Transitions and Geometric Evolution of Cu309 Nanocluster during Slow Cooling Process
Abstract
:1. Introduction
2. Simulation Details
3. Results and Discussion
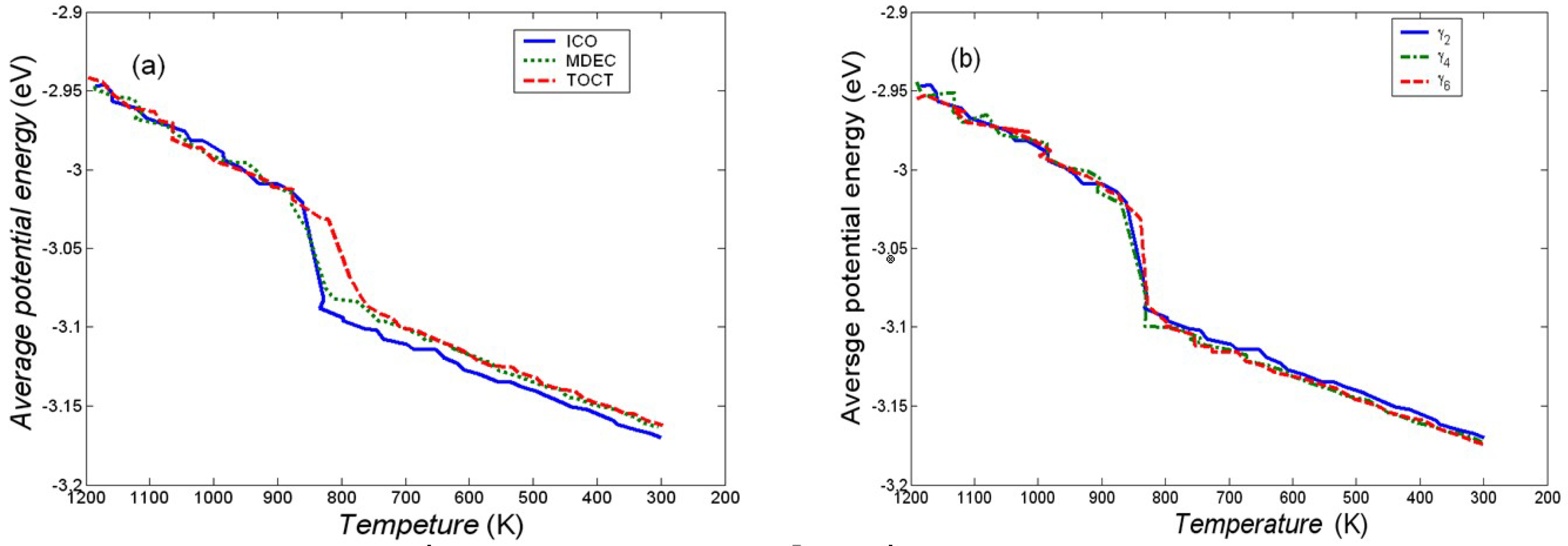
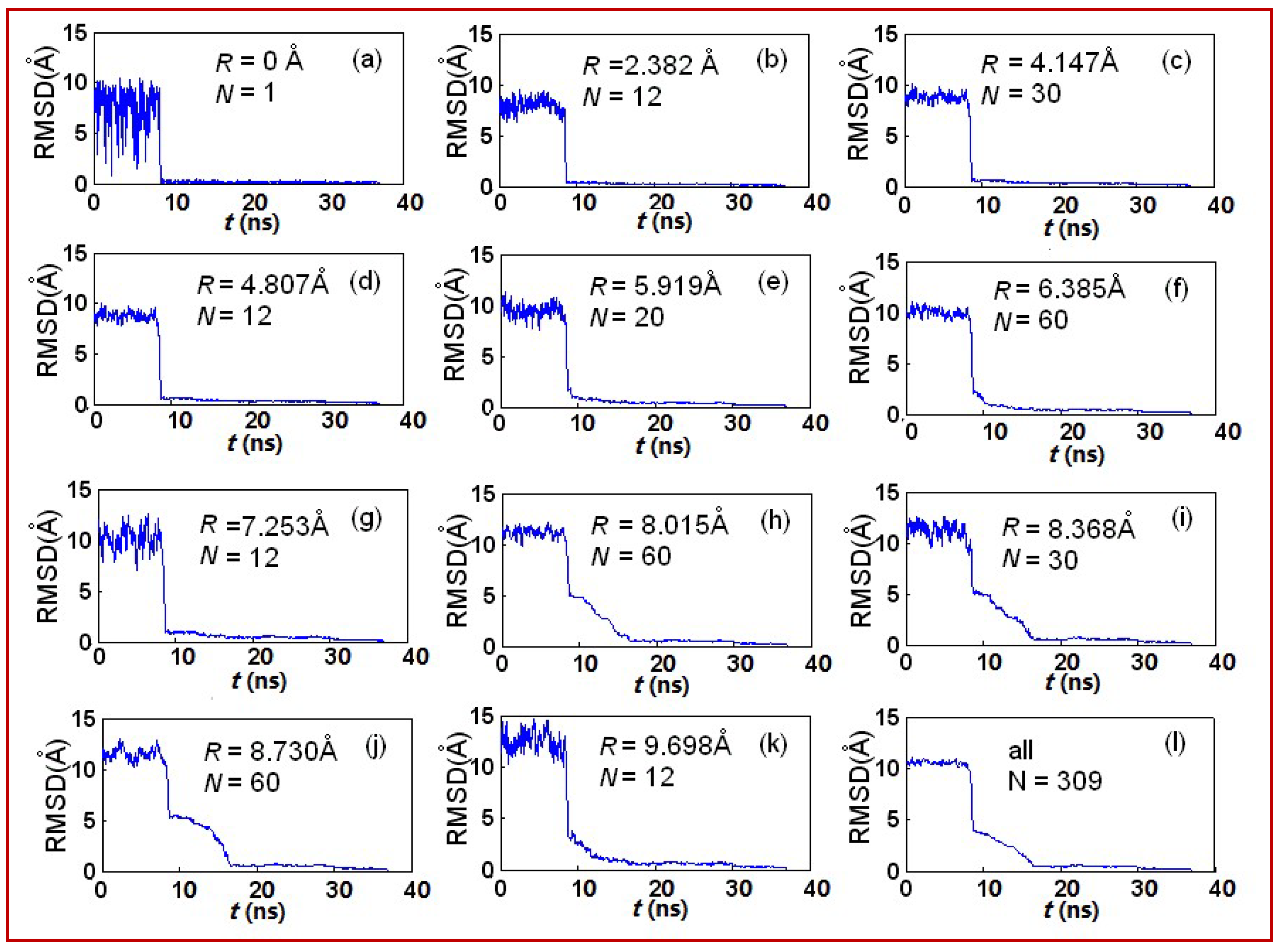
3.1. Effect of Cooling Rate on Structures
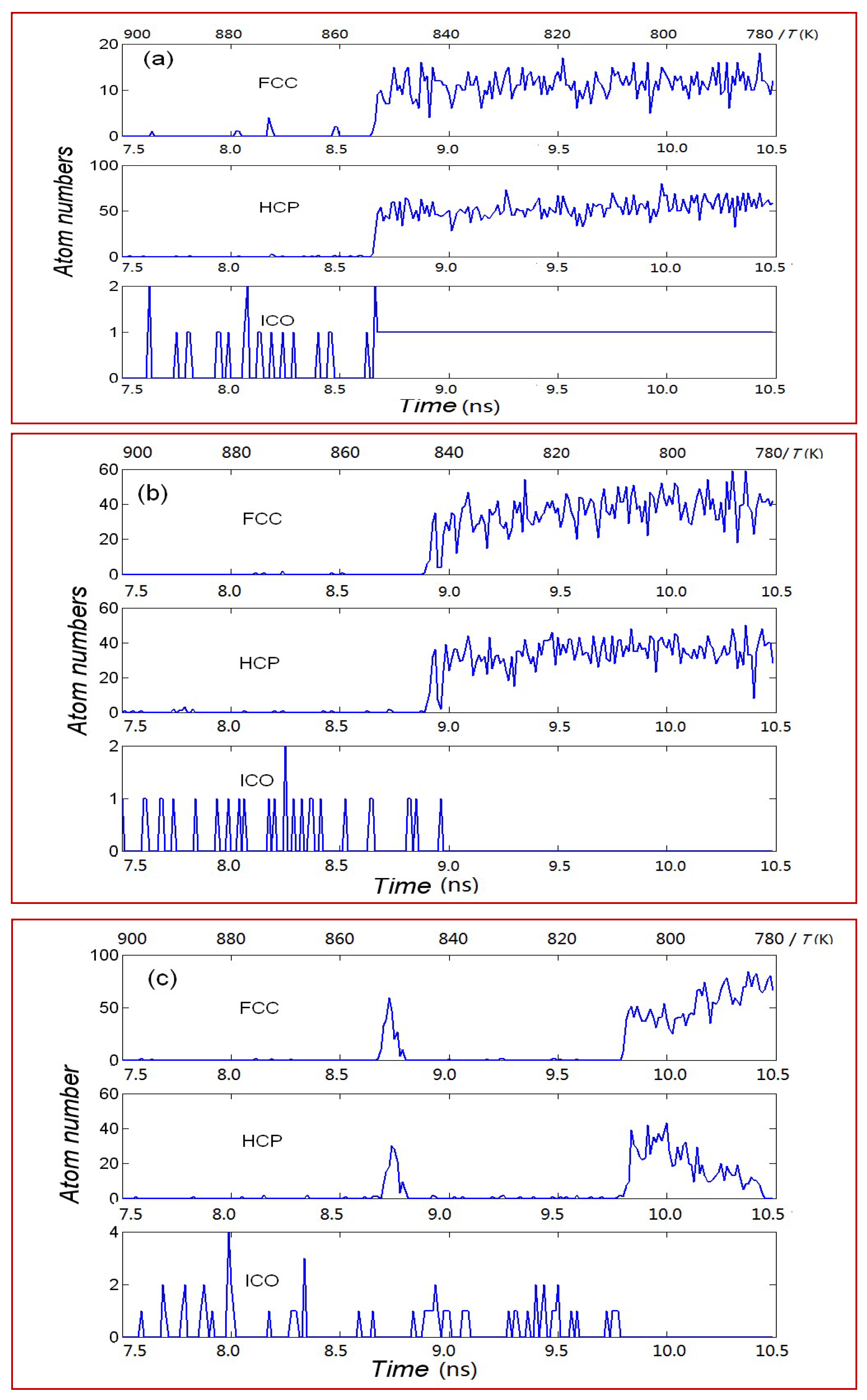
3.2. Structural Transition Process
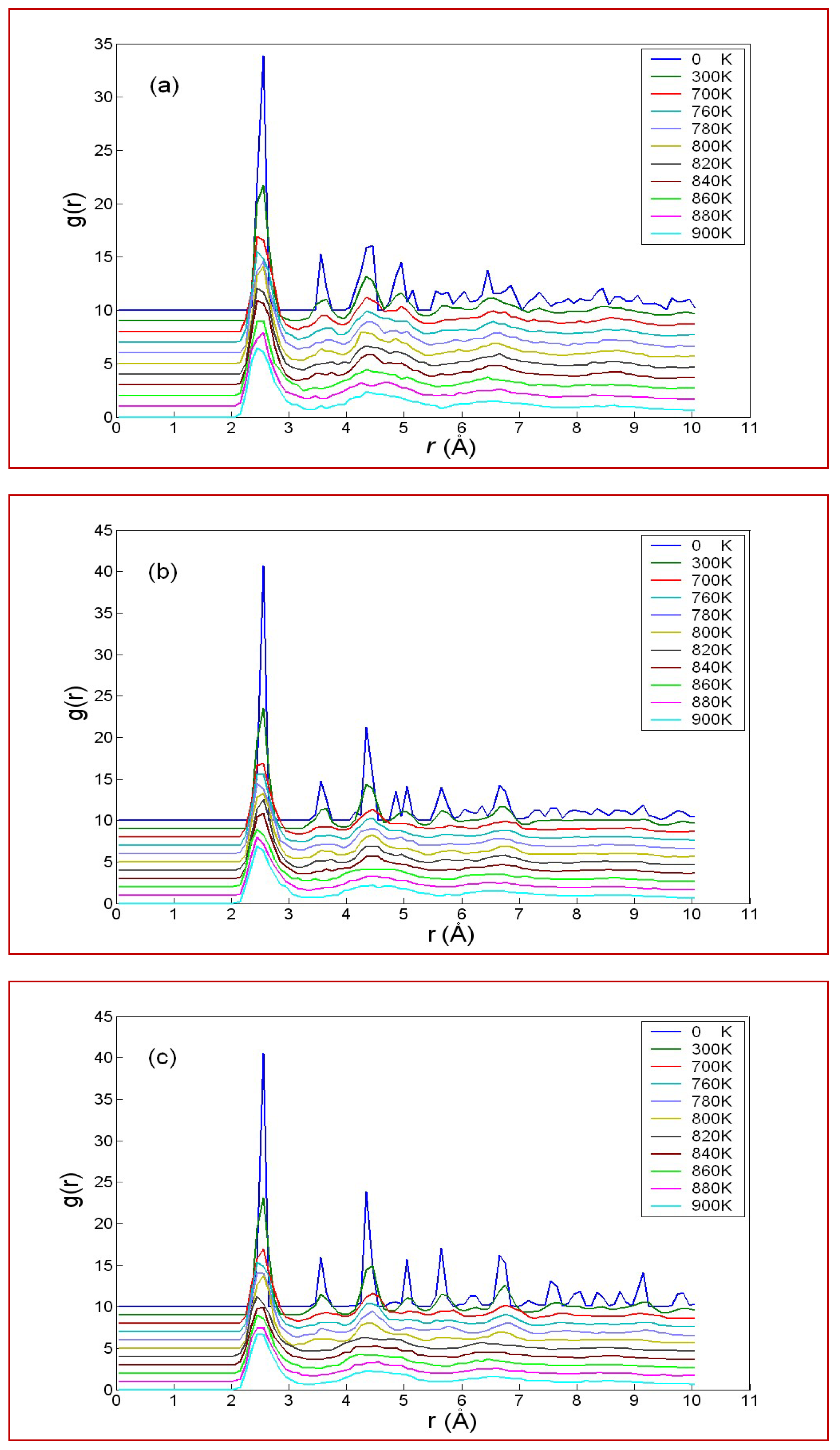
3.3. Geometric Evolution of Icosahedra
3.4. Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Weber, A.P.; Davoodi, P.; Seipenbusch, M.; Kasper, G. Catalytic behaviour of nickel nanoparticles: Gasborne vs. supported state. J. Nanopart. Res. 2006, 8, 445–453. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fissan, H.; Kennedy, M.K.; Krinke, T.J.; Kruis, F.E. Nanoparticles from the gas phase as building blocks for electrical devices. J. Nanopart. Res. 2003, 5, 299–310. [Google Scholar] [CrossRef]
- Lee, T.; Liu, J.; Chen, N.P.; Andres, R.P.; Janes, D.B.; Reifenberge, R. Electronic properties of metallic nanoclusters on semiconductor surfaces: Implications for nanoelectronic device applications. J. Nanopart. Res. 2000, 2, 345–362. [Google Scholar] [CrossRef]
- Shibuta, Y.; Suzuki, T. Melting and nucleation of iron nanoparticles: A molecular dynamics study. Chem. Phys. Lett. 2007, 445, 265–270. [Google Scholar] [CrossRef]
- Castelletto, S.; Boretti, A. Noble metal nanoparticles in thin film solar cells. Nanosci. Nanotechnol. Lett. 2013, 5, 36–40. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered Carbon Nanopipes with Tunable Diameter Exhibiting Extraordinary High Dispersion of Pt Nanoparticles. Nature (London) 2001, 412, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Gittins, D.I.; Bethell, D.; Schiffrin, D.J.; Nichols, R.J. A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature (London) 2000, 408, 67–69. [Google Scholar]
- Gusev, A.I. Nanomaterialy, Nanostruktury, Nanotekhnologii (Nanomaterials, Nanostructures, Nanotechnologies); Fizmatlit: Moscow, Russia, 2007. [Google Scholar]
- Andreeva, L.V.; Koshkin, A.V.; Lebedev-Stepanov, P.V. Crystallization of solutes from droplets. Tech. Phys. 2007, 52, 164–172. [Google Scholar] [CrossRef]
- Huang, C.J.; Chiu, P.H.; Wang, Y.H.; Chen, W.R.; Meen, T.H.; Yang, C.F. Preparation and Characterization of Gold Nanodumbbels. Nanotecnology 2006, 17, 5355–5362. [Google Scholar] [CrossRef]
- Sergeev, B.M.; Kiryukhin, M.V.; Bakhov, F.N.; Sergeev, V.G. Photochemical Synthesis of Silver Nanoparticles in Water Solutions of Polycarbon Acids. Matrix Effect on Size and Form of Particles. Vestn. Mosk. Univ. Ser. 2: Khim. 2001, 42, 308–314. [Google Scholar]
- Kauffeldt, E.; Kauffeldt, T. Thermodynamic-controlled gas phase process for the synthesis of nickel nanoparticles of adjustable size and morphology. J. Nanopart. Res. 2006, 8, 477–488. [Google Scholar] [CrossRef]
- Chushak, Y.G.; Bartell, L.S. Melting and Freezing of Gold Nanoclusters. J. Phys. Chem. B 2001, 105, 11605. [Google Scholar] [CrossRef]
- Yang, Q.W.; Zhu, R.Z. Freezing of Cu nanoclusters studied by molecular dynamics simulation. Acta Phys. Sin. 2005, 54, 4245–4250. [Google Scholar]
- Song, H.J.; Li, X.H. Molecular Dynamics Studies on the Kinetics of Phase Changes of Nanoparticles: Properties, Structures, and Crystal Nucleation of Copper Nanoparticle Cu453. Chin. J. Chem. 2006, 24, 273–278. [Google Scholar] [CrossRef]
- Zhang, C.L.; Li, C.P.; Bai, J.; Li, H.Y. A Cu Nanoparticle Embedded in Electrospundoped Carbon Nanofibers as Efficient Catalysts for Ullmann O-Arylation of Aryl Halides with arious Phenols. Catal. Lett. 2015, 145, 1764–1770. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, J.T.; Lai, C.H. Influence of embedding Cu nano-particles into a Cu/SiO2/Pt structure on its resistive switching. Nanoscale Res. Lett. 2013, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Du, C.; Peng, S.; Shi, D. Plasmon Peak Sensitivity Investigation of Individual Cu and Cu@Cu2O Core–Shell Nanoparticle Sensors. Plasmonics 2016, 11, 1197–1200. [Google Scholar] [CrossRef]
- Korenchenkoa, A.E.; Vorontsova, A.G.; Gelchinskiib, B.R. Statistical Analysis of Formation and Relaxation of Atomic Clusters Based on Data of Molecular-Dynamic Modeling of Gas-Phase Nucleation of Metallic Nanoparticles. High Temp. 2016, 54, 229–234. [Google Scholar] [CrossRef]
- Chen, K.Y.; Liu, H.B.; Li, X.P. Molecular dynamics simulation of local structure of aluminum and copper in supercooled liquid and solid state by using EAM. J. Phys.-Condens. Matter 1995, 7, 2379–2394. [Google Scholar]
- Doye, J.P.K.; Wales, D.J. Magic numbers and growth sequences of small face-centred-cubic and decahedral clusters. Chem. Phys. Lett. 1995, 247, 339–347. [Google Scholar] [CrossRef]
- Baletto, F.; Ferrando, R.; Fortunelli, R.; Montalenti, F.; Mottet, C. Crossover among structural motifs in transition and noble-metal clusters. J. Chem. Phys. 2002, 116, 3856–3863. [Google Scholar] [CrossRef]
- Wei, C.M.; Cheng, C.; Chang, C.M. A structural path for the icosahedra fcc–structural transition in clusters. J. Mol. Liquids 2017, 230, 271–279. [Google Scholar]
- Kimizuka, H.; Kaburaki, H.; Shimizu, F.; Li, J. Crack-tip dislocation nanostructures in dynamical fracture of fcc metals: A molecular dynamics study. J. Comput. Aided Mater. Des. 2003, 10, 143–154. [Google Scholar] [CrossRef]
- Wu, Z.X.; Zhang, Y.W.; Jhon, M.H.; Srolovitz, D.J. Nanostructure and surface effects on yield in Cu nanowires. Acta Mater. 2013, 61, 1831–1842. [Google Scholar] [CrossRef]
- Yuan, Y.Q.; Zeng, X.G.; Chen, H.Y. Molecular dynamics simulation on microstructure evolution during during solidification of copper nanoparticles. J. Korean Phys. Soc. 2013, 62, 1645–1651. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Mishin, Y.; Mehl, M.J.; Papaconstantopoulos, D.A.; Voter, A.F.; Kress, J.D. Structural Stability and Lattice Defects in Copper: Ab Initio, Tight-Binding, and Embedded-Atom Calculations. Phys. Rev. B 2001, 63, 224106. [Google Scholar] [CrossRef]
- Marchenko, A.; Zhang, H. Effects of location of twin boundaries and grain size on plastic deformation of nanocrystalline copper. Metall. Mater. Trans. A 2012, 43, 3547–3555. [Google Scholar] [CrossRef]
- Wu, Z.X.; Zhang, Y.M.; Jhon, M.H.; Gao, H.J.; Srolovitz, D.J. Nanowire Failure:Long = Brittle and Short = Ductile. Nano Lett. 2012, 12, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Marinica, M.C.; Barreteau, C.; Spanjaard, D.; Desjonqueres, M.C. Diffusion rates of Cu adatoms on Cu (111) in the presence of an adisland nucleated at FCC or HCP sites. Phys. Rev. B 2004, 72, 115402. [Google Scholar] [CrossRef]
- Lei, H. Melting of free copper clusters. Condens. Matter 2001, 13, 3023–3030. [Google Scholar] [CrossRef]
- Valkealahti, S.; Manninen, M. Melting of copper clusters. Comput. Mater. Sci. 1993, 1, 123–134. [Google Scholar] [CrossRef]
- Hou, M. Solid–liquid and liquid–solid transitions in metal nanoparticles. Phys. Chem. Chem. Phys. 2017, 19, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Faken, D.; Jonsson, H. Systematic analysis of local atomic structure combined with 3D computer graphics. Comput. Mater. Sci. 1994, 2, 279–286. [Google Scholar] [CrossRef]
- Tsuzuki, H.; Branicio, P.S.; Rino, J.P. Structural characterization of deformed crystals by analysis of common atomic neighborhood. Comput. Phys. Commun. 2007, 177, 518–523. [Google Scholar] [CrossRef]
- Kelchner, C.L.; Plimpton, S.J.; Hamilton, J.C. Dislocation nucleation and defect structure during surface indentation. Phys. Rev. B 1998, 58, 11085. [Google Scholar] [CrossRef]
- Shibuta, Y.; Suzuki, T. A molecular dynamics study of cooling rate during solidification of metal nanoparticles. Chem. Phys. Lett. 2011, 502, 82–86. [Google Scholar] [CrossRef]
- Gafner, S.L.; Redel, L.V.; Gafner, Y.Y. Molecular_Dynamics Simulation of the Heat Capacity for Nickel and Copper Clusters: Shape and Size Effects. J. Exp. Theor. Phys. 2012, 114, 428–439. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.N.; Zhang, C.B.; Qi, Y. Molecular dynamics investigations of structural changes accompanying with freezing a molten Cu135 cluster on cooling. Comput. Mater. Sci. 2009, 47, 162–167. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Q.N. Effect of quenching temperature and size on atom movement and local structural change for small copper clusters containing 51–54 atoms during quenching processes. Indian J. Phys. 2016, 90, 9–20. [Google Scholar] [CrossRef]
- Glezer, A.M.; Blinova, E.N.; Pozdnyakov, V.A.; Shelyakov, A.V. Martensite transformation in nanoparticles and nanomaterials. J. Nanopart. Res. 2003, 5, 551–560. [Google Scholar] [CrossRef]
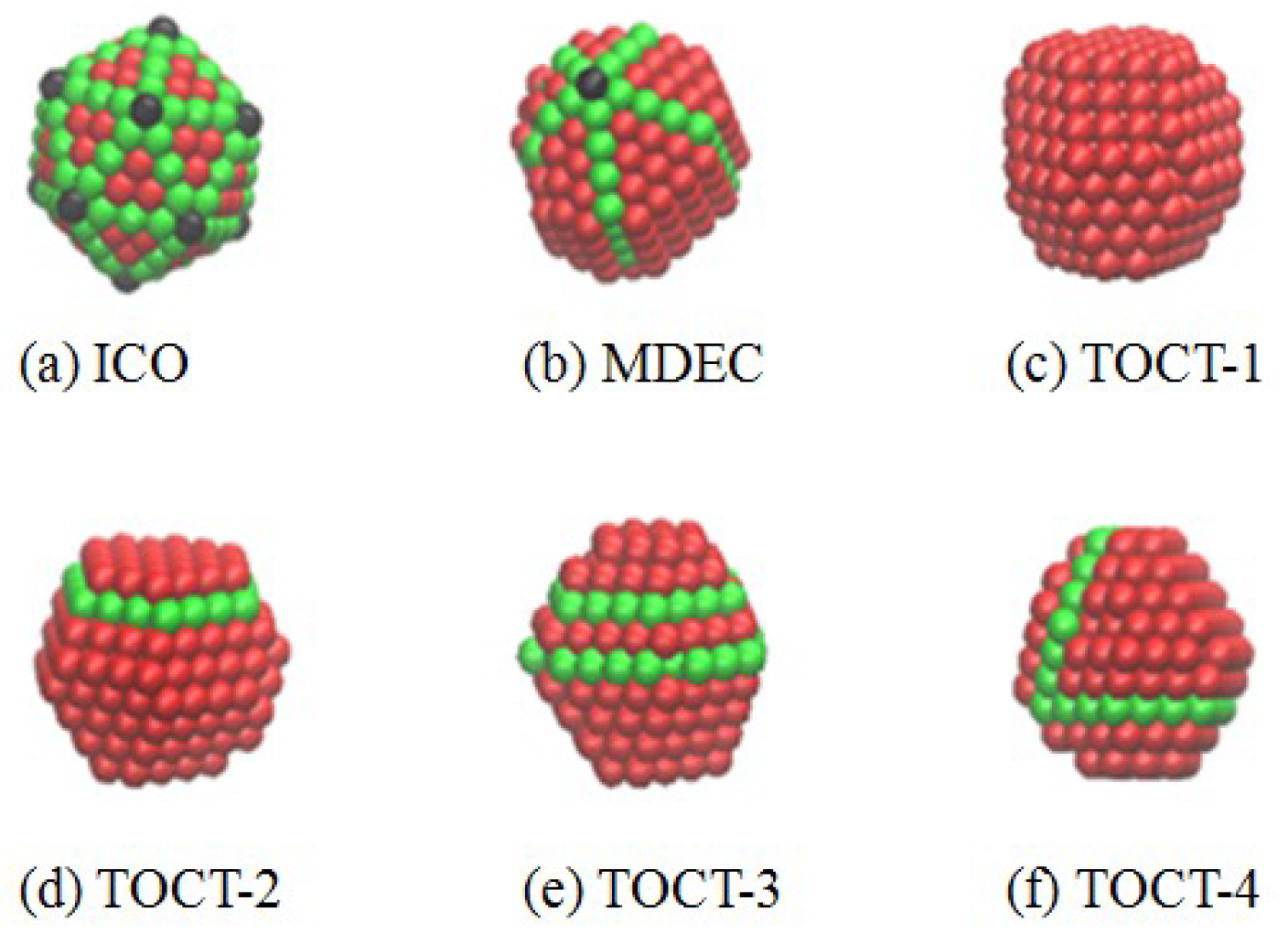
| ICO | 6 | 5 | 6 | 8 | 8 | 10 |
| MDEC | 2 | 2 | 3 | 0 | 1 | 0 |
| TOCT | 2 | 3 | 1 | 2 | 1 | 0 |
| Ri/Å | 0 | 2.382 | 4.147 | 4.807 | 5.919 | 6.385 | 7.253 | 8.015 | 8.368 | 8.730 | 9.698 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1 | 12 | 30 | 12 | 20 | 60 | 12 | 60 | 30 | 60 | 12 |
| Local structure | ICO | 5-fold | HCP | 5-fold | FCC | HCP | 5-fold | FCC | FCC | HCP | 5-fold |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, P.; Zhao, Y.; Wan, M.; He, J.; Tian, M.; Song, Y.; Li, Y. Transitions and Geometric Evolution of Cu309 Nanocluster during Slow Cooling Process. Crystals 2018, 8, 231. https://doi.org/10.3390/cryst8050231
Ji P, Zhao Y, Wan M, He J, Tian M, Song Y, Li Y. Transitions and Geometric Evolution of Cu309 Nanocluster during Slow Cooling Process. Crystals. 2018; 8(5):231. https://doi.org/10.3390/cryst8050231
Chicago/Turabian StyleJi, Pengfei, Yi Zhao, Mingli Wan, Jinna He, Mingli Tian, Yueli Song, and Yong Li. 2018. "Transitions and Geometric Evolution of Cu309 Nanocluster during Slow Cooling Process" Crystals 8, no. 5: 231. https://doi.org/10.3390/cryst8050231
APA StyleJi, P., Zhao, Y., Wan, M., He, J., Tian, M., Song, Y., & Li, Y. (2018). Transitions and Geometric Evolution of Cu309 Nanocluster during Slow Cooling Process. Crystals, 8(5), 231. https://doi.org/10.3390/cryst8050231





