The Interplay between Various σ- and π-Hole Interactions of Trigonal Boron and Trigonal Pyramidal Arsenic Triiodides
Abstract
1. Introduction
2. Methods
2.1. Experimental
2.2. Computations
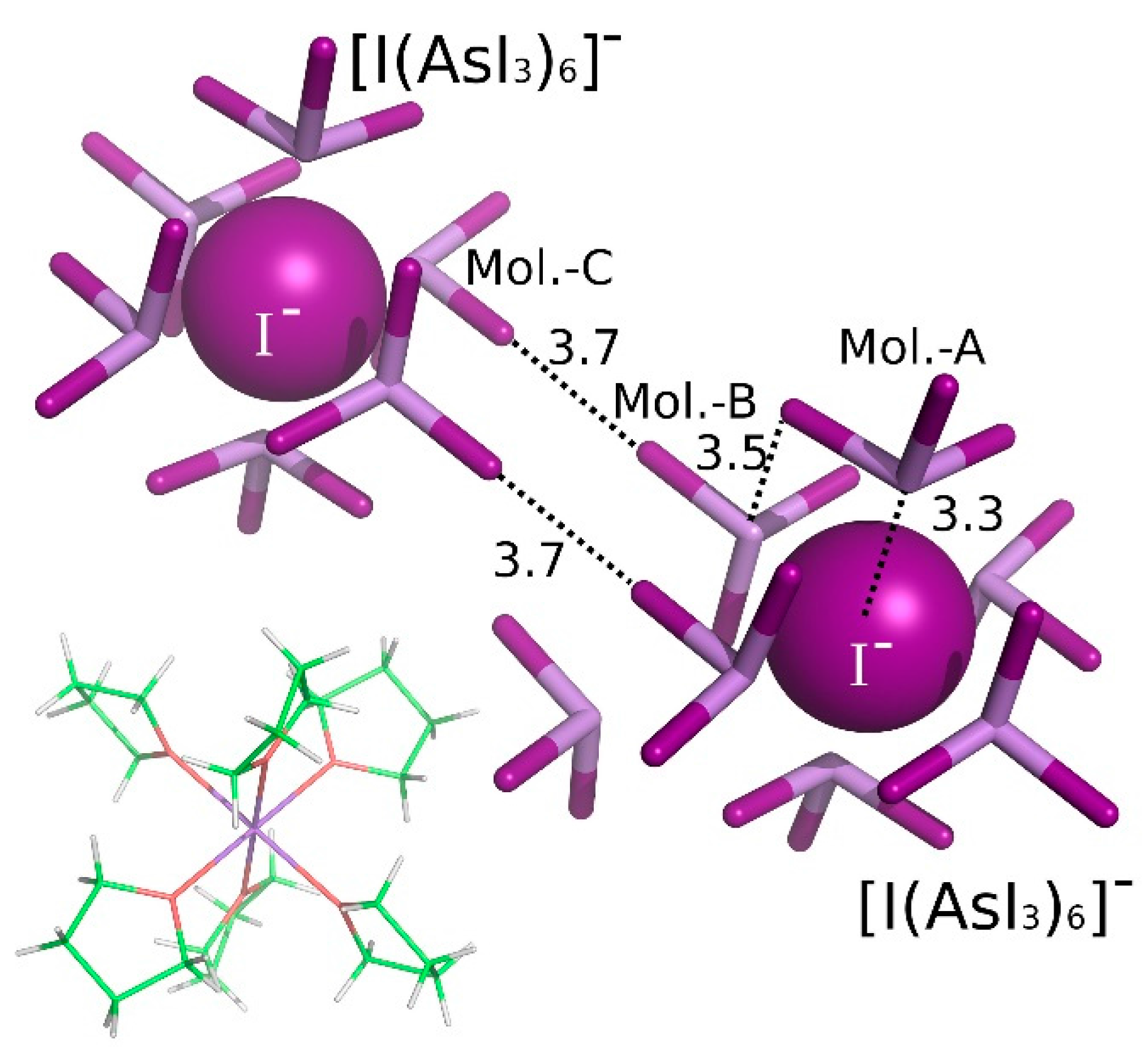
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Cavall, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Primagi, A.; Ransani, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-hole strengths and interactions of F3MX molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.; Hostaš, J.; Hobza, P. The strength and directionality of a halogen bond are co-determined by the magnitude and size of the σ-hole. Phys. Chem. Chem. Phys. 2014, 16, 23279–23280. [Google Scholar]
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen Bond Tunability I: The Effects of Aromatic Fluorine Substitution on the Strengths of Halogen-Bonding Interactions Involving Chlorine, Bromine, and Iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Herdegger, L.A.; Kuhn, B.; Spinnler, B.; Anselm, L.; Ecabert, R.; Stihle, M.; Gsell, B.; Thoma, R.; Diez, J.; Benz, J.; et al. Systematic Investigation of Halogen Bonding in Protein−Ligand Interactions. Angew. Chem. Int. Ed. 2011, 50, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Fanfrlík, J.; Kolář, M.; Kamlar, M.; Hurny, D.; Ruiz, F.X.; Cousido-Siah, A.; Mitschler, A.; Řezáč, J.; Munusamy, E.; Lepšík, M.; et al. The Modulation of Aldose Reductase Inhibition by Halogen Bond Tuning. ACS Chem. Biol. 2013, 8, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Ring, M.A.; Donnay, J.D.H.; Koski, W.S. The Crystal Structure of Boron Triiodide. Inorg. Chem. 1962, 1, 109–111. [Google Scholar] [CrossRef]
- Trotter, J. The crystal structure of arsenic triiodide, AsI3. Z. Kristallogr. Cryst. Mater. 1965, 121, 81–86. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bergner, M.; Dolg, M.; Küchle, W.; Stoll, H.; Preuss, H. Ab initio energy-adjusted pseudopotentials for elements of groups 13–17. Mol. Phys. 1993, 80, 1431–1441. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Flűkiger, P.; Lűthi, H.P.; Portmann, S.; Weber, J. MOLEKEL 4.3; Swiss Center for Scientific Computing: Manno, Switzerland, 2000. [Google Scholar]
- Portmann, S.; Luthi, H.P. MOLEKEL: An Interactive Molecular Graphic Tool. CHIMIA Int. J. Chem. 2000, 54, 766–770. [Google Scholar]
- Riley, K.E.; Tran, K.-A.; Lane, P.; Murray, J.S.; Politzer, P. Comparative analysis of electrostatic potential maxima and minima on molecular surfaces, as determined by three methods and a variety of basis sets. J. Comput. Sci. 2016, 17, 273–284. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bar, M.; Haser, M.; Horn, H.; Kolmel, C. Electronic Structure Calculations on Workstation Computers: The Program System Turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Řezáč, J. Cuby: An integrative framework for computational chemistry. J. Comput. Chem. 2016, 37, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Halkier, A.; Helgaker, T.; Jørgensen, P.; Klopper, W.; Koch, H.; Olsen, J.; Wilson, A.K. Basis-Set Convergence in Correlated Calculations on Ne, N2, and H2O. Chem. Phys. Lett. 1998, 286, 243–252. [Google Scholar] [CrossRef]
- Foord, A.; Beagley, B.; Reader, W.; Steer, I.A. A gas-phase electron-diffraction study of trvinylborane. J. Mol. Struct. 1975, 24, 131–137. [Google Scholar] [CrossRef]
- Cornu, D.; Miele, P.; Faure, R.; Bonnetot, B.; Mongeot, H.; Bouix, J. Conversion of B (NHCH3)3 into boron nitride and polyborazine fibres and tubular BN structures derived therefrom. J. Mater. Chem. 1999, 9, 757–761. [Google Scholar] [CrossRef]
- Lo, R.; Svec, P.; Ruzickova, Z.; Ruzicka, A.; Hobza, P. On the nature of the stabilisation of the E···π pnicogen bond in the SbCl3···toluene complex. Chem. Commun. 2016, 52, 3500–3503. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Schier, A. π-Complexation of Post-Transition Metals by Neutral Aromatic Hydrocarbons: The Road from Observations in the 19th Century to New Aspects of Supramolecular Chemistry. Organometallics 2008, 27, 2361–2395. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Sedlak, R.; Pecina, A.; Rulíšek, L.; Dostál, L.; Moncóľ, J.; Růžička, A.; Hobza, P. The non-planarity of the benzene molecule in the X-ray structure of the chelated bismuth(III) heteroboroxine complex is not supported by quantum mechanical calculations. J. Chem. Soc. Dalton Trans. 2016, 45, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.C.; Hanusch, K.; Wolf, H.U. Arsenic and Arsenic Compounds, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Scholes, R.S. Arsenic in Glass. Ind. Eng. Chem. 1912, 4, 16–17. [Google Scholar] [CrossRef]
- Delgado-Friedrichs, O.; Foster, M.D.; O’Keeffe, M.; Proserpio, D.M.; Treacy, M.M.J.; Yaghi, O.M. What do we know about three-periodic nets? J. Solid State Chem. 2005, 178, 2533–2554. [Google Scholar] [CrossRef]
- Haiduc, I. Inverse coordination—An emerging new chemical concept. Oxygen and other chalcogens as coordination centers. Coord. Chem. Rev. 2017, 338, 1–26. [Google Scholar] [CrossRef]
- Honda, D.; Ikegami, S.; Inoue, T.; Ozeki, T.; Yagasaki, A. Protonation and methylation of an Anderson-type polyoxoanion [IMo6O24]5−. Inorg. Chem. 2007, 46, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Long, D.L.; Song, Y.F.; Wilson, E.F.; Kögerler, P.; Guo, S.X.; Bond, A.M.; Hargreaves, J.S.; Cronin, L. Capture of Periodate in a {W18O54} Cluster Cage Yielding a Catalytically Active Polyoxometalate [H3W18O56(IO6)]6− Embedded with High-Valent Iodine. Angew. Chem. Int. Ed. 2008, 47, 4384–4387. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Chen, W.L.; Wang, E.B.; Li, Y.G.; Feng, X.J.; Liu, L. Three new polyoxometalate-based hybrids prepared from choline chloride/urea deep eutectic mixture at room temperature. Inorg. Chem. Commun. 2010, 13, 972–975. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, C.; Comotti, A.; Ward, M.D. Supramolecular Archimedean cages assembled with 72 hydrogen bonds. Science 2011, 333, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Krautscheid, H.; Lode, C.; Vielsack, F.; Vollmer, H. Synthesis and crystal structures of iodoplumbate chains, ribbons and rods with new structural types. J. Chem. Soc. Dalton Trans. 2001, 7, 1099–1104. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
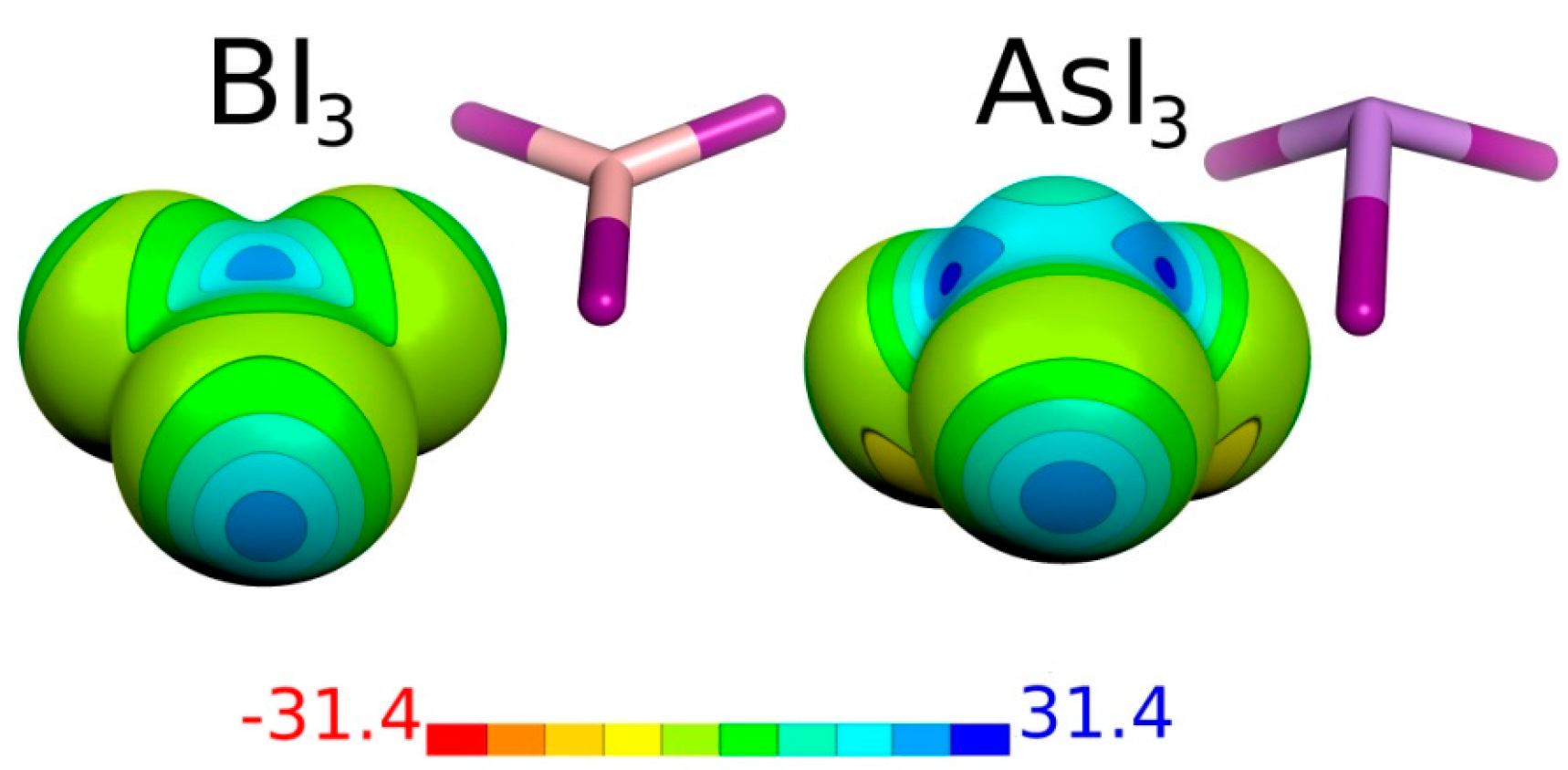
| Molecule | Atom | Vmax | μ |
|---|---|---|---|
| BI3 | B | 2 × 23.5 | 0.00 |
| I | 22.3 | ||
| AsI3 | As | 3 × 25.7 | 0.74 |
| I | 23.2 |
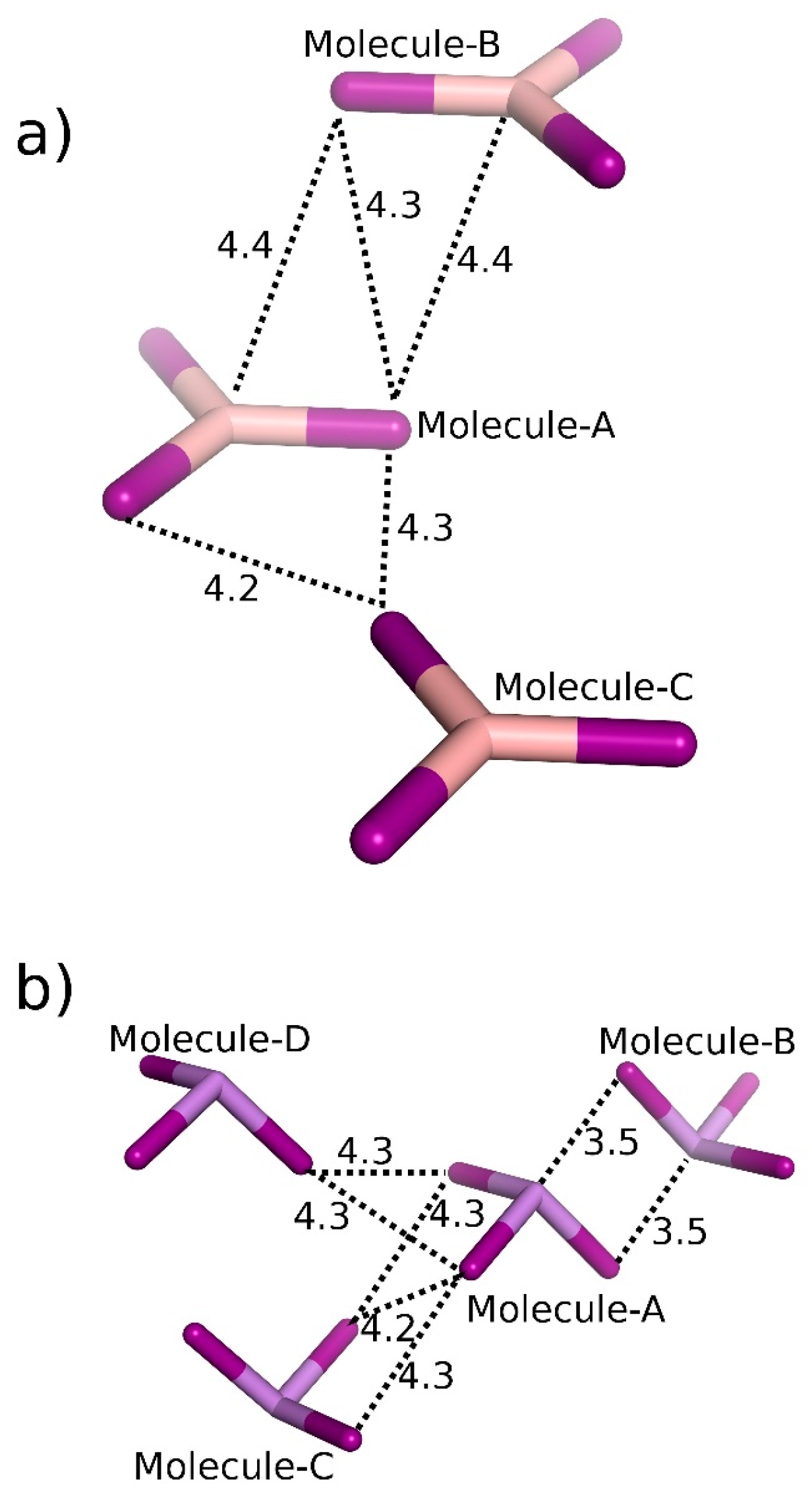
| Crystal | Motif | Interaction | CCSD(T)/CBS |
|---|---|---|---|
| BI3 | A···B | 2 × π-hole bonding | −6.13 |
| A···C | Bifurcated diX-bond | −2.78 | |
| AsI3 | A···B | 2 × Pn-bonding | −7.52 |
| A···C | 2 × Bifurcated diX-bonding | −4.43 | |
| A···D | Bifurcated diX-bonding | −3.26 | |
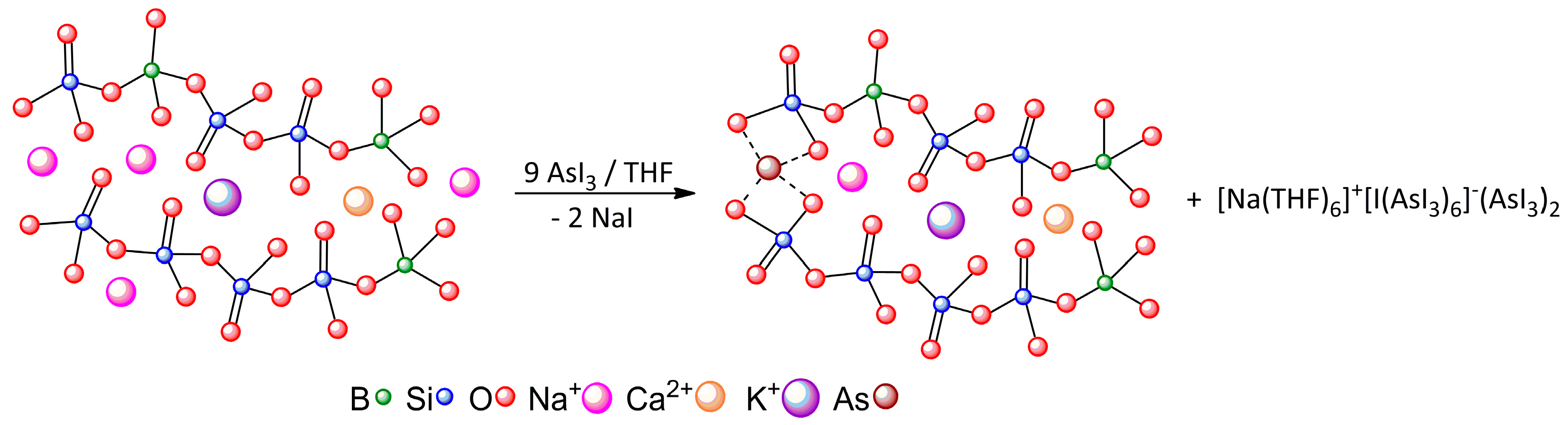
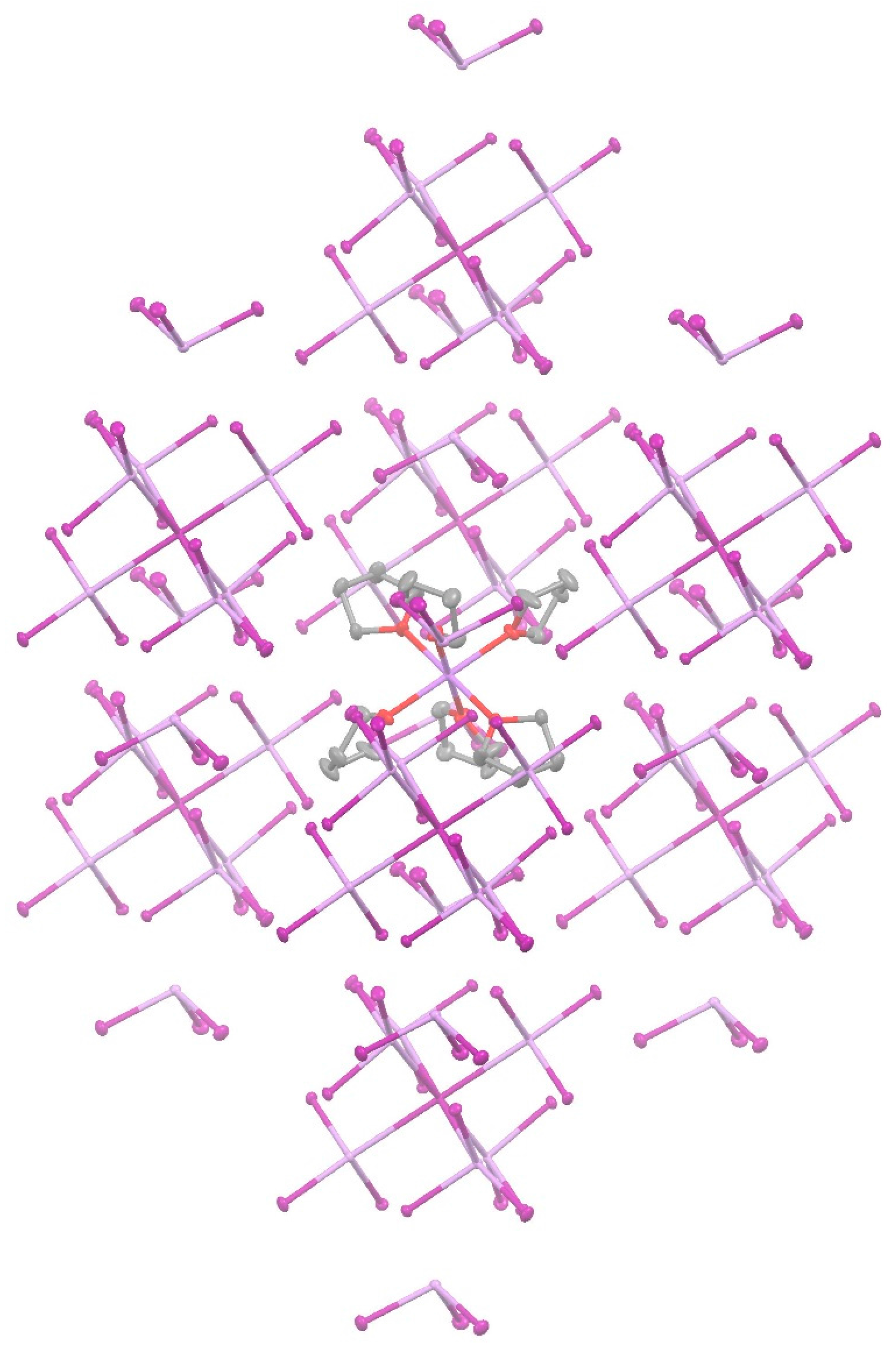
| [Na(THF)6]+[I(AsI3)6]−(AsI3)2 | A···I− | Pn-bonding | −23.59 |
| A···B | Pn-bonding | −4.64 | |
| B···C | diX-bonding | −2.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanfrlík, J.; Švec, P.; Růžičková, Z.; Hnyk, D.; Růžička, A.; Hobza, P. The Interplay between Various σ- and π-Hole Interactions of Trigonal Boron and Trigonal Pyramidal Arsenic Triiodides. Crystals 2017, 7, 225. https://doi.org/10.3390/cryst7070225
Fanfrlík J, Švec P, Růžičková Z, Hnyk D, Růžička A, Hobza P. The Interplay between Various σ- and π-Hole Interactions of Trigonal Boron and Trigonal Pyramidal Arsenic Triiodides. Crystals. 2017; 7(7):225. https://doi.org/10.3390/cryst7070225
Chicago/Turabian StyleFanfrlík, Jindřich, Petr Švec, Zdeňka Růžičková, Drahomír Hnyk, Aleš Růžička, and Pavel Hobza. 2017. "The Interplay between Various σ- and π-Hole Interactions of Trigonal Boron and Trigonal Pyramidal Arsenic Triiodides" Crystals 7, no. 7: 225. https://doi.org/10.3390/cryst7070225
APA StyleFanfrlík, J., Švec, P., Růžičková, Z., Hnyk, D., Růžička, A., & Hobza, P. (2017). The Interplay between Various σ- and π-Hole Interactions of Trigonal Boron and Trigonal Pyramidal Arsenic Triiodides. Crystals, 7(7), 225. https://doi.org/10.3390/cryst7070225






