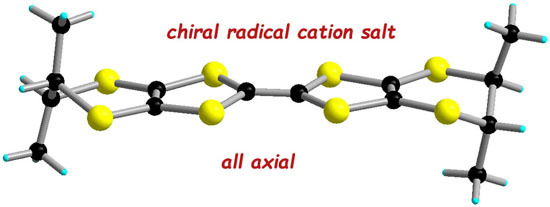
Enantiopure Radical Cation Salt Based on Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene and Hexanuclear Rhenium Cluster
Abstract
:1. Introduction
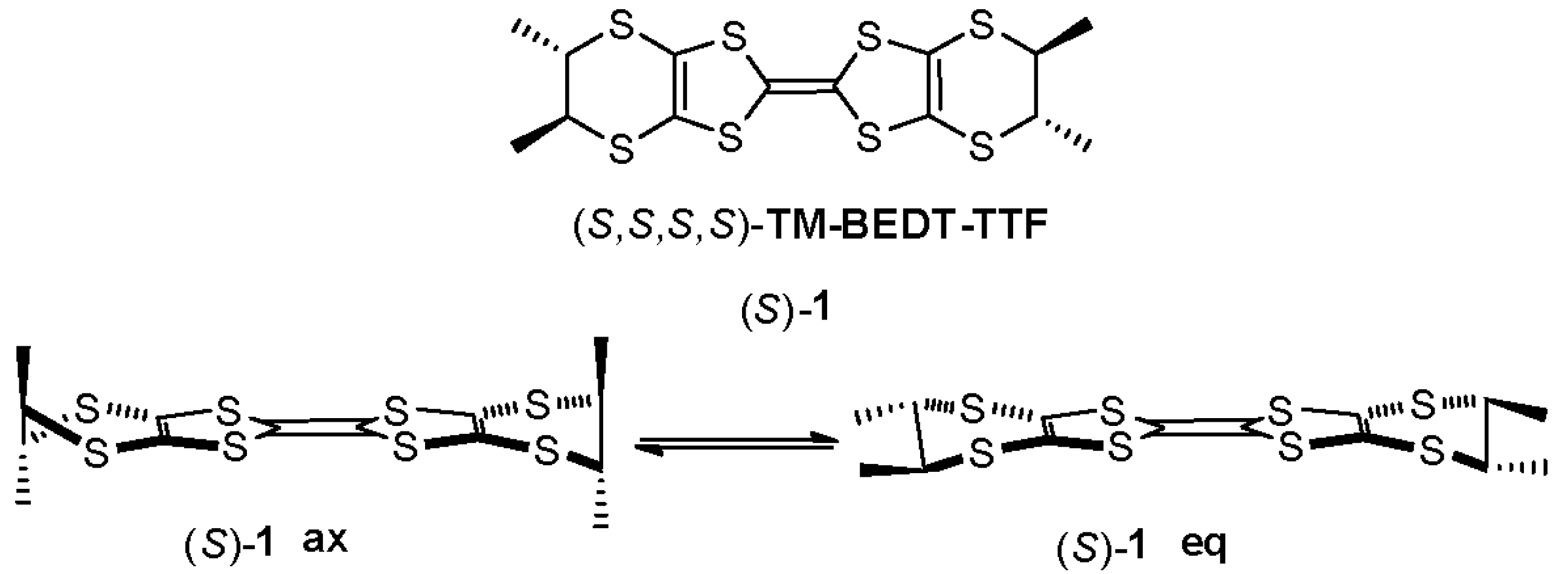
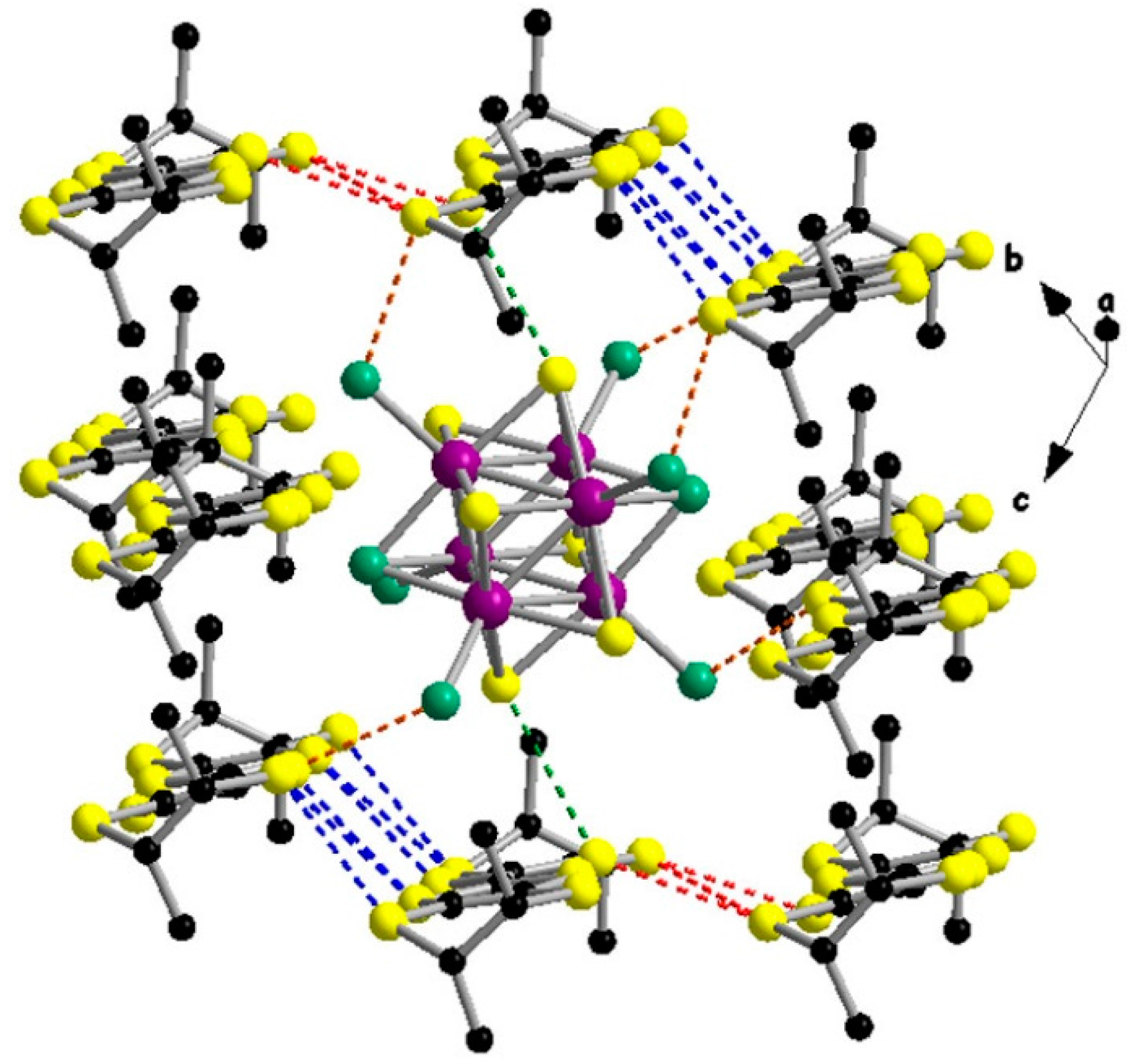
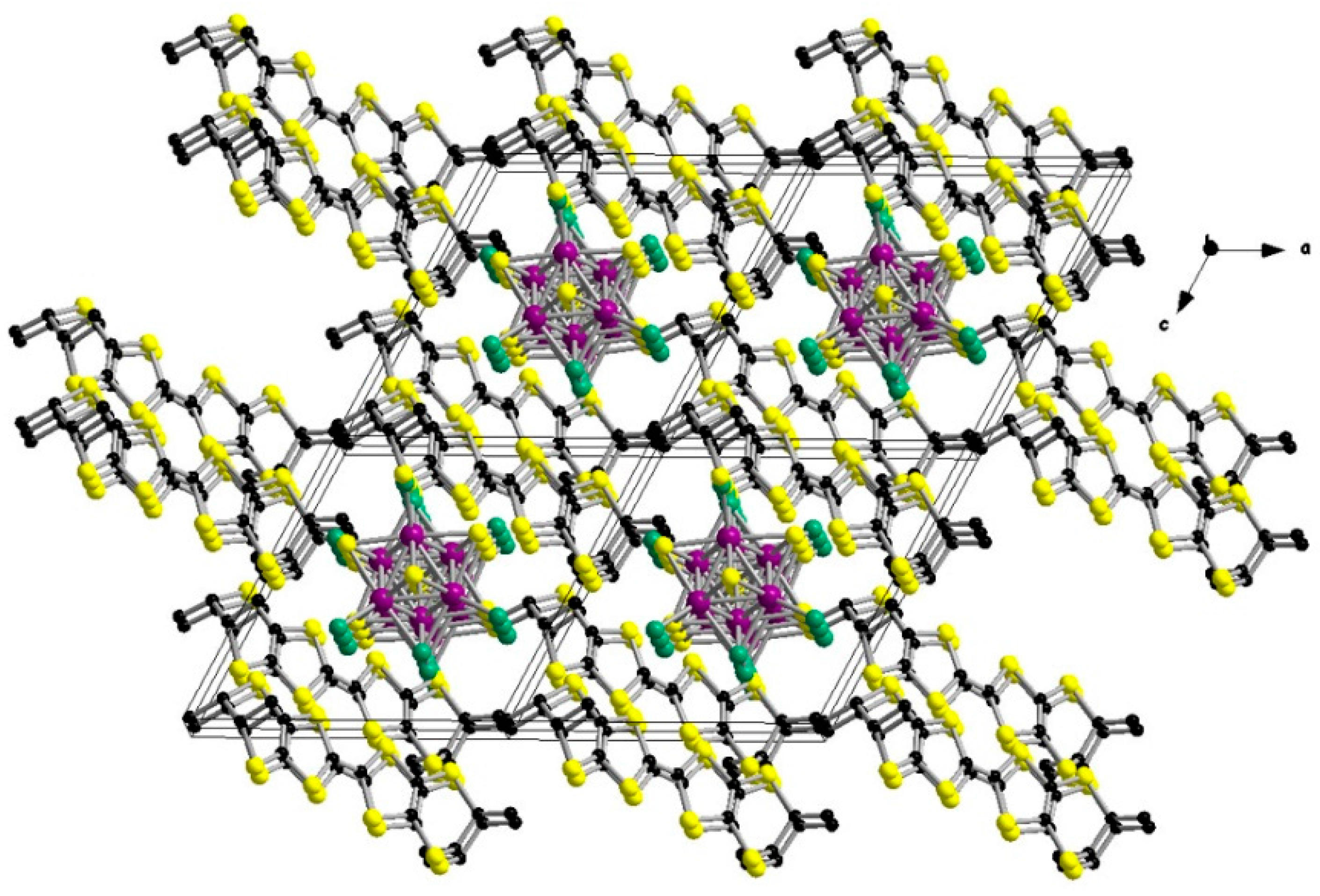
2. Results and Discussion
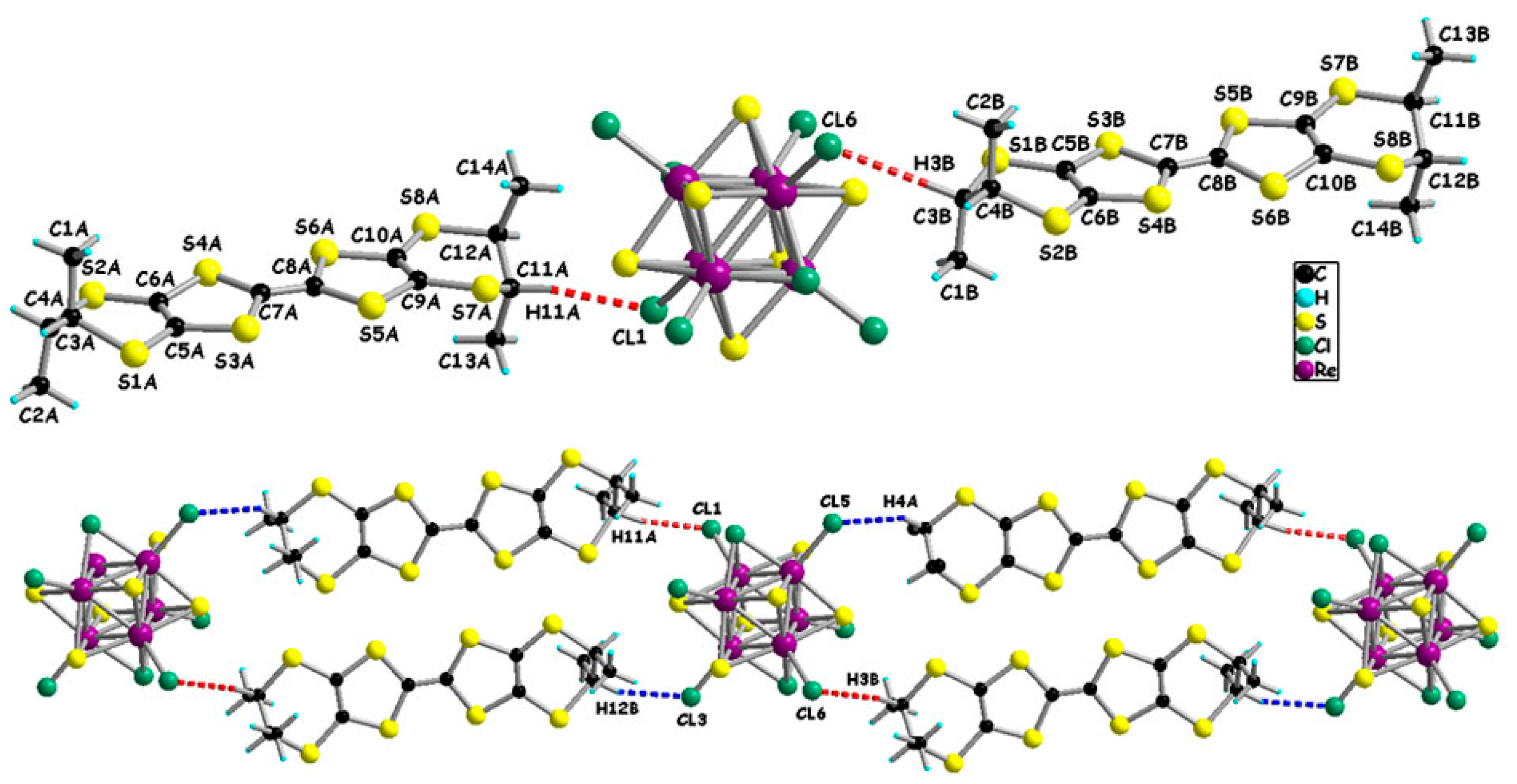
| Compound | Orientation of Methyl Group | Displacements of CH Atoms/Å |
|---|---|---|
| (R)-1-ax [2] | axial | +0.563, −0.331 |
| axial | +0.285, −0.593 | |
| [(S)-1]2·Re6S6Cl8 | axial (A) | +0.045, −0.788 |
| axial (A) | +0.708, −0.118 | |
| axial (B) | +0.109, −0.715 | |
| axial (B) | +0.542, −0.320 |
3. Experimental Section
X-ray structure determination
| Compound | [(S)-1]2·Re6S6Cl8 |
| empirical formula | C28H32Cl8Re6S22 |
| fw | 2474.66 |
| T (K) | 293(2) |
| wavelength (Å) | 0.71073 |
| crystal system | triclinic |
| space group | P1 |
| unit cell dimens | |
| a (Å) | 11.9422(4) |
| b (Å) | 12.2034(5) |
| c (Å) | 12.3025(5) |
| α (deg) | 108.613(4) |
| β (deg) | 110.882(4) |
| γ (deg) | 105.463(3) |
| V (Å3) | 1433.57(10) |
| Z | 1 |
| Dc (g·cm−3) | 2.866 |
| abs coeff (mm−1) | 13.817 |
| θ range for data collection (deg) | 3.5–34.15 |
| reflns collected | 44218 |
| indep reflns | 14,253 |
| completeness (%) | 99.5 |
| data/restraints/param | 22683/4/577 |
| structure Flack parameter | −0.006(8) |
| GOF on F 2 | 1.011 |
| final R indices [I > 2σ(I)] | R1 = 0.039, wR2 = 0.051 |
| R indices (all data) | R1 = 0.093, wR2 = 0.062 |
| largest diff. peak and hole (e·Å−3) | 1.071 and −1.207 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pop, F.; Laroussi, S.; Cauchy, T.; Gómez-García, C.J.; Wallis, J.D.; Avarvari, N. Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene (TM-BEDT-TTF) Revisited: Crystal Structures, Chiroptical Properties, Theoretical Calculations, and a Complete Series of Conducting Radical Cation Salts. Chirality 2013, 25, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Pop, F.; Melan, C.; Brooks, A.C.; Martin, L.; Horton, P.; Auban-Senzier, P.; Rikken, G.L.J.A.; Avarvari, N.; Wallis, J.D. Charge transfer complexes and radical cation salts of chiral methylated organosulfur donors. CrystEngComm 2014, 16, 3906–3916. [Google Scholar] [CrossRef]
- Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y. Effect of Methyl Substitution on Conformation and Molecular Arrangement of BEDT-TTF Derivatives in the Crystalline Environment. Bull. Chem. Soc. Jpn. 1993, 66, 513–522. [Google Scholar] [CrossRef]
- Wallis, J.D.; Karrer, A.; Dunitz, J.D. Chiral metals? A chiral substrate for organic conductors and superconductors. Helv. Chim. Acta 1986, 69, 69–70. [Google Scholar] [CrossRef]
- Karrer, A.; Wallis, J.D.; Dunitz, J.D.; Hilti, B.; Mayer, C.W.; Bürkle, M.; Pfeiffer, J. Structures and Electrical Properties of Some New Organic Conductors Derived from the Donor Molecule TMET (S,S,S,S-Bis(dimethylethylenedithio) tetrathiafulvalene). Helv. Chim. Acta 1987, 70, 942–953. [Google Scholar] [CrossRef]
- Galán-Mascarós, J.R.; Coronado, E.; Goddard, P.A.; Singleton, J.; Coldea, A.I.; Wallis, J.D.; Coles, S.J.; Alberola, A. A Chiral Ferromagnetic Molecular Metal. J. Am. Chem. Soc. 2010, 132, 9271–9273. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Pop, F.; Auban-Senzier, P.; Clérac, R.; Canadell, E.; Mercuri, M.L.; Avarvari, N. Complete Series of Chiral Paramagnetic Molecular Conductors Based on Tetramethyl-bis(ethylenedithio)-tetrathiafulvalene (TM-BEDT-TTF) and Chloranilate-Bridged Heterobimetallic Honeycomb Layers. Inorg. Chem. 2015, 54, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Avarvari, N.; Wallis, J.D. Strategies towards chiral molecular conductors. J. Mater. Chem. 2009, 19, 4061–4076. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Fölling, J.; Wyder, P. Electrical Magnetochiral Anisotropy. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Auban-Senzier, P.; Canadell, E.; Rikken, G.L.J.A.; Avarvari, N. Electrical magneto-chiral anisotropy in a bulk chiral molecular conductor. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Réthoré, C.; Fourmigué, M.; Avarvari, N. Tetrathiafulvalene based phosphino-oxazolines: A new family of redox active chiral ligands. Chem. Commun. 2004, 12, 1384–1385. [Google Scholar] [CrossRef] [PubMed]
- Réthoré, C.; Fourmigué, M.; Avarvari, N. Chiral tetrathiafulvalene-hydroxyamides and -oxazolines: Hydrogen bonding, chirality, and a radical cation salt. Tetrahedron 2005, 61, 10935–10942. [Google Scholar] [CrossRef]
- Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. Chiral Molecular Metals: Syntheses, Structures and Properties of the AsF6− Salts of Racemic (+/−)-, (R)- and (S)-Tetrathiafulvalene-Oxazoline Derivatives. J. Am. Chem. Soc. 2005, 127, 5748–5749. [Google Scholar] [CrossRef] [PubMed]
- Madalan, A.M.; Réthoré, C.; Fourmigué, M.; Canadell, E.; Lopes, E.B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Order versus Disorder in Chiral Tetrathiafulvalene–Oxazolines Radical Cation Salts: Structural, Theoretical Investigations and Physical Properties. Chem. Eur. J. 2010, 16, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Auban-Senzier, P.; Frąckowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J.D.; Canadell, E.; Avarvari, N. Chirality Driven Metallic versus Semiconducting Behavior in a Complete Series of Radical Cation Salts Based on Dimethyl-Ethylenedithio-Tetrathiafulvalene (DM-EDT-TTF). J. Am. Chem. Soc. 2013, 135, 17176–17186. [Google Scholar] [CrossRef] [PubMed]
- Biet, T.; Fihey, A.; Cauchy, T.; Vanthuyne, N.; Roussel, C.; Crassous, J.; Avarvari, N. Ethylenedithio-Tetrathiafulvalene-Helicenes: Electroactive Helical Precursors with Switchable Chiroptical Properties. Chem. Eur. J. 2013, 19, 13160–13167. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, K.; Hasegawa, M.; Sasaki, H.; Endo, J.; Matsuzawa, H.; Sako, K.; Yoshida, J.; Mazaki, Y. Dimeric Tetrathiafulvalene Linked to pseudo-ortho-[2.2]Paracyclophane: Chiral Electrochromic Properties and Use as a Chiral Dopant. Chem. Asian J. 2014, 9, 2751–2754. [Google Scholar] [CrossRef] [PubMed]
- Tatewaki, Y.; Hatanaka, T.; Tsunashima, R.; Nakamura, T.; Kimura, M.; Shirai, H. Conductive Nanoscopic Fibrous Assemblies Containing Helical Tetrathiafulvalene Stacks. Chem. Asian J. 2009, 4, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Danila, I.; Riobé, F.; Piron, F.; Puigmartí-Luis, J.; Wallis, J.D.; Linares, M.; Ågren, H.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchichal chiral expression from the nano to meso-scale in supramolecular helical fibres of a non-amphiphilic C3-symmetrical π-functional molecule. J. Am. Chem. Soc. 2011, 133, 8344–8353. [Google Scholar] [CrossRef] [PubMed]
- Danila, I.; Pop, F.; Escudero, C.; Feldborg, L.N.; Puigmartí-Luis, J.; Riobé, F.; Avarvari, N.; Amabilino, D.B. Twists and turns in the hierarchical self-assembly pathways of a non-amphiphilic chiral supramolecular material. Chem. Commun. 2012, 48, 4552–4554. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Melan, C.; Danila, I.; Linares, M.; Beljonne, D.; Amabilino, D.B.; Avarvari, N. Hierarchical Self-Assembly of Supramolecular Helical Fibres from Amphiphilic C3-Symmetrical Functional Tris(tetrathiafulvalenes). Chem. Eur. J. 2014, 20, 17443–17453. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, S.; Izuoka, A.; Sugawara, T.; Taruishi, T.; Kawada, Y.; Tokumoto, M. Crystal Structure and Conductivity of Chiral Radical Ion Salts (Me2ET)2X. Bull. Chem. Soc. Jpn. 1993, 66, 1949–1954. [Google Scholar] [CrossRef]
- Pop, F.; Allain, M.; Auban-Senzier, P.; Martínez-Lillo, J.; Lloret, F.; Julve, M.; Canadell, E.; Avarvari, N. Enantiopure Conducting Salts of Dimethylbis(ethylenedithio)tetrathiafulvalene (DM-BEDTTTF) with the Hexachlororhenate(IV) Anion. Eur. J. Inorg. Chem. 2014, 3855–3862. [Google Scholar] [CrossRef]
- Pop, F.; Avarvari, N. Regioselective synthesis of chiral dimethyl-bis(ethylenedithio)-tetrathiafulvalene sulfones. Beil. J. Org. Chem. 2015, 11, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Lacour, J.; Avarvari, N. [4+2] Cycloadducts between enantiopure tetramethyl-BEDT-TTF and ortho-chloranil: Conformational issues in the solid state. Rev. Roum. Chim. 2012, 57, 457–462. [Google Scholar]
- Gabriel, J.C.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef] [PubMed]
- Pénicaud, A.; Boubekeur, K.; Batail, P.; Canadell, E.; Auban-Senzier, P.; Jérôme, D. Hydrogen-Bond Tuning of Macroscopic Transport Properties from the Neutral Molecular Component Site along the Series of Metallic Organic-Inorganic Solvates (BEDT-TTF)4Re6Se5C19·[guest], [guest = DMF, THF, dioxane]. J. Am. Chem. Soc. 1993, 115, 4101–4112. [Google Scholar] [CrossRef]
- Deluzet, A.; Rousseau, R.; Guilbaud, C.; Granger, I.; Boubekeur, K.; Batail, P.; Canadell, E.; Auban-Senzier, P.; Jérôme, D. An In-Depth Correlation of the Perturbation of the Organic–Inorganic Interface Topology, Electronic Structure, and Transport Properties within an Extended Series of 21 Metallic Pseudopolymorphs, β′′-(BEDT-TTF)4·(guest)n·[Re6Q6Cl8], (Q=S, Se). Chem. Eur. J. 2002, 8, 3884–3900. [Google Scholar] [CrossRef]
- Perruchas, S.; Boubekeur, K.; Batail, P. Hydrogen Bonds in Radical Cation Salts of TTF(CH2OH)4: First Complete Series with the Octahedral Rhenium Cluster Anions [Re6S8-nCl6+n]n−4 (n = 0, 1, 2, 3). Cryst. Growth Des. 2005, 5, 1585–1596. [Google Scholar] [CrossRef]
- Perruchas, S.; Boubekeur, K.; Canadell, E.; Misaki, Y.; Auban-Senzier, P.; Pasquier, C.; Batail, P. Modulating the framework negative charge density in the system BDT-TTP+/Re6S5Cl91−/Re6(S/Se)6Cl82−/Re6S7Cl73−: Templating by isosteric cluster anions of identical symmetry and shape, variations of incommensurate band filling, and electronic structure in 2D metals. J. Am. Chem. Soc. 2008, 130, 3335–3348. [Google Scholar] [PubMed]
- Sheldrick, G.M. Programs for the Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, F.; Batail, P.; Avarvari, N. Enantiopure Radical Cation Salt Based on Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene and Hexanuclear Rhenium Cluster. Crystals 2016, 6, 8. https://doi.org/10.3390/cryst6010008
Pop F, Batail P, Avarvari N. Enantiopure Radical Cation Salt Based on Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene and Hexanuclear Rhenium Cluster. Crystals. 2016; 6(1):8. https://doi.org/10.3390/cryst6010008
Chicago/Turabian StylePop, Flavia, Patrick Batail, and Narcis Avarvari. 2016. "Enantiopure Radical Cation Salt Based on Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene and Hexanuclear Rhenium Cluster" Crystals 6, no. 1: 8. https://doi.org/10.3390/cryst6010008
APA StylePop, F., Batail, P., & Avarvari, N. (2016). Enantiopure Radical Cation Salt Based on Tetramethyl-Bis(ethylenedithio)-Tetrathiafulvalene and Hexanuclear Rhenium Cluster. Crystals, 6(1), 8. https://doi.org/10.3390/cryst6010008