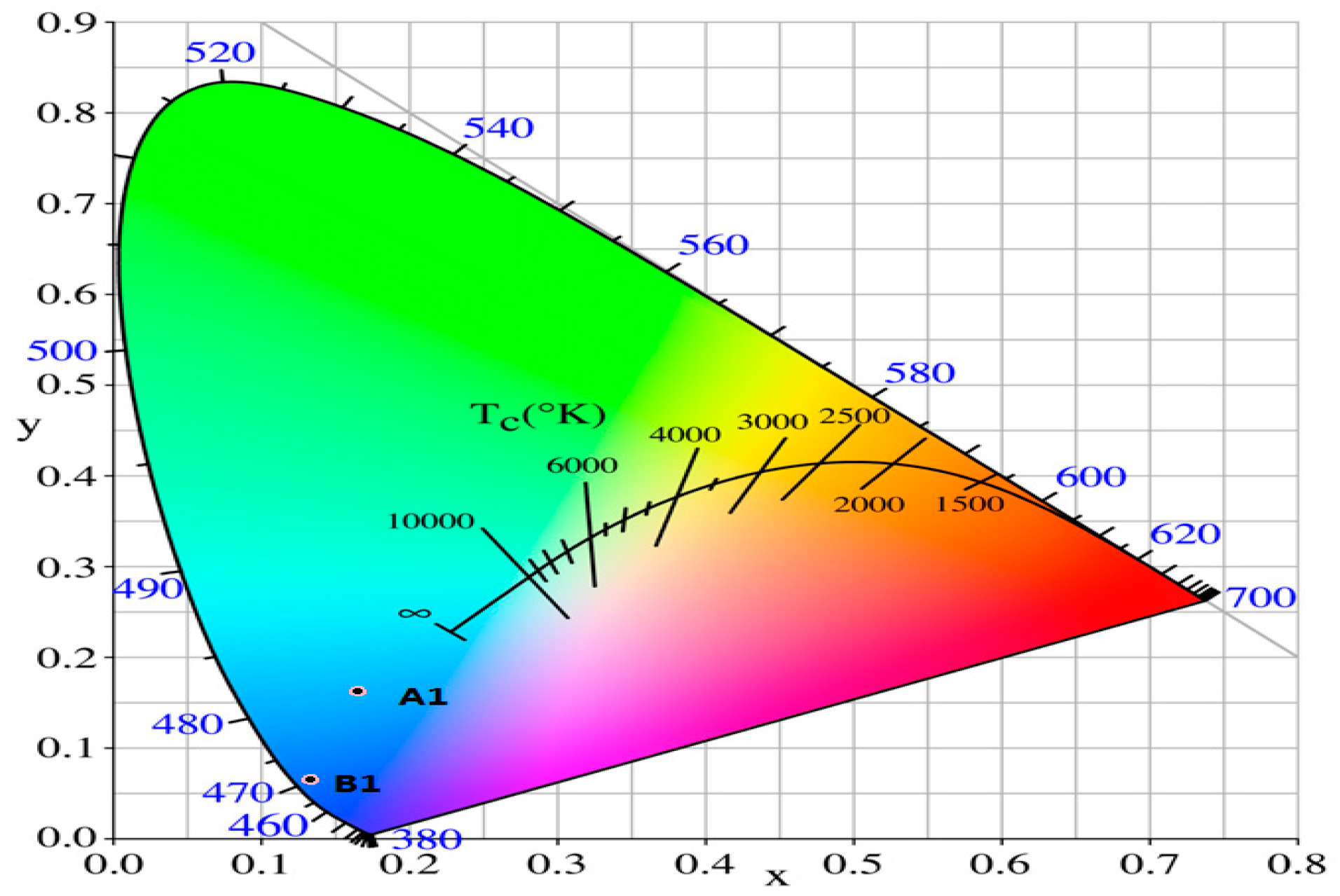
3.2. Spectroscopic Properties of A1 and B1
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Horley, N.J.; Beresford, K.J.M.; Chawla, T.; McCann, G.J.P.; Rupareli, K.C.; Gatchie, L.; Sonawane, V.R.; Williams, I.S.; Tan, H.L.; Joshi, P.; et al. Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur. J. Med. Chem. 2017, 129, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, J.; Polo, S.; Insuasty, B.; Quiroga, J. Design, facile synthesis, and evaluation of novel spiro- and pyrazolo[1,5-c]quinazolines as cholinesterase inhibitors: Molecular docking and MM/GBSA studies. Comput. Biol. Chem. 2018, 74, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K.A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; et al. Flupyrimin: A novel insecticide acting at the nicotinic acetylcholine receptors. J. Agric. Food Chem. 2017, 65, 7865–7873. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, A.N.; Wheeler, S.E. Stacking interactions of heterocyclic drug fragments with protein amide backbones. Chem. Med. Chem. 2018, 13, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Namieciñska, E.; Sobiesiak, M.; Małecka, M.; Guga, P.; Rozalska, B.; Budzisz, E. Antimicrobial and structural properties of metal ions complexes with thiosemicarbazide motif and related heterocyclic compounds. Curr. Med. Chem. 2018, 25, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Sun, Q.; Zhang, Z.Z.; Tang, L.L.; Shen, X.; Xue, S.F.; Yang, W.J. New frog-type Dibenzo[a,c][1,2,5]thiadiazolo[3,4-i]phenazine heterocyclic derivatives with aggregation-enhanced one- and two-photon excitation NIR fluorescence. Dyes Pigments 2018, 153, 233–240. [Google Scholar] [CrossRef]
- Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Heterocyclic amino acids as fluorescent reporters for transition metals: Synthesis and evaluation of novel furyl-benzoxazol-5-yl-l-alanines. New J. Chem. 2018, 42, 3483–3492. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, X.X.; Zhang, J.; Zhou, Y.; Fan, S.M.; Sheng, H.; Cao, Y.; Hu, Y.H. Hydroxyphenylquinazolinone-based turn-on fluorescent probe for â-galactosidase activity detection and application in living cells. Dyes Pigments 2018, 156, 100–107. [Google Scholar] [CrossRef]
- Gao, H.Q.; Wu, X.W. A 3-hydroxyflavone derivative as fluorescence chemosensor for copper and zinc ions. Chem. Heterocycl. Compd. 2018, 54, 125–129. [Google Scholar] [CrossRef]
- He, Y.; Yin, J.; Wang, G. New selective “on-off” fluorescence chemosensor based on carbazole schiff base for Fe3+ detection. Chem. Heterocycl. Compd. 2018, 54, 146–152. [Google Scholar] [CrossRef]
- Tang, M.C.; Lee, C.H.; Ng, M.; Wong, Y.C.; Chan, M.Y.; Yam, V.W.W. Highly emissive fused heterocyclic alkynylgold(III) complexes for multiple color emission spanning from green to red for solution-processable organic light-emitting devices. Angew. Chem. Int. Ed. 2018, 57, 5463–5466. [Google Scholar] [CrossRef] [PubMed]
- Karuppusamy, A.; Kannan, P. Bluish green emission from pyrene-pyrazoline containing heterocyclic materials and their electronic properties. J. Lumin. 2018, 194, 718–728. [Google Scholar] [CrossRef]
- Chen, W.C.; Yuan, Y.; Zhu, Z.L.; Ni, S.F.; Jiang, Z.Q.; Liao, L.S.; Wong, F.L.; Lee, C.S. A novel spiro-annulated benzimidazole host for highly efficient blue phosphorescent organic light-emitting devices. Chem. Commun. 2018, 54, 4541–4544. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.L.; Jian, F.; Guo, H.M.; Zhang, H.X. Synthesis, characterization, crystal structure and DFT studies on 3-(1H-benzo[d][1,2,3]triazol-1-yl)-1-(4-ethylphenyl)-1-oxopropan-2-yl-4-ethylbenzoate. Spectrochim. Acta Part A 2010, 75, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.L.; Zhang, X.M. Synthesis and crystal structure of 4,5-Bis(2-chloroethylthio)phthalonitrile. Chin. J. Struct. Chem. 2012, 31, 635–638. [Google Scholar]
- Zeng, W.L.; Jiang, J.H. Synthesis and crystal ctructure of a new hydrated benzimidazolium salt containing spiro structure. Crystals 2017, 7, 303. [Google Scholar] [CrossRef]
- Zeng, W.L.; Jiang, J.H. Synthesis and Crystal structures of two Novel O,N-containing spiro compounds. Crystals 2016, 6, 69. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Chang, J.H.; Dong, J.G. Spectrum Principle and Analysis; Science Press: Beijing, China, 2012; Volume 3, pp. 15–16. [Google Scholar]


| Compounds | A1 | B1 |
|---|---|---|
| Formula | C21H29NO8 | C19H19NO4 |
| CCDC No. | 1843487 | 1489653 |
| Mr | 423.45 | 301.33 |
| Crystal System, Space group | Triclinic, P-1 | Triclinic, P-1 |
| a, b, c (Å) | 9.399 (3), 9.708 (3), 12.512 (4) | 6.9229 (14), 8.4594 (17), 13.795 (3) |
| α, β, γ (°) | 89.624 (5), 76.334 (5), 75.042 (5) | 104.19 (3), 90.29 (3), 101.27 (3) |
| Crystal Size (mm) | 0.35 × 0.31 × 0.23 | 0.25 × 0.16 × 0.14 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| θ Ranges (°) | 3.170~27.485 | 3.00~27.480 |
| V (Å3) | 1069.9 (6) | 766.9 (3) |
| Z | 2 | 2 |
| F(000) | 452 | 320 |
| D/g·cm−3 | 1.314 | 1.305 |
| μ/ mm−1 | 0.101 | 0.097 |
| −h, h/−k, k/−l, l | −11: 12; −12: 12; −16: 16 | −8: 8; −10: 10; −17: 17 |
| Total, unique and [I > 2σ(I)] reflections | 9715, 4749, 2971 | 7496, 3490, 2171 |
| No. of reflections, restraints, parameters | 4749, 1, 273 | 3490, 0, 199 |
| R(int) | 0.0443 | 0.0320 |
| S | 0.980 | 1.030 |
| R1/wR2 (I > 2σ(I)) R1/wR2 (all data) | 0.0574/0.1333 0.0840/0.1489 | 0.0555/0.1474 0.0813/0.1769 |
| ∆ρmax/∆ρmin/e Å−3 | 0.323/−0.202 | 0.437/−0.250 |
| A1 | B1 | ||
|---|---|---|---|
| Bond | Dist. | Bond | Dist. |
| C(8)–C(10) | 1.373(2) | C(8)–C(10) | 1.380(2) |
| C(10)–C(11) | 1.396(2) | C(8)–C(9) | 1.443(2) |
| O(8)–C(13) | 1.355(2) | C(8)–C(7) | 1.443(3) |
| O(1)–C(1) | 1.432(2) | N(1)–C(10) | 1.314(2) |
| O(1)–C(7) | 1.359(2) | N(1)–C(11) | 1.418(2) |
| C(1)–O(2) | 1.440(2) | O(1)–C(7) | 1.211(2) |
| O(2)–C(9) | 1.365(2) | O(2)–C(9) | 1.216(2) |
| Angle | (°) | Angle | (°) |
| C(8)–C(10)–C(11) | 131.80(16) | N(1)–C(10)–C(8) | 125.12(16) |
| C(10)–C(8)–C(7) | 124.20(17) | C(10)–N(1)–C(11) | 126.83(14) |
| C(10)–C(11)–C(12) | 123.86(15) | C(10)–C(8)–C(9) | 121.40(15) |
| C(10)–C(11)–C(13) | 117.04(15) | C(10)–C(8)–C(7) | 117.80(15) |
| C(13)–C(11)–C(12) | 117.18(15) | C(9)–C(8)–C(7) | 120.24(14) |
| C(7)–C(8)–C(9) | 117.93(16) | C(12)–C(11)–C(16) | 120.87(15) |
| C(10)–C(8)–C(7) | 124.20(17) | C(12)–C(11)–N(1) | 120.38(14) |
| C(10)–C(8)–C(9) | 117.50(16) | C(16)–C(11)–N(1) | 118.75(14) |
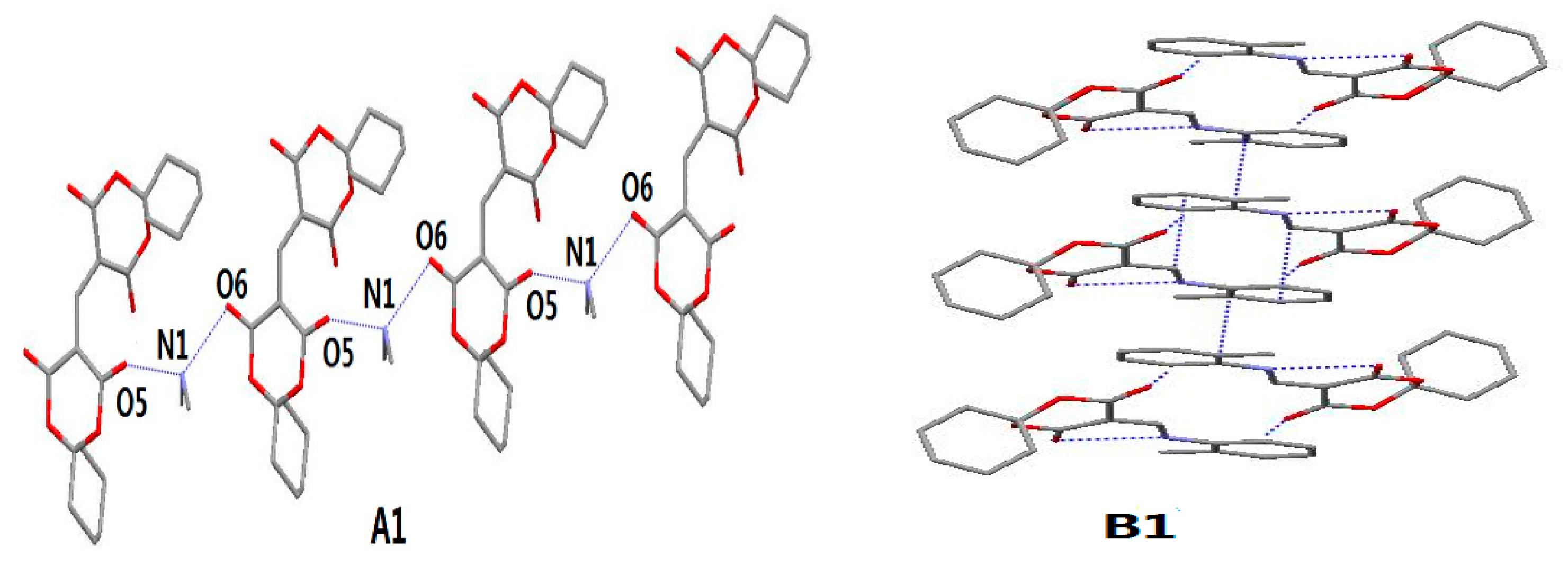
| D–H···A | Symmetry | D–H (Å) | H···A (Å) | D···A (Å) | ∠D–H···A (°) |
|---|---|---|---|---|---|
| N(1)–H(1A)···O(5) A1 | Intra | 0.89 | 1.95 | 2.750 (2) | 149.4 |
| N(1)–H(1B)···O(6) A1 | x−1,y,z | 0.89 | 1.89 | 2.776 (2) | 170.5 |
| N(1)–H(1A)···O(2) B1 | Intra | 0.8600 | 2.0696 | 2.7267 (2) | 132.65 |
| C(12)–H(12A)···O(1) B1 | x−1, 2−y, x−z | 0.9300 | 2.3182 | 3.2265 (2) | 165.33 |
| ring | Symmetry | Dihedral angels (°) | dπ···π(Å) | dc–c (Å) | |
| Cg(3)···Cg(3) a B1 | –x, 1−y, x−z | 0 | 4.354 | 3.370 | |
| Cg(3)···Cg(3) a B1 | x−1, 1−y, x−z | 0 | 4.685 | 3.543 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Jiang, J.; Jiang, G.; Li, Y. Synthesis, Characterization, and Fluorescence Properties of Two New Heterocyclic Compounds Containing 1,5-Dioxaspiro Group. Crystals 2018, 8, 269. https://doi.org/10.3390/cryst8070269
Zeng W, Jiang J, Jiang G, Li Y. Synthesis, Characterization, and Fluorescence Properties of Two New Heterocyclic Compounds Containing 1,5-Dioxaspiro Group. Crystals. 2018; 8(7):269. https://doi.org/10.3390/cryst8070269
Chicago/Turabian StyleZeng, Wulan, Jinhe Jiang, Guanyu Jiang, and Yuehui Li. 2018. "Synthesis, Characterization, and Fluorescence Properties of Two New Heterocyclic Compounds Containing 1,5-Dioxaspiro Group" Crystals 8, no. 7: 269. https://doi.org/10.3390/cryst8070269
APA StyleZeng, W., Jiang, J., Jiang, G., & Li, Y. (2018). Synthesis, Characterization, and Fluorescence Properties of Two New Heterocyclic Compounds Containing 1,5-Dioxaspiro Group. Crystals, 8(7), 269. https://doi.org/10.3390/cryst8070269