Synthesis, Crystal Structure, and Cytotoxic Activity of a Novel Eight-Coordinated Dinuclear Ca(II)-Schiff Base Complex
Abstract
:1. Introduction
2. Results and Discussion
2.1. Properties of 1
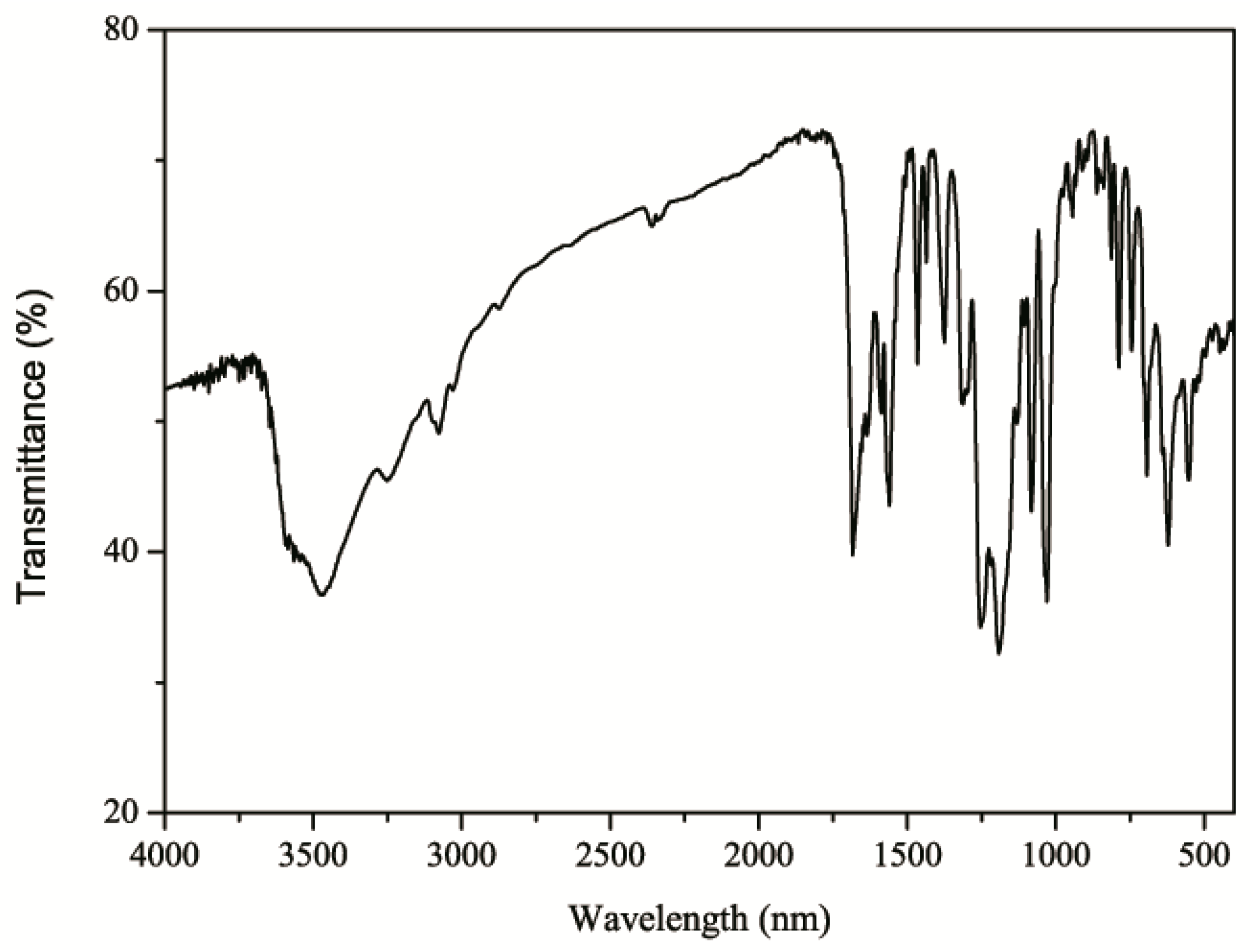
2.2. IR Spectrum of 1
2.3. 1HNMR and 13CNMR Spectra of 1
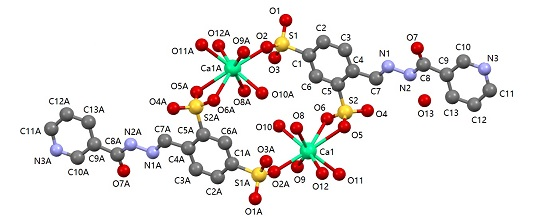
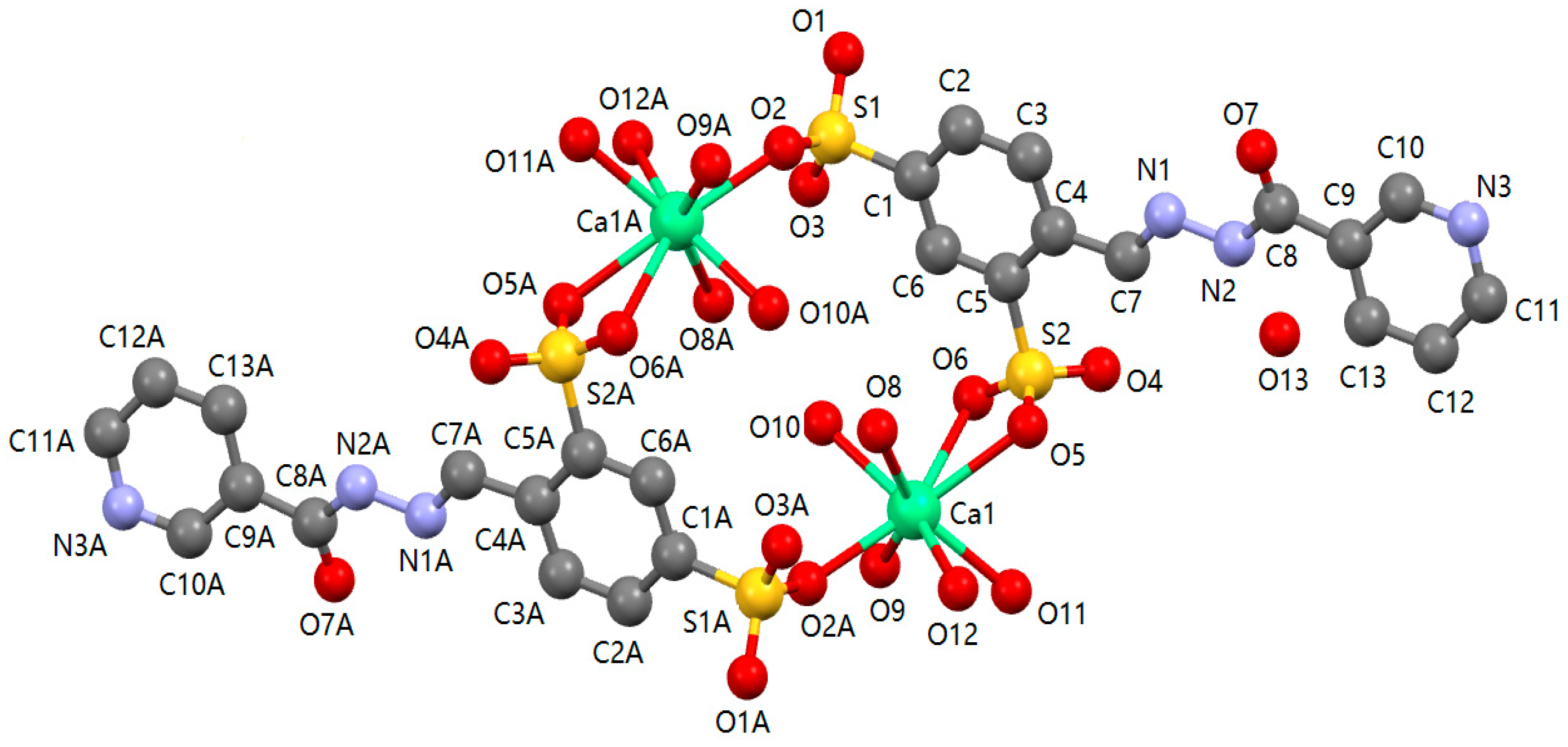
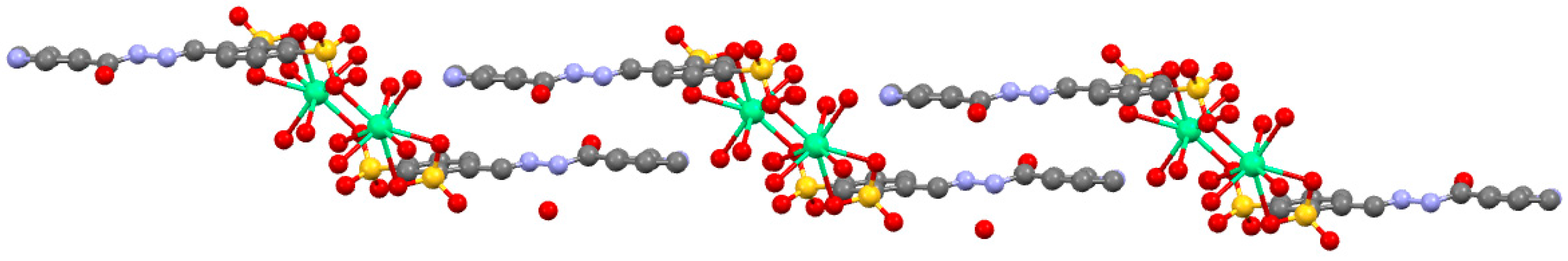
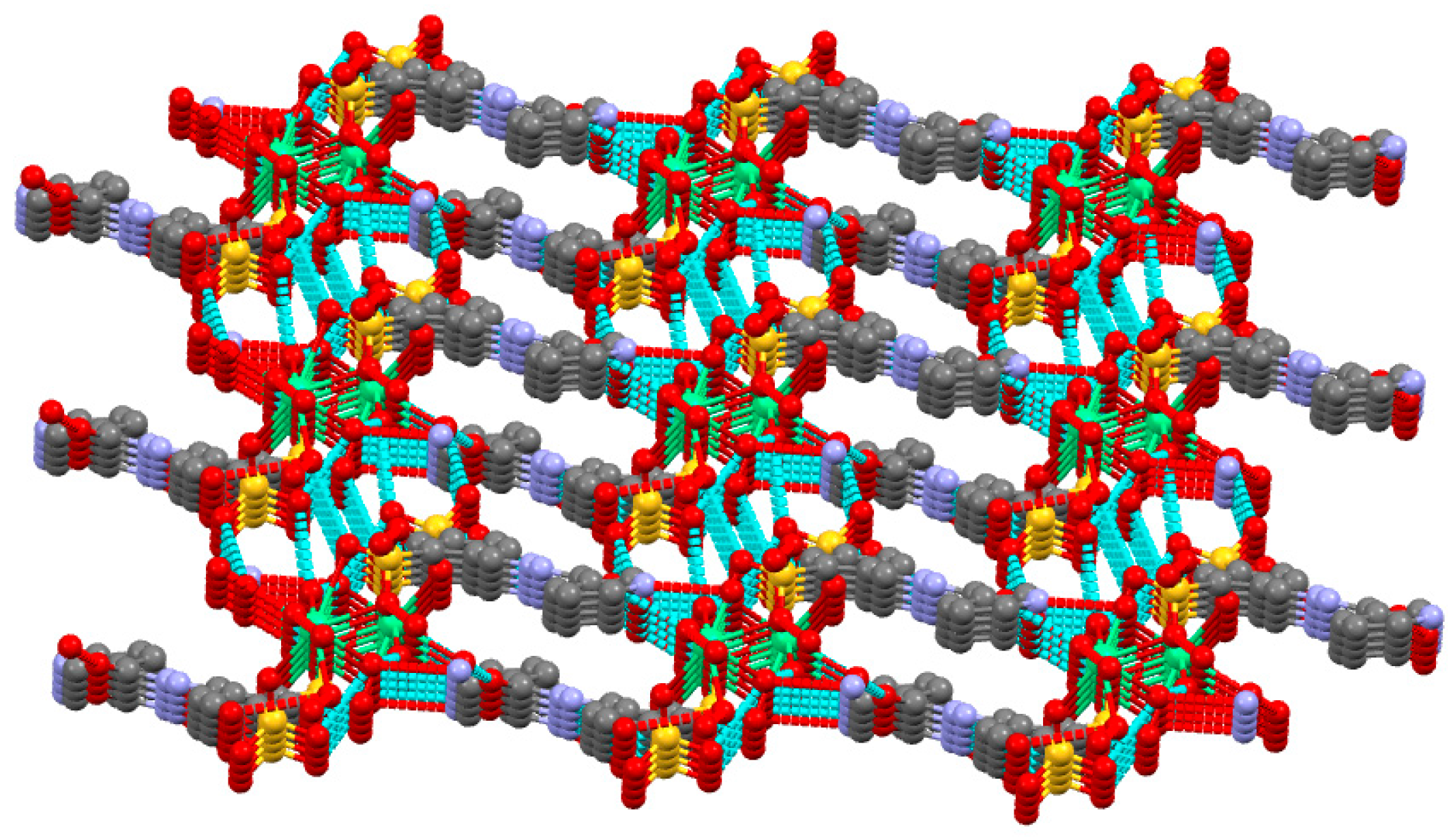
2.4. Description of 1
2.5. In Vitro Cytotoxic Activity
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of [Ca2(L)2(H2O)10]·H2O (1)
3.3. Crystal Structure Determination
3.4. In Vitro Cytotoxic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Bindary, A.A.; El-Sonbati, A.Z.; Shoair, A.F.; Mohamed, A.S. Spectroscopic, thermal analysis and antimicrobial activities of supramolecular Cu(II) complexes. J. Mol. Liq. 2016, 220, 409–425. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Y.; Li, Q.Y.; Ma, T.L.; Xu, J.; Zhu, T.F.; Xie, J.; Zhu, W.J.; Cao, Z.H.; Dong, K.; et al. Ternary dinuclear copper(II) complexes of a reduced Schiff base ligand with diimine coligand: DNA binding, cytotoxic cell apoptosis, and apoptotic mechanism. Chem. Bio. Drug Des. 2016, 87, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Sherif, Y.E.; Gouda, M.A.; El-Asmy, A.A. Synthesis, characterization, analgesic and anti-inflammation activity of new phthalazines and their Cu(II) and Zn(II) complexes. Med. Chem. Res. 2015, 24, 3853–3862. [Google Scholar] [CrossRef]
- Zhao, J.A.; Guo, Y.; Hua, J.Y.; Yu, H.B.; Zhi, S.C.; Zhang, J.S. Potential anticancer activity of benzimidazole-based mono/dinuclear Zn(II) complexes towards human carcinoma cells. Polyhedron 2015, 102, 163–172. [Google Scholar] [CrossRef]
- Amirnasr, M.; Erami, R.S.; Mereiter, K.; Joß, K.S.; Meghdadi, S.; Abbasi, S. Syntheses, characterization, X-ray crystal structures, and antibacterial activities of Co(II), Ni(II), and Zn(II) complexes of the Schiff base derived from 5-nitro-2-hydroxybenzaldehyde and benzylamine. J. Coord. Chem. 2015, 68, 616–631. [Google Scholar] [CrossRef]
- Tai, X.S.; Yin, J.; Liu, Y.Y. Synthesis, crystal structure and antitumor activity of a Zn(II) complex with 1,2-phenylenedioxydiacetic acid. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 15, 297–305. [Google Scholar]
- Thamilarasan, V.; Sengottuvelan, N.; Stalin, N.; Srinivasan, P.; Chakkaravarthi, G. Synthesis, interactions, molecular structure, biological properties and molecular docking studies on Mn, Co, Zn complexes containing acetylacetone and pyridine ligands with DNA duplex. J. Photochem. Photobiol. B. 2016, 160, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lin, Q.Y.; Zheng, X.L.; Zhang, L.L.; Yang, Q.; Gu, J.Y. Crystal structures, interactions with biomacromolecules and anticancer activities of Mn(II), Ni(II), Cu(II) complexes of demethylcantharate and 2-aminopyridine. J. Fluoresc. 2012, 22, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.; Khan, N.H.; Prathap, K.J.; Kureshy, R.I.; Abdi, S.H.R.; Mishra, S.; Bajaj, H.C. DNA binding, antioxidant activity, and DNA damage protection of chiral macrocyclic Mn(II) salen complexes. Chirality 2012, 24, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Zangrando, E.; Islam, M.T.; Islam, M.A.A.A.; Sheikh, M.C.; Tarafder, M.T.H.; Miyatake, R.; Zahan, R.; Hossain, M.A. Synthesis, characterization and bio-activity of nickel(II) and copper(II) complexes of a bidentate NS Schiff base of S-benzyl dithiocarbazate. Inorg. Chim. Acta 2015, 427, 278–284. [Google Scholar] [CrossRef]
- Tyagi, P.; Chandra, S.; Saraswat, B.S.; Yadav, D. Design, spectral characterization, thermal, DFT studies and anticancer cell line activities of Co(II), Ni(II) and Cu(II) complexes of Schiff bases derived from 4-amino-5-(pyridine-4-yl)-4H-1,2,4-triazole-3-thiol. Spectrochim. Acta A 2015, 145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Kong, S.L.; Wang, Z.C.; Pan, C.X.; Zhu, H.L. Ni(II) ternary complex based on antimicrobial drug enoxacin: Synthesis and biological properties. Chin. J. Chem. 2014, 32, 1069–1175. [Google Scholar] [CrossRef]
- Mihorianu, M.; Franz, M.H.; Jones, P.G.; Freytag, M.; Kelter, G.; Fiebig, H.H.; Tamm, M.; Neda, I. N-heterocyclic carbenes derived from imidazo-[1,5-a]pyridines related to natural products: synthesis, structure and potential biological activity of some corresponding gold(I0 and silver(I) complexes. Appl. Organomet. Chem. 2016, 30, 581–589. [Google Scholar] [CrossRef]
- Haque, R.A.; Choo, S.Y.; Budagumpi, S.; Iqbal, M.A.; Amirul, A.A. Silver(I) complexes of mono- and bidentate N-heterocyclic carbine ligands: synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Eur. J. Med. Chem. 2014, 90, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Tailor, S.M.; Patel, U.H. Synthesis, spectroscopic characterization, antimicrobial activity and crystal structure of [Ag2(C10H10N3O3S2)2(C5H5N)3]. J. Mol. Struct. 2015, 1088, 161–168. [Google Scholar] [CrossRef]
- Min, L.J.; Shi, Y.X.; Yang, M.Y.; Zhai, Z.W.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Microwave assisted synthesis and antifungal activity of some novel hydrazones containing pyridine moiety. Lett. Drug Des. Discov. 2016, 13, 324–328. [Google Scholar] [CrossRef]
- Sundaree, S.; Vaddula, B.R.; Tantak, M.P.; Khandagale, S.B.; Shi, C.; Shah, K.; Kumar, D. Synthesis and anticancer activity study of indolyl hydrazide-hydrazones. Med. Chem. Res. 2016, 25, 941–950. [Google Scholar] [CrossRef]
- Velezheva, V.; Brennan, P.; Ivanov, P.; Kornienko, A.; Lyubimov, S.; Kazarian, K.; Nikonenko, B.; Majorov, K.; Apt, A. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorgan. Med. Chem. Lett. 2016, 26, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of Ca(II) coordination polymer based on 1,5-naphthalenedisulfonate. J. Inorg. Organomet. Polym. 2013, 23, 1354–1357. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis and Crystal Structure of a 1D Chained Coordination Polymer Constructed from Ca2+ and 2-[(E)-(2-Furoylhydrazono)methyl]benzenesulfonate. Crystals 2015, 5, 458–465. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis, characterization and antitumor activity of a Ca (II) cordination polymer based on 3-amino-2-pyrazinecarboxylic acid. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 253–259. [Google Scholar]
- Tai, X.S.; Zhang, Y.P.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of a dinuclear calcium complex based on coordination polymer based on 1,5-naphthalenedisulfonate and 2,2′-bipyridine ligand. Res. Chem. Intermed. 2015, 41, 4339–4347. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H.; Li, F.H. Synthesis, crystal structure and antitumor activity of a Ca(II) coordination polymer constructed by N-benzenesulphonyl-L-phenylalanine. Chin. J. Inorg. Chem. 2013, 29, 2200–2204. [Google Scholar]
- Tai, X.S.; Zhao, W.H.; Li, F.H. Synthesis, structural characterization and antitumor activity of a Ca(II)-schiff base complex. Chin. J. Inorg. Chem. 2013, 29, 1328–1332. [Google Scholar]
- Tai, X.S.; Yin, J.; Feng, Y.M.; Kong, F.Y. Synthesis and crystal structure of Ca(II) complex with salicylaldehyde-4-aminobenzene sulfonic acid. Chin. J. Inorg. Chem. 2007, 23, 1812–1814. [Google Scholar]
- Nakamoto, K. Infrared and Ramen Spectra of Inorganic and Coordination Compounds, 3rd ed.; Wiley: New York, NY, USA, 1978; Volume 1, pp. 359–368. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Ca1-O11 | 2.3882 (18) | Ca1-O6 | 2.688 (2) |
| Ca1-O10 | 2.4110 (17) | S2-O4 | 1.4396 (14) |
| Ca1-O2i | 2.4221 (15) | S2-O6 | 1.4504 (15) |
| Ca1-O8 | 2.4333 (18) | S2-O5 | 1.4556 (17) |
| Ca1-O9 | 2.4448 (19) | S1-O1 | 1.4454 (16) |
| Ca1-O12 | 2.4799 (19) | S1-O2 | 1.4501 (16) |
| Ca1-O5 | 2.5823 (16) | S1-O3 | 1.4546 (17) |
| C7-N1 | 1.266 (3) | N1-N2 | 1.381 (2) |
| Angle | ° | Angle | ° |
| O11-Ca1-O10 | 139.72 (7) | O11-Ca1-O2i | 114.65 (7) |
| O10-Ca1-O2i | 79.62 (6) | O11-Ca1-O8 | 140.87 (6) |
| O10-Ca1-O8 | 76.19 (7) | O8-Ca1-O2i | 80.77 (6) |
| O11-Ca1-O9 | 72.33 (6) | O10-Ca1-O9 | 73.73 (6) |
| O9-Ca1-O2i | 80.05 (6) | O8-Ca1-O9 | 146.67 (5) |
| O11-Ca1-O12 | 72.44 (6) | O12-Ca1-O10 | 147.10 (6) |
| O12-Ca1-O2i | 78.36 (6) | O8-Ca1-O12 | 76.39 (6) |
| O12-Ca1-O9 | 125.36 (6) | O11-Ca1-O5 | 75.96 (6) |
| O10-Ca1-O5 | 108.77 (6) | O5-Ca1-O2i | 153.19 (6) |
| O8-Ca1-O5 | 76.89 (6) | O9-Ca1-O5 | 126.56 (6) |
| O5-Ca1-O12 | 82.12 (6) | O11-Ca1-O6 | 84.33 (6) |
| O10-Ca1-O6 | 69.68 (5) | O6-Ca1-O2i | 147.52 (95) |
| O8-Ca1-O6 | 101.30 (6) | O9-Ca1-O6 | 81.40 (6) |
| O12-Ca1-O6 | 133.92 (6) | O5-Ca1-O9 | 53.40 (5) |
| Hydrogen Bonds | d(D-H) | d(H…A) | d(D…A) | DHA | Symmetry Code |
|---|---|---|---|---|---|
| O(8)-H(8WB)…O(3) | 0.853 | 2.05 | 2.812(2) | 149 | 1 − x, 2 − y, −z |
| O(8)-H(8WA)…N(3) | 0.849 | 1.94 | 2.771(3) | 166 | 1 − x, 3 − y, 1 − z |
| N(2)-H(2B)…O(13) | 0.86 | 1.97 | 2.766(3) | 153 | x, y, z |
| O(9)-H(9WB)…O(7) | 0.856 | 1.99 | 2.815(2) | 159 | x, −1 + y, z |
| O(9)-H(9WA)…O(1) | 0.85 | 2.03 | 2.869(2) | 170 | 2 − x, 2 − y, −z |
| O(11)-H(1WB)…O(5) | 0.85 | 2.01 | 2.817(2) | 160 | 1 − x, 2 − y, 1 − z |
| O(11)-H(1WA)…O(7) | 0.85 | 1.90 | 2.717(3) | 163 | x, −1 + y, z |
| O(12)-H(2WA)…O(6) | 0.86 | 2.06 | 2.910(3) | 169 | −1 + x, y, z |
| O(12)-H(2WB)…O(4) | 0.85 | 2.06 | 2.887(2) | 163 | 1 − x, 2 − y, 1 − z |
| O(10)-H(10C)…O(8) | 0.85 | 2.14 | 2.900(2) | 149 | 1 − x, 2 − y, − z |
| O(10)-H(10D)…O(3) | 0.86 | 1.95 | 2.801(3) | 170 | 2 − x, 2 − y, − z |
| O(13)-H(13B)…O(1) | 0.96 | 2.45 | 3.187(3) | 133 | x, y, 1 + z |
| O(13)-H(13B)…O(2) | 0.96 | 2.41 | 3.079(3) | 126 | x, y, 1 + z |
| O(13)-H(13C)…O(12) | 0.96 | 2.16 | 3.039(3) | 152 | 1 − x, 2 − y, 1 − z |
| Compound | IC50 (μg/mL) | |
| HL-60 | MLTC-1 | |
| Ca(II) complex | 27.68 ± 0.9 | 19.51 ± 1.2 |
| Empirical Formula | C26H42Ca2N6O26S4 |
|---|---|
| Formula weight | 1063.06 |
| Temperature/K | 296(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 7.186(3) |
| b/Å | 11.978(5) |
| c/Å | 12.263(5) |
| α/° | 90.318(5) |
| β/° | 91.922(5) |
| γ/° | 96.797(5) |
| Volume/Å3 | 1047.5(8) |
| Z | 1 |
| ρcalcmg/mm3 | 1.685 |
| μ/mm‑1 | 0.572 |
| S | 1.093 |
| F(000) | 552 |
| Index ranges | −8 ≤ h ≤ 8, −14 ≤ k ≤ 13, −14 ≤ l ≤ 13 |
| Reflections collected | 5175 |
| Independent reflections | 3646 [R(int) = 0.0169] |
| Data/restraints/parameters | 3646/10/329 |
| Goodness-of-fit on F2 | 1.094 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0308, wR2 = 0.0770 |
| Final R indexes [all data] | R1 = 0.0355, wR2 = 0.0792 |
| Largest diff. peak/hole / e Å−3 | 0.354/−0.358 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, X.-S.; Meng, Q.-G.; Liu, L.-L. Synthesis, Crystal Structure, and Cytotoxic Activity of a Novel Eight-Coordinated Dinuclear Ca(II)-Schiff Base Complex. Crystals 2016, 6, 109. https://doi.org/10.3390/cryst6090109
Tai X-S, Meng Q-G, Liu L-L. Synthesis, Crystal Structure, and Cytotoxic Activity of a Novel Eight-Coordinated Dinuclear Ca(II)-Schiff Base Complex. Crystals. 2016; 6(9):109. https://doi.org/10.3390/cryst6090109
Chicago/Turabian StyleTai, Xi-Shi, Qing-Guo Meng, and Li-Li Liu. 2016. "Synthesis, Crystal Structure, and Cytotoxic Activity of a Novel Eight-Coordinated Dinuclear Ca(II)-Schiff Base Complex" Crystals 6, no. 9: 109. https://doi.org/10.3390/cryst6090109
APA StyleTai, X.-S., Meng, Q.-G., & Liu, L.-L. (2016). Synthesis, Crystal Structure, and Cytotoxic Activity of a Novel Eight-Coordinated Dinuclear Ca(II)-Schiff Base Complex. Crystals, 6(9), 109. https://doi.org/10.3390/cryst6090109