Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
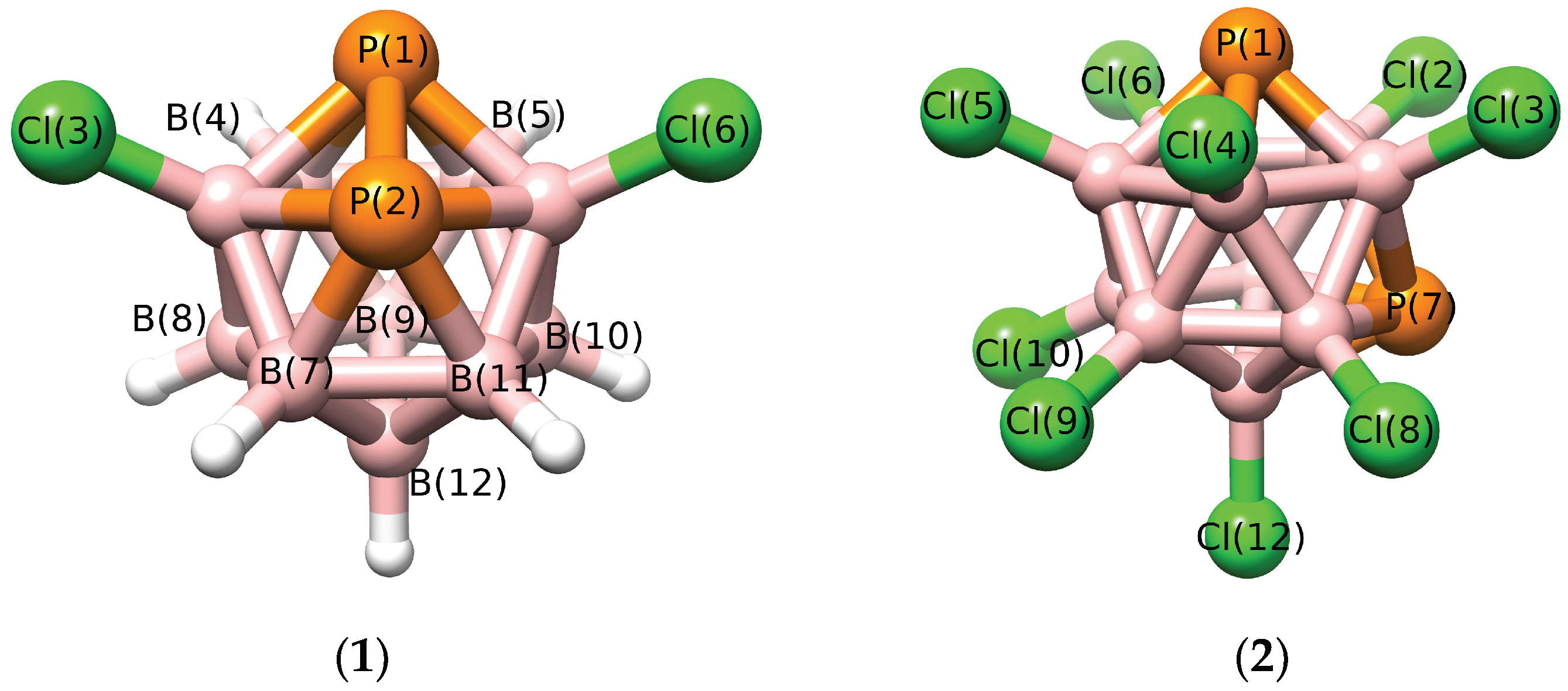
3.1. The Properties of Isolated Molecules
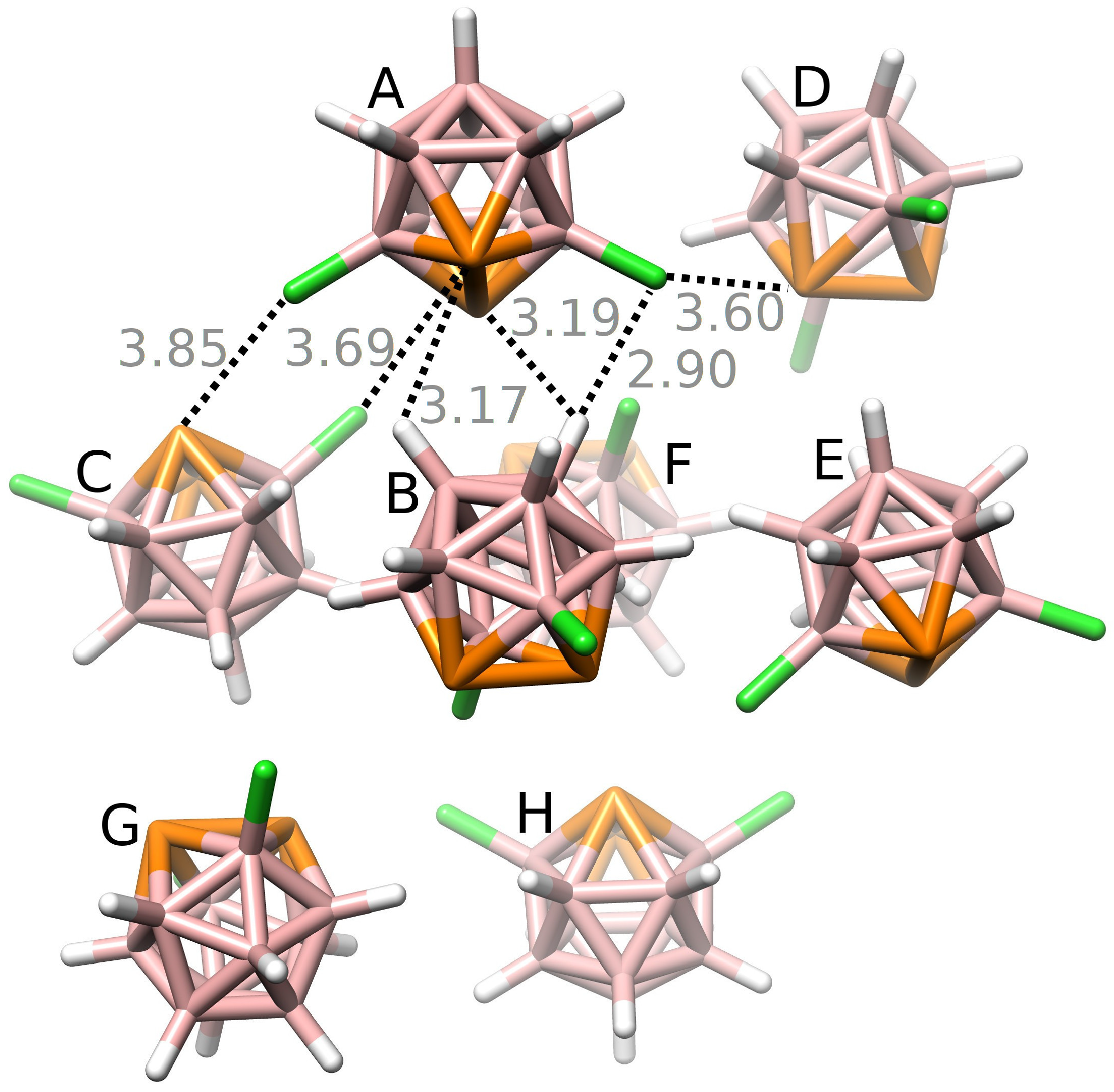
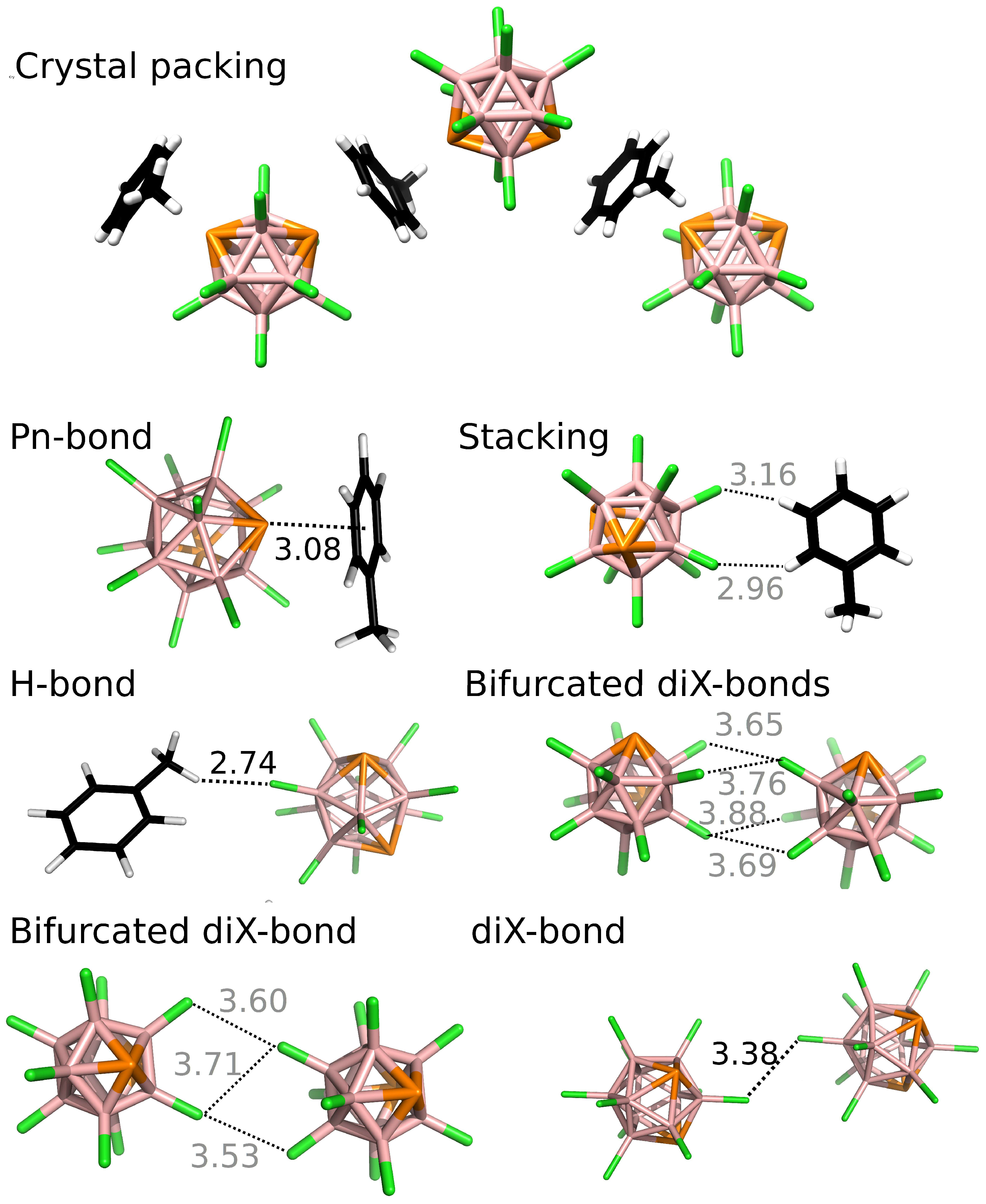
3.2. Interactions in the Single Crystal of 1 and the Crystal of 2•Toluene
Author Contributions
Funding
Conflicts of Interest
References
- Grüner, B.; Hnyk, D.; Císařová, I.; Plzák, Z.; Štíbr, B. Phosphaborane Chemistry. Syntheses and Calculated Molecular Structures of Mono- and Di-chloro Derivatives of 1,2-Diphospha-closo-dodecaborane(10). J. Chem. Soc. Dalton Trans. 2002, 15, 2954–2959. [Google Scholar] [CrossRef]
- Štíbr, B.; Holub, J.; Bakardjiev, M.; Hnyk, D.; Tok, O.L.; Milius, W.; Wrackmeyer, B. Phosphacarborane Chemistry: The Synthesis of the Parent Phosphadicarbaboranes nido-7,8,9-PC2B8H11 and [nido-7,8,9-PC2B8H10]−, and Their 10-Cl Derivatives—Analogs of the Cyclopentadiene Anion. Eur. J. Inorg. Chem. 2002, 9, 2320–2326. [Google Scholar] [CrossRef]
- Little, J.L.; Whitesell, M.A.; Chapman, R.W.; Kester, J.G.; Huffman, J.C.; Todd, L.J. Synthesis of Some 10-, 11-, and 12-Atom Phosphaboranes. Crystal Structure of 2-(Trimethylamine)-1-PB11H10. Inorg. Chem. 1993, 32, 3369–3372. [Google Scholar] [CrossRef]
- Keller, W.; Sawitzki, G.; Haubold, W. Synthesis of Halogenated Polyhedral Phosphaboranes. Crystal Structures of closo-1,7-P2B10Cl10. Inorg. Chem. 2000, 39, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Melichar, P.; Hnyk, D.; Fanfrlík, J. Systematic Examination of Classical and Multi-Center Bonding in Heteroborane Clusters. Phys. Chem. Chem. Phys. 2018, 20, 4666–4675. [Google Scholar] [CrossRef] [PubMed]
- Hnyk, D.; Všetečka, V.; Drož, L.; Exner, O. Charge Distribution within 1,2-Dicarba-closo-dodecaborane: Dipole Moments of its Phenyl Derivatives. Collect. Czech. Chem. Commun. 2001, 66, 1375–1379. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Pecina, A.; Lepšík, M.; Hnyk, D.; Hobza, P.; Fanfrlík, J. Chalcogen and Pnicogen Bonds in Complexes of Neutral Icosahedral and Biccaped Square-Antiprismatic Heteroboranes. J. Phys. Chem. A 2015, 119, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Fanfrlík, J.; Přáda, A.; Padělková, Z.; Pecina, A.; Macháček, J.; Lepšík, M.; Holub, J.; Růžička, A.; Hnyk, D.; Hobza, P. The Dominant Role of Chalcogen Bonding in the Crystal Packing of 2D/3D Aromatics. Angew. Chem. Int. Ed. 2014, 53, 10139–10142. [Google Scholar] [CrossRef] [PubMed]
- Fanfrlík, J.; Hnyk, D. Chalcogens Act as Inner and Outer Heteroatoms in Borane Cages with Possible Consequences for σ-Hole Interactions. CrystEngComm 2016, 47, 8973–9162. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Holub, J.; Růžičková, Z.; Řezáč, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Růžička, A.; Hobza, P. Competition between Halogen, Hydrogen and Dihydrogen Bonding in Brominated Carboranes. ChemPhysChem 2016, 17, 3373–3376. [Google Scholar]
- Holub, J.; Melichar, P.; Růžičková, Z.; Vrána, J.; Wann, D.A.; Fanfrlík, J.; Hnyk, D.; Růžička, A. A Novel Stibacarbaborane Cluster with Adjacent Antimony Atoms Exhibiting Unique Pnictogen Bond Formation that Dominates Its Crystal Packing. Dalton Trans. 2017, 46, 13714–13719. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.; Růžičková, Z.; Hobza, P.; Fanfrlík, J.; Hnyk, D.; Růžička, A. Various Types of Non-covalent Interactions Contributing Towards Crystal Packing of Halogenated Diphospha-dicarborane with an Open Pentagonal Belt. New J. Chem. 2018, 42, 10481–10483. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Flűkiger, P.; Lűthi, H.P.; Portmann, S.; Weber, J. MOLEKEL 4.3; Swiss Center for Scientific Computing: Manno, Switzerland, 2000. [Google Scholar]
- Portmann, S.; Luthi, H.P. MOLEKEL: An Interactive Molecular Graphic Tool. CHIMIA Int. J. Chem. 2000, 54, 766–770. [Google Scholar]
- Riley, K.E.; Tran, K.A.; Lane, P.; Murray, J.S.; Politzer, P. Comparative Analysis of Electrostatic Potential Maxima and Minima on Molecular Surfaces, as Determined by Three Methods and a Variety of Basis Sets. J. Comput. Sci. 2016, 17, 273–284. [Google Scholar] [CrossRef]
- Hostaš, J.; Řezáč, J. Accurate DFT-D3 Calculations in a Small Basis Set. J. Chem. Theory Comput. 2017, 13, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, J.; Hobza, P. Advanced Corrections of Hydrogen Bonding and Dispersion for Semiempirical Quantum Mechanical Methods. J. Chem. Theory Comput. 2012, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, J.; Huang, Y.; Hobza, P.; Beran, J.O.G. Benchmark Calculations of Three-Body Intermolecular Interactions and the Performance of Low-Cost Electronic Structure Methods. J. Chem. Theory Comput. 2015, 11, 3065–3079. [Google Scholar] [CrossRef] [PubMed]
- Pitonak, M.; Neogady, P.; Cerny, J.; Grimme, S.; Hobza, P. Scaled MP3 Non-Covalent Interaction Energies Agree Closely with Accurate CCSD(T) Benchmark Data. ChemPhysChem 2009, 10, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Halkier, A.; Helgaker, T.; Jørgensen, P.; Klopper, W.; Koch, H.; Olsen, J.; Wilson, A.K. Basis-Set Convergence in Correlated Calculations on Ne, N2, and H2O. Chem. Phys. Lett. 1998, 286, 243–252. [Google Scholar] [CrossRef]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Parker, T.M.; Burns, L.A.; Parrish, R.M.; Ryno, A.G.; Sherrill, C.D. Levels of Symmetry Adapted Perturbation Theory (SAPT). I. Efficiency and Performance for Interaction Energies. J. Chem. Phys. 2014, 140, 094106. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. Psi4: An Open-Source Ab Initio Electronic Structure Program. WIREs Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Stewart, J.P. Optimization of parameters for semiempirical methods IV: Extension of MNDO, AM1, and PM3 to more main group elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, J. Cuby: An Integrative Framework for Computational Chemistry. J. Comput. Chem. 2016, 37, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Macháček, J.; Plešek, J.; Holub, J.; Hnyk, D.; Všetečka, V.; Císařová, I.; Kaupp, M.; Štíbr, B. New Route to 1-Thia-closo-dodecaborane(11), closo-1-SB11H11, and Its Halogenation Reactions. The Effect of the Halogen on the Dipole Moments and the NMR Spectra and the Importance of Spin-Orbit Coupling for the 11B Chemical Shifts. Dalton Trans. 2006, 1024–1029. [Google Scholar]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Nowak, R.; Steigelmann, O.; Muller, G. π-Complexes of p-Block Elements: Synthesis and Structures of Adducts of Arsenic and Antimony Halides with Alkylated Benzenes. Chemische Berichte 1990, 123, 1221–1226. [Google Scholar] [CrossRef]
- Burford, N.; Clyburne, J.A.C.; Wiles, J.A.; Cameron, T.S.; Robertson, K.N. Tethered Diarenes as Four-Site Donors to SbCl3. Organometallics 1996, 15, 361–364. [Google Scholar] [CrossRef]
- Burford, N.; Clyburne, J.A.C.; Bakshi, P.K.; Cameron, T.S. η6-Arene Complexation to a Phosphenium Cation. J. Am. Chem. Soc. 1993, 115, 8829–8830. [Google Scholar] [CrossRef]
- McGrath, T.D.; Welch, A.J. Steric Effects in Heteroboranes. IV. 1-Ph-2-Br-1,2-closo-C2B10H10. Acta Cryst. 1995, C51, 649–651. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Halogen Bonding versus Chalcogen and Pnicogen Bonding: A Combined Cambridge Structural Database and Theoretical Study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willet, R.D.; Peterson, K.A.; Twamley, B. The Nature of Halogen···Halogen Synthons: Crystalographic and Theoretical Studies. Chem. Eur. J. 2006, 12, 8952–8960. [Google Scholar] [CrossRef] [PubMed]
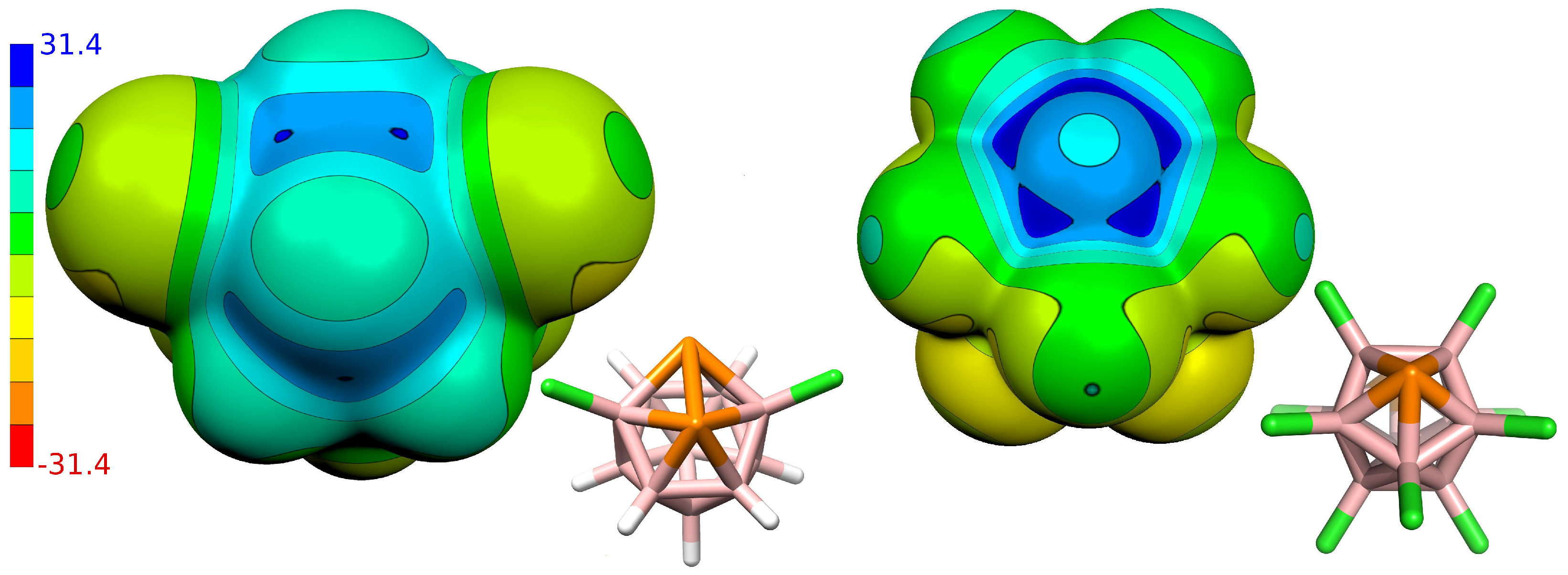
| Compound | Atom | VS,max [kcal mol−1] |
|---|---|---|
| 3,6-Cl2-closo-1,2-P2B10H8 (1) | P(1,2) | 2 × 25.2; 25.1 |
| Cl(3,6) 1 | 2.3 | |
| closo-1,7-P2B10Cl10 (2) | P(1,7) | 30.2; 2 × 28.9; 2 × 27.8 |
| Cl(2,3) | 13.1 | |
| Cl(9,10) 2 | 1.5 |
| Interaction | DFT-D3/MP2.5 | SAPT0/jun-cc-pVDZ | ||||
|---|---|---|---|---|---|---|
| Total | Eelec | Eind | Edisp | Eexch | ||
| A···B | −4.85/−4.23 | −4.83 | −2.28 (21%) | −0.81 (8%) | −7.67 (71%) | 5.94 |
| A···C | −4.09/−3.90 | −4.01 | −2.26 (27%) | −0.61 (7%) | −5.42 (65%) | 4.28 |
| A···D | −3.85/−3.35 | −3.70 | −2.11 (24%) | −0.65 (8%) | −5.87 (68%) | 4.92 |
| Interaction | DFT-D3/MP2.5 | SAPT0/jun-cc-pVDZ | ||||
|---|---|---|---|---|---|---|
| Total | Eelec | Eind | Edisp | Eexch | ||
| 2···toluene Pn-bond | −9.94/−10.55 | −11.56 | −9.80 (35%) | −3.96 (14%) | −14.61 (52%) | 16.81 |
| 2···toluene stacking | −2.51/−2.25 | −1.96 | −0.84 (22%) | −0.21 (6%) | −2.75 (72%) | 1.84 |
| 2···toluene H-bond | −0.87/−0.80 | −0.51 | −0.35 (16 %) | −0.21 (10%) | −1.58 (74%) | 1.63 |
| 2···2 bifurcated diX-bonds | −3.67 | −3.27 | −1.17 (16%) | −0.32 (4%) | −5.73 (79%) | 3.95 |
| 2···2 bifurcated diX-bond | −2.94 | −3.01 | −1.78 (26%) | −0.40 (6%) | −4.67 (68%) | 3.84 |
| 2···2 diX-bond | −2.32/−2.65 | −2.13 | −1.51 (25%) | −0.35 (6%) | −4.13 (69%) | 3.86 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanfrlík, J.; Hnyk, D. Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes. Crystals 2018, 8, 390. https://doi.org/10.3390/cryst8100390
Fanfrlík J, Hnyk D. Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes. Crystals. 2018; 8(10):390. https://doi.org/10.3390/cryst8100390
Chicago/Turabian StyleFanfrlík, Jindřich, and Drahomír Hnyk. 2018. "Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes" Crystals 8, no. 10: 390. https://doi.org/10.3390/cryst8100390
APA StyleFanfrlík, J., & Hnyk, D. (2018). Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes. Crystals, 8(10), 390. https://doi.org/10.3390/cryst8100390