Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure
Abstract
:1. Introduction
2. Materials and Methods
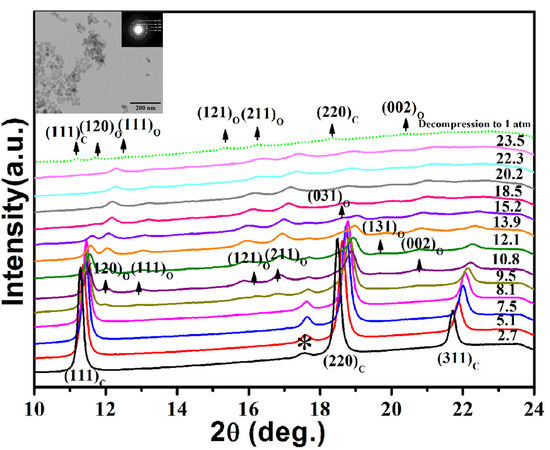
3. Results and Discussion
4. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Peng, Q.; Li, Y. Upconversion Luminescence of Monodisperse CaF2: Yb3+/Er3+ Nanocrystals. J. Am. Chem. Soc. 2009, 131, 14200–14201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Peng, C.; Chai, R.; Huang, S.; Yang, D.; Cheng, Z.; Lin, J. Facile and Controllable Synthesis of Monodisperse CaF2 and CaF2: Ce3+/Tb3+ Hollow Spheres as Efficient Luminescent Materials and Smart Drug Carriers. Chem. Eur. J. 2010, 16, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, C.; Roming, M.; Trampert, K. Polyol-Mediated Synthesis of Nanoscale CaF2 and CaF2: Ce, Tb. Small 2006, 2, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Gerward, L.; Olsen, J.S.; Steenstrup, S.; Sbrink, S.A.; Waskowska, A. X-ray diffraction investigations of CaF2 at high pressure. J. Appl. Crystallogr. 1992, 25, 578–581. [Google Scholar] [CrossRef]
- Kavner, A. Radial diffraction strength and elastic behavior of CaF2 in low-and high-pressure phases. Phys. Rev. B 2008, 77, 224102. [Google Scholar] [CrossRef]
- Cui, S.X.; Feng, W.X.; Hua, H.Q. Structural stabilities, electronic and optical properties of CaF2 under high pressure: A first-principles study. Comput. Mater. Sci. 2009, 47, 41–45. [Google Scholar] [CrossRef]
- Wu, X.; Qin, S.; Wu, Z.Y. First-principles study of structural stabilities, and electronic and optical properties of CaF2 under high pressure. Phys. Rev. B 2006, 73, 134103. [Google Scholar] [CrossRef]
- Dorfman, S.M.; Jiang, F.; Mao, Z.; Kubo, A.; Meng, Y.; Prakapenka, V.B.; Duffy, T.S. Phase transitions and equations of state of alkaline earth fluorides CaF2, SrF2, and BaF2 to Mbar pressures. Phys. Rev. B 2010, 81, 174121. [Google Scholar] [CrossRef]
- Boulfelfel, S.E.; Zahn, D.; Hochrein, O.; Grin, Y.; Leoni, S. Low-dimensional sublattice melting by pressure: Superionic conduction in the phase interfaces of the fluorite-to-cotunnite transition of CaF2. Phys. Rev. B 2006, 74, 094106. [Google Scholar] [CrossRef]
- Hu, T.; Cui, X.; Wang, J.; Zhong, X.; Chen, Y.; Zhang, J.; Li, X.; Yang, J.; Gao, C. The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure. Crystals 2018, 8, 98. [Google Scholar] [CrossRef]
- Hu, T.; Cui, X.; Wang, J.; Zhang, J.; Li, X.; Yang, J.; Gao, C. Transport properties of mixing conduction in CaF2 nanocrystals under high pressure. Chin. Phys. B 2018, 27, 016401. [Google Scholar] [CrossRef]
- Cazorla, C.; Errandonea, D. Superionicity and polymorphism in calcium fluoride at high pressure. Phys. Rev. Lett. 2014, 113, 235902. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, C.; Errandonea, D. Giant Mechanocaloric Effects in Fluorite-Structured Superionic Materials. Nano Lett. 2016, 16, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Ma, H.; Cui, Y.; Mao, W.L. Compressional Behavior of Bulk and Nanorod LiMn2O4 under Nonhydrostatic Stress. J. Phys. Chem. C 2011, 115, 9844–9849. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.W.; Fan, H.Y. Stress-Induced Nanoparticle Crystallization. J. Am. Chem. Soc. 2014, 136, 7634–7636. [Google Scholar] [CrossRef] [PubMed]
- Bian, K.; Wang, Z.; Hanrath, T. Comparing the Structural Stability of PbS Nanocrystals Assembled in fcc and bcc Superlattice Allotropes. J. Am. Chem. Soc. 2012, 134, 10787–10790. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, S.H.; Alivisatos, A.P. Size Dependence of a First Order Solid-Solid Phase Transition: The Wurtzite to Rock Salt Transformation in CdSe Nanocrystals. Science 1994, 265, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, S.H.; Alivisatos, A.P. High-Pressure Structural Transformations in Semiconductor Nanocrystals. Annu. Rev. Phys. Chem. 1995, 46, 595–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Q. Size-Dependent Structural Stability and Tuning Mechanism: A Case of Zinc Sulfide. J. Phys. Chem. C 2009, 113, 4286–4295. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Gerward, L.; Olsen, J.S. Pressure induced phase transformation in nanocrystal SnO2. Scr. Mater. 2001, 44, 1983–1986. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Ding, Y.; Ren, Y.; Xiao, S.; Liu, B.; Sinogeikin, S.V.; Meng, Y.; Gosztola, D.J.; Shen, G.; et al. Size-Dependent Amorphization of Nanoscale Y2O3 at High Pressure. Phys. Rev. Lett. 2010, 105, 095701. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Alexei, K.; Dubrovinsky, L.S.; Mcmillan, P.F.; Prakapenka, V.B.; Shen, G.; Muddle, B.C. Size-Dependent Pressure-Induced Amorphization in Nanoscale TiO2. Phys. Rev. Lett. 2006, 96, 135702. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B.; Wang, L.; Li, D.; Liu, R.; Zou, B.; Cui, T.; Zou, G. Pressure-Induced Amorphization and Polyamorphism in One-Dimensional Single-Crystal TiO2 Nanomaterials. J. Phys. Chem. Lett. 2010, 1, 309–314. [Google Scholar] [CrossRef]
- Quan, Z.W.; Wang, Y.X.; Bae, I.T.; Loc, W.S.; Wang, C.Y.; Wang, Z.W.; Fang, J.Y. Reversal of Hall–Petch Effect in Structural Stability of PbTe Nanocrystals and Associated Variation of Phase Transformation. Nano Lett. 2011, 11, 5531–5536. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, Q.; Hu, T.; Yang, J.; Li, X.; Liu, Y.; Liu, B.; Zhao, W.; Zhu, H.; Yang, L. Pressure-Induced Amorphization in BaF2 Nanoparticles. J. Phys. Chem. C 2016, 120, 12249–12253. [Google Scholar] [CrossRef]
- Lv, H.; Yao, M.; Li, Q.; Li, Z.; Liu, B.; Liu, R.; Lu, S.; Li, D.; Mao, J.; Ji, X.; et al. Effect of Grain Size on Pressure-Induced Structural Transition in Mn3O4. J. Phys. Chem. C 2012, 116, 2165–2171. [Google Scholar] [CrossRef]
- Wieligor, M.; Wang, Y.; Zerda, T.W. Raman spectra of silicon carbide small particles and nanowires. J. Phys. Condens. Matter 2005, 17, 2387–2395. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wu, J.; Coffer, J.L.; Lin, Z.; Sinogeikin, S.V.; Yang, W.; Zhao, Y. Phase transition and compressibility in silicon nanowires. Nano Lett. 2008, 8, 2891–2895. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Zhao, Y. Strength weakening by nanocrystals in ceramic materials. Nano Lett. 2007, 7, 3196–3199. [Google Scholar] [CrossRef] [PubMed]
- Senter, R.A.; Pantea, C.; Wang, Y.; Liu, H.; Zerda, T.W.; Coffer, J.L. Structural Influence of Erbium Centers on Silicon Nanocrystal Phase Transitions. Phys. Rev. Lett. 2004, 93, 175502. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Coffer, J.L.; Wang, Y.; Schulze, R. Oxidized Germanium as a Broad-Band Sensitizer for Er-Doped SnO2 Nanofibers. J. Phys. Chem. C 2009, 113, 12–16. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Zhang, J.; Xu, H.; Wang, L.; Luo, S.; Daemen, L. In situ phase transition study of nano- and coarse-grained TiO2 under high pressure/temperature conditions. J. Phys. Condens. Matter 2008, 20, 125224. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Zou, G.; Wu, J.; Coffer, J.L.; Sinogeikin, S.V.; Zhang, J. Anomalous Surface Doping Effect in Semiconductor Nanowires. J. Phys. Chem. C 2017, 121, 11824–11830. [Google Scholar] [CrossRef]
- Wang, J.; Hao, J.; Wang, Q.; Jin, Y.; Li, F.; Liu, B.; Li, Q.; Liu, B.; Cui, Q. Pressure-induced structural transition in CaF2 nanocrystals. Phys. Status Solidi B 2011, 248, 1115–1118. [Google Scholar] [CrossRef]
- Birch, F. Finite Strain Isotherm and Velocities for Single-Crystal and Polycrystalline NaCl at High Pressures and 300 K. J. Geophys. Res. 1978, 83, 1257–1268. [Google Scholar] [CrossRef]
- Wang, J.; Ma, C.; Zhou, D.; Xu, Y.; Zhang, M.; Gao, W.; Zhu, H.; Cui, Q. Structural phase transitions of SrF2 at high pressure. J. Solid State Chem. 2012, 186, 231–234. [Google Scholar] [CrossRef]
- Popescu, C.; Sans, J.A.; Errandonea, D.; Segura, A.; Villanueva, R.; Sapiña, F. Compressibility and structural stability of nanocrystalline TiO2 anatase synthesized from freeze-dried precursors. Inorg. Chem. 2014, 53, 11598–11603. [Google Scholar] [CrossRef] [PubMed]
- Grinblat, F.; Ferrari, S.; Pampillo, L.G.; Saccone, F.; Errandonea, D.; Santamaria-Perez, D.; Segura, A.; Vilaplana, R.; Popescu, C. Compressibility and structural behavior of pure and Fe-doped SnO2 nanocrystals. Solid State Sci. 2017, 64, 91–98. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proceedings of the Physical Society. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]
- Petch, N. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Angel, R.J. The high-pressure, high-temperature equation of state of calcium fluoride, CaF2. J. Phys. Condens. Matter 1993, 5, L141. [Google Scholar] [CrossRef]

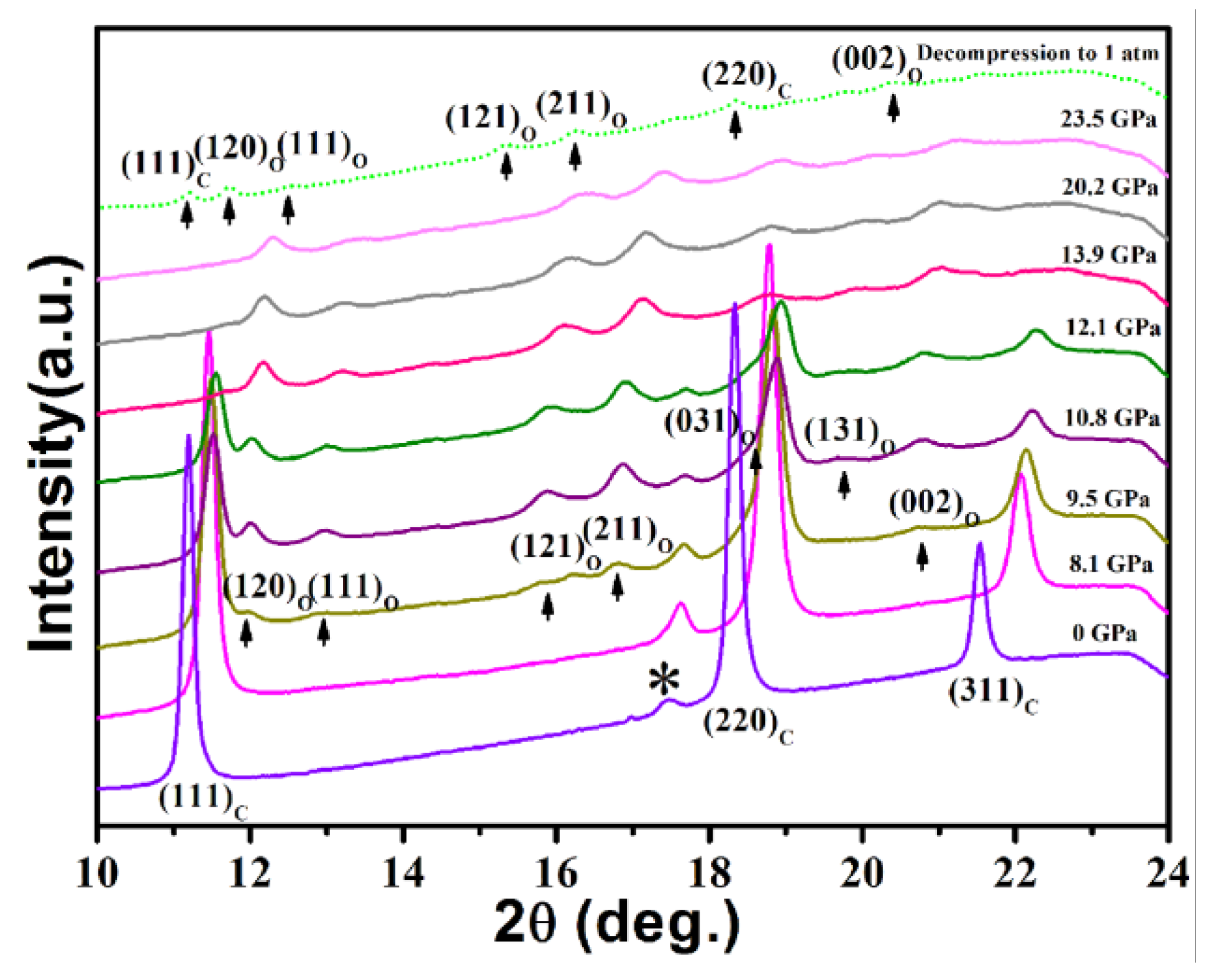
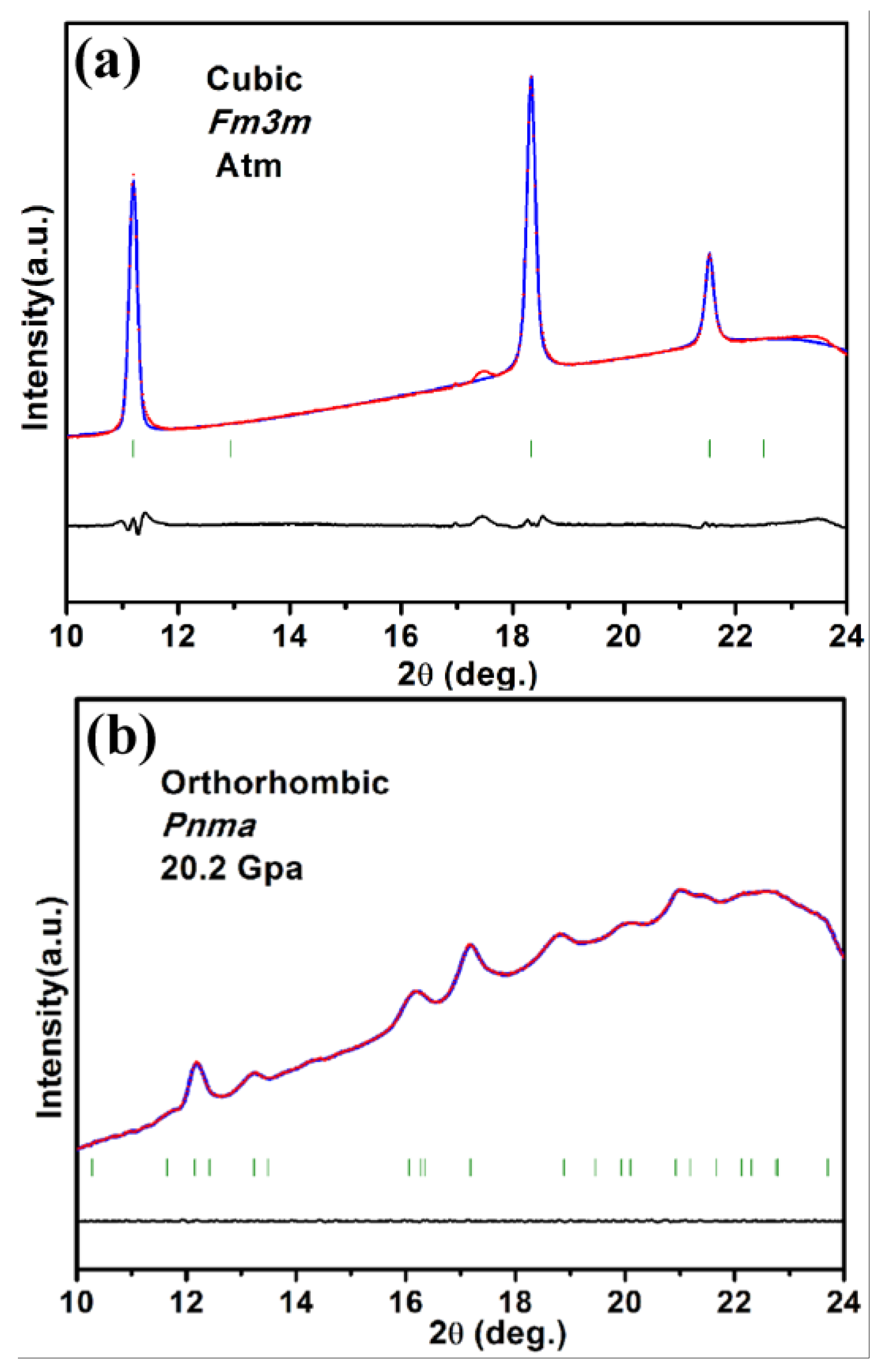
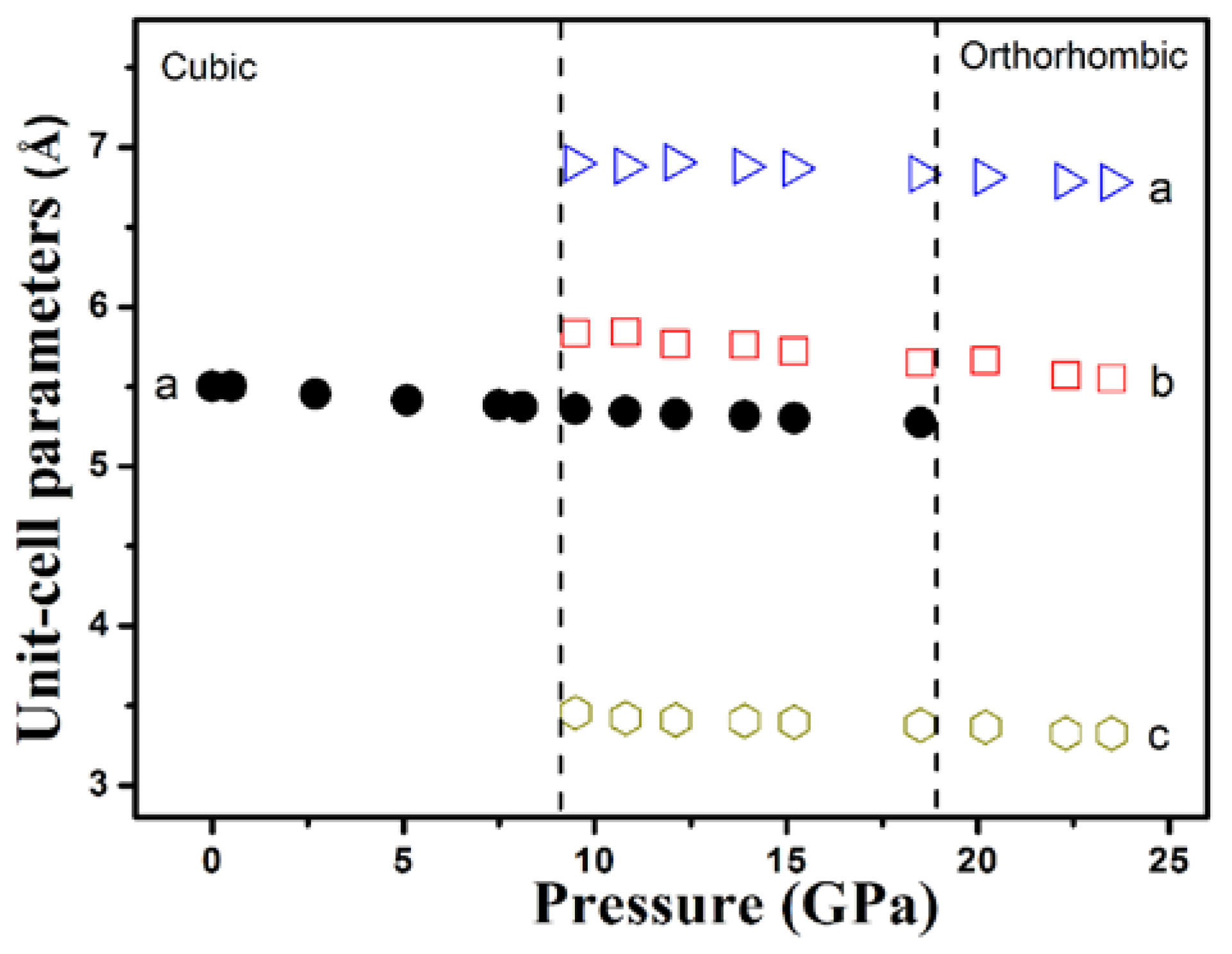


| Morphology | Size | PT (GPa) | B0 (GPa) | B0′ | ||
|---|---|---|---|---|---|---|
| Fm3m | Pnma | Fm3m | Pnma | |||
| Bulk | Micro | 9.5 1 | 87(5) 1 | 74(5) 2 | 5 | 4.7 |
| 9 3 | 81(1) 3 | 5.22 | ||||
| 8.1 4 | 79.54 4 | 70.92 4 | 4.54 | 4.38 | ||
| Nanocrystals | 8 nm | 14 5 | -- | -- | -- | -- |
| This work | 23 nm | 9.5 | 103(2) | 78(2) | 5 | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, J.; Hu, T.; Chen, X.; Lang, J.; Wu, X.; Zhang, J.; Zhao, H.; Yang, J.; Cui, Q. Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure. Crystals 2018, 8, 199. https://doi.org/10.3390/cryst8050199
Wang J, Yang J, Hu T, Chen X, Lang J, Wu X, Zhang J, Zhao H, Yang J, Cui Q. Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure. Crystals. 2018; 8(5):199. https://doi.org/10.3390/cryst8050199
Chicago/Turabian StyleWang, Jingshu, Jinghan Yang, Tingjing Hu, Xiangshan Chen, Jihui Lang, Xiaoxin Wu, Junkai Zhang, Haiying Zhao, Jinghai Yang, and Qiliang Cui. 2018. "Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure" Crystals 8, no. 5: 199. https://doi.org/10.3390/cryst8050199
APA StyleWang, J., Yang, J., Hu, T., Chen, X., Lang, J., Wu, X., Zhang, J., Zhao, H., Yang, J., & Cui, Q. (2018). Structural Phase Transition and Compressibility of CaF2 Nanocrystals under High Pressure. Crystals, 8(5), 199. https://doi.org/10.3390/cryst8050199