Enthalpies of Formation of Transition Metal Diborides: A First Principles Study
Abstract
:1. Introduction
| Compound | Pearson Symbol | Space Group | Lattice Parameters | Positions |
|---|---|---|---|---|
| TiB2 AlB2-type | hP3 | P6/mmm N°191 | Exp. [5] a = 3.032 Å c = 3.229 Å | B (2d) 1/3, 2/3, 1/2 Ti (1a) 0, 0, 0 |
| Exp. [6] a = 3.0316 Å c = 3.2301 Å | ||||
| Calc. * a = 3.0337 Å c = 3.2260 Å | ||||
| MoB2 Prot MoB2 or Prot CaSi2 6-lay | hP18 | R(-)3m N°166 | Exp. [7] a = 3.0119 Å c = 20.931 Å | B1 (6c) 0, 0, 0.33306 B2 (6c) 0, 0, 0.18155 Mo (6c) 0, 0, 0.0750 |
| hP18 | Exp. [8] a = 3.0136 Å c = 20.939 Å | B1 (6c) 0, 0, 0.33230 B2 (6c) 0, 0, 0.18184 Mo (6c) 0, 0, 0.07569 | ||
| hP18 | Calc. * a = 3.0201 Å c = 21.0353 Å | B1 (6c) 0, 0, 0.33214 B2 (6c) 0, 0, 0.18147 Mo (6c) 0, 0, 0.07599 | ||
| hR6 | R(-)3m N°166 | Calc. * a = 7.2266 Å α = 24.1286 Å | B1 (2c) 0.33219, 0.33219, 0.33219 B2 (2c) 0.18146, 0.18146, 0.18146 Mo (2c) 0.07595, 0.07595, 0.07595 | |
| Prot WB2 HT | hP12 | P63/mmc N° 194 | Exp. [7] [Frotscher] a = 2.9864 Å c = 13.896 Å | B (2b)0, 0, 1/4 B (2c) 1/3, 2/3, 1/4 B (4f) 1/3, 2/3, 0.0243 Mo (4f) 1/3, 2/3, 0.63759 |
| Exp. [9] a = 2.9872 Å c = 13.8823 Å | B (2b)0, 0, 1/4 B (2c) 1/3, 2/3, 1/4 B (4f) 1/3, 2/3, 0.0243 Mo (4f) 1/3, 2/3, 0.63759 | |||
| Calc. * a = 3.0196 Å c = 14.0937 Å | B (2b)0, 0, 1/4 B (2c) 1/3, 2/3, 1/4 B (4f) 1/3, 2/3, 0.02276 Mo (4f) 1/3, 2/3, 0.63481 | |||
| Prot ReB2 | hP6 | P63/mmc N° 194 | Exp. [10] a = 2.9005 Å c = 7.4772 Å | B (4f) 1/3, 2/3, 0.54783 Re (2c) 1/3, 2/3, 1/4 |
| Exp. [11] a = 2.8982 Å c = 7.4723 Å | B (4f) 1/3, 2/3, 0.547 Re (2c) 1/3, 2/3, 1/4 | |||
| Calc. * a = 2.9178 Å c = 7.5014 Å | B (4f) 1/3, 2/3, 0.5478 Re (2c) 1/3, 2/3, 1/4 | |||
| Prot RuB2 | oP6 | Pmmn N° 59 | Exp. [10] a = 2.8651 Å b = 4.6448 Å c = 4.0456 Å | B (4e) 1/4, 0.0544, 0.1385 Ru (2a) 1/4, 1/4, 0.6505 |
| Exp. [12] a = 2.8657 Å b = 4.6457 Å c = 4.0462 Å | B (4e) 1/4, 0.063, 0.138 Ru (2a) 1/4, 1/4, 0.649 | |||
| Calc. * a = 2.8804 Å b = 4.6637 Å c = 4.0582 Å | B (4e) 1/4, 0.0544, 0.1364 Ru (2a) 1/4, 1/4, 0.6518 | |||
| Prot ThSi2 | tI12 | I41/amd N° 141 | Exp. [13] a = 4.127 Å c = 14.194 Å | Si (8e) 0, 1/4, 0.2915 Th (4a) 0, 3/4, 1/8 |
| Prot CaSi2 3-lay | hR9 | R(-3)m N° 166 | Exp. [13] a = 3.8295 Å c = 15.904 Å | Si (6c) 0, 0, 0.19733 Ca (3a) 0, 0, 0 |
2. Computational Details
3. Results

| Compound | TiB2 | VB2 | CrB2 | MnB2 | FeB2 |
|---|---|---|---|---|---|
| Experimental | hP3 | hP3 | hP3 | hP3 | hP3 |
| Ab-initio | hP3 | hP3 | hP12 | hP6 | oP12 |
| Compound | ZrB2 | NbB2 | MoB2 | TcB2 | RuB2 |
| Experimental | hP3 | hP3 | hR6, hP3(HT) | hP6 | oP6 |
| Ab-initio | hP3 | hP3 | hR6, hP12 | hP6 | oP6 |
| Compound | HfB2 | TaB2 | WB2 | ReB2 | OsB2 |
| Experimental | hP3 | hP3 | hP3, hP12 | hP6 | oP6 |
| Ab-initio | hP3 | hP12 | hP6 | hP6 | oP6, hP6 |
| Compound | Experimental T = 298 K∆fH (kJ/mol of Atoms) | Method | Calculated T = 0 K Present Work (kJ/mol of Atoms) | |
|---|---|---|---|---|
| ScB2 | −102.3 | SSD [43] | hP3: −80.99 | |
| TiB2 | −69.7 | Calo. [44] | hP3: −102.30 | |
| −107.9 | OBC [45] | |||
| −109.5 | Calo. [46] | |||
| −98 | Calo. [47] | |||
| −105 | Equil. [48] | |||
| −109.5 | SSD [43] | |||
| −107.3, −107.8 | EMF [49] | |||
| VB2 | −67.9 | Equil. [50] | hP3: −71.33 | |
| −70.7 | SSD [43] | |||
| CrB2 | −39.8 | SSD [51] | hP3: −31.90 | |
| hP12: −41.13 | ||||
| MnB2 | −21.1 | SC [52] | hP3: −14.06 | |
| hP6: −35.52 | ||||
| YB2 | −35.7 | DSC [53] | hP3: −54.59 | |
| ZrB2 | −97.6 | Vap. Press. [54] | hP3: −95.87 | |
| −107.7 | OBC [55] | |||
| −103.3 | Vap. Press. [56] | |||
| −108.9 | FBC [57] | |||
| −93 | Calo. [47] | |||
| NbB2 | −82.4 | OBC [58] | hP3: −70.71 | |
| −65.9 | Calo. [59] | |||
| −85.3 | FBC [60] | |||
| −73 | Calo. [47] | |||
| −60.3 | DSC [61] | |||
| HfB2 | −106.55 | Vap. Press. [62] | hP3: −98.90 | |
| −109.5 | FBC [57] | |||
| −85 | Calo. [47] | |||
| TaB2 | −62.9 | FBC [60] | hP3: −62.58, | |
| −64.9 | Calo. [47] | hP12: −64.32 | ||
| −53.3 | DSC [53] | |||
| ReB2 | −21.5 | DSC [61] | hP6: −41.50 | |
| OsB2.5 | −11.4 | DSC [63] | oP6: −20.77 | |

4. Discussion
| Atom | Env. | Nb. | Distance (Å) |
|---|---|---|---|
| B | B | 3 | 1.751 |
| Ti | 6 | 2.381 | |
| Ti | B | 12 | 2.381 |
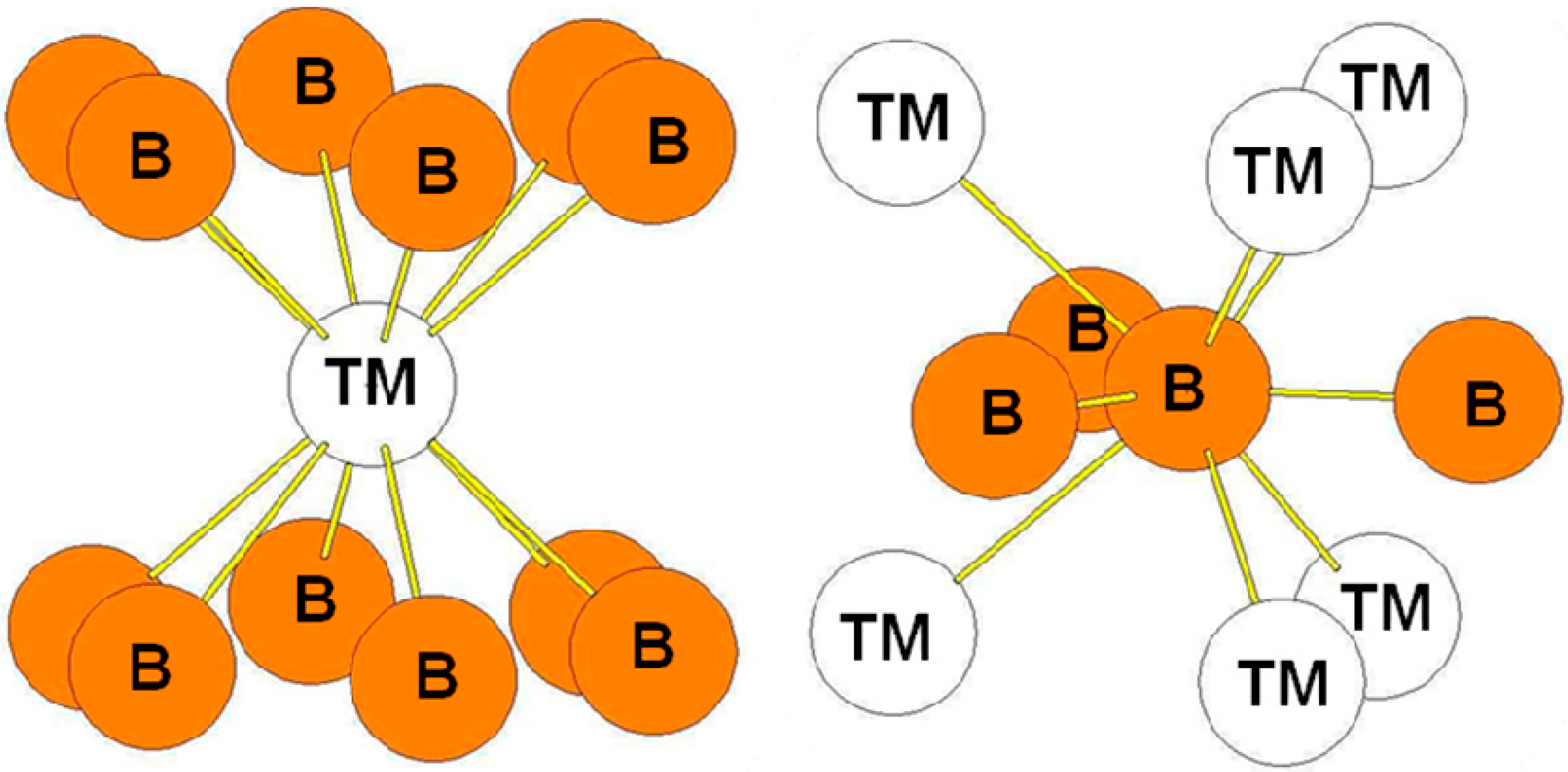
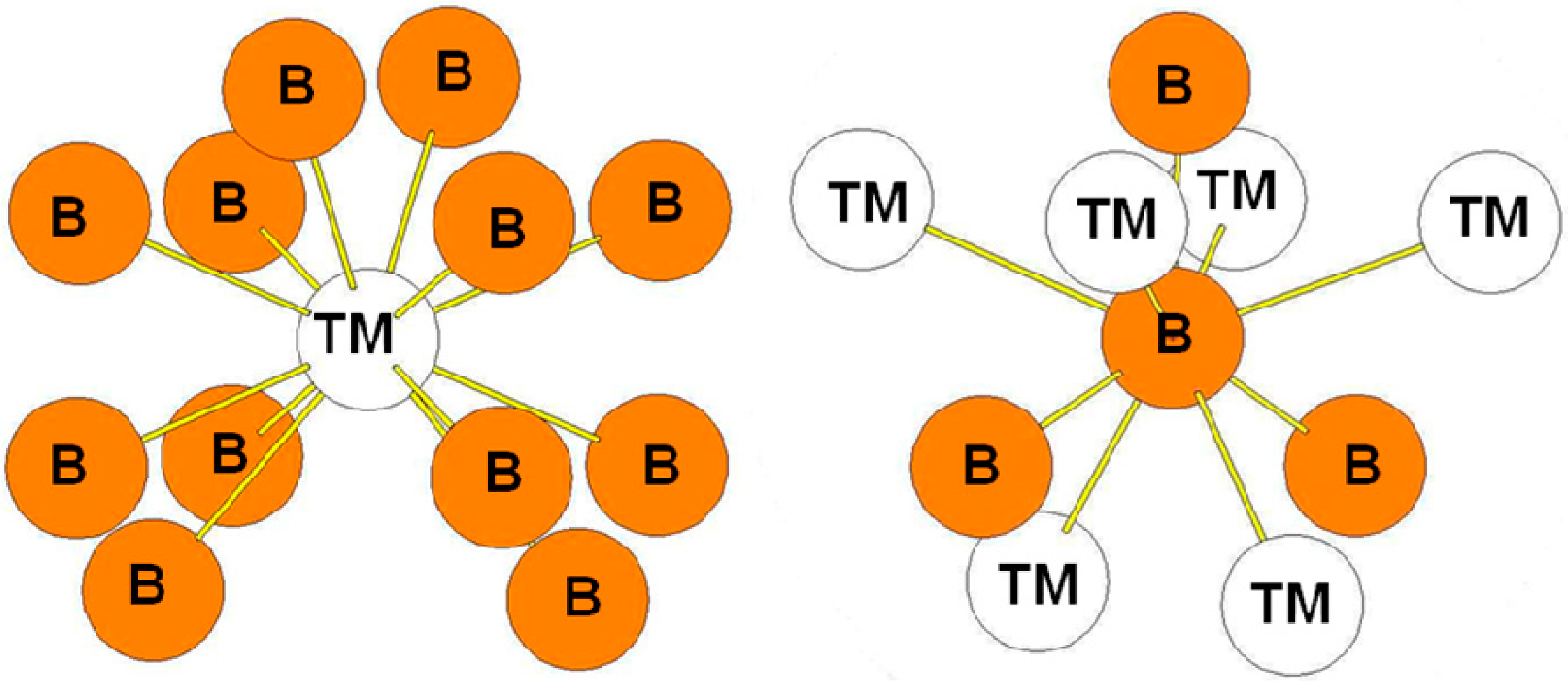
| Atom | Env. | Number | Distance(Å) |
|---|---|---|---|
| B1 | B1 | 3 | 1.745 |
| Mo | 3 | 2.382 | |
| Mo | 3 | 2.349 | |
| B2 | B2 | 3 | 1.852 |
| Mo | 1 | 2.22 | |
| Mo | 3 | 2.365 | |
| Mo | B1 | 3 | 2.382 |
| B1 | 3 | 2.349 | |
| B2 | 1 | 2.22 | |
| B2 | 3 | 2.365 | |
| Mo | 6 | 3.021 |

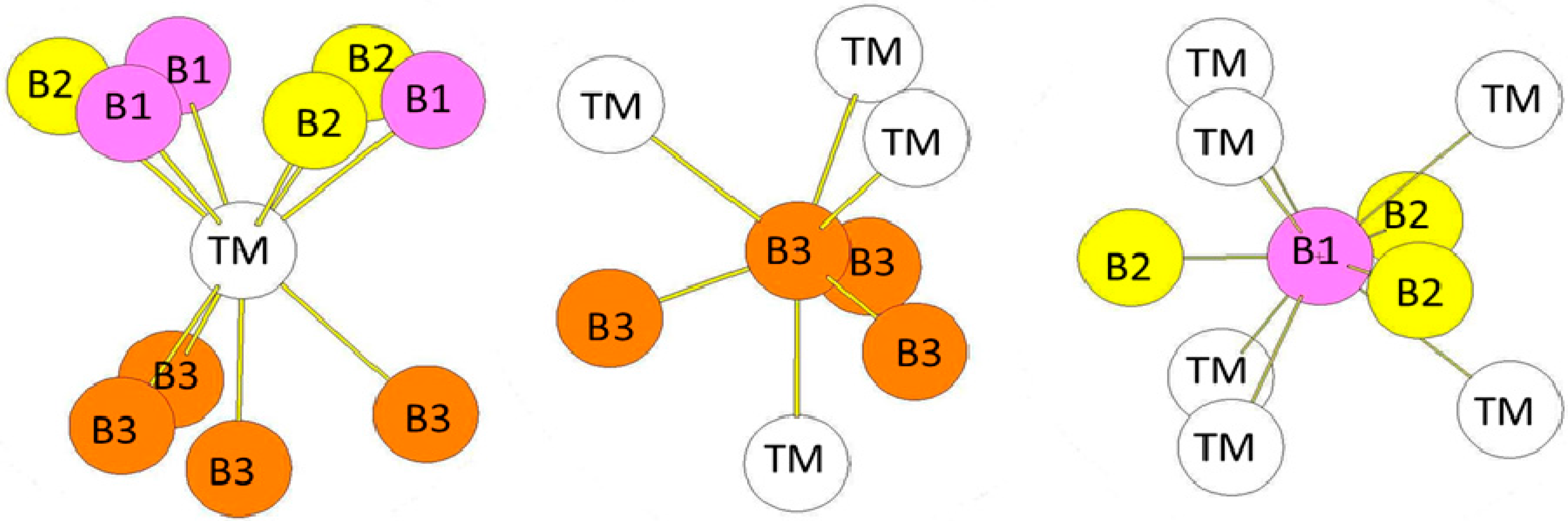
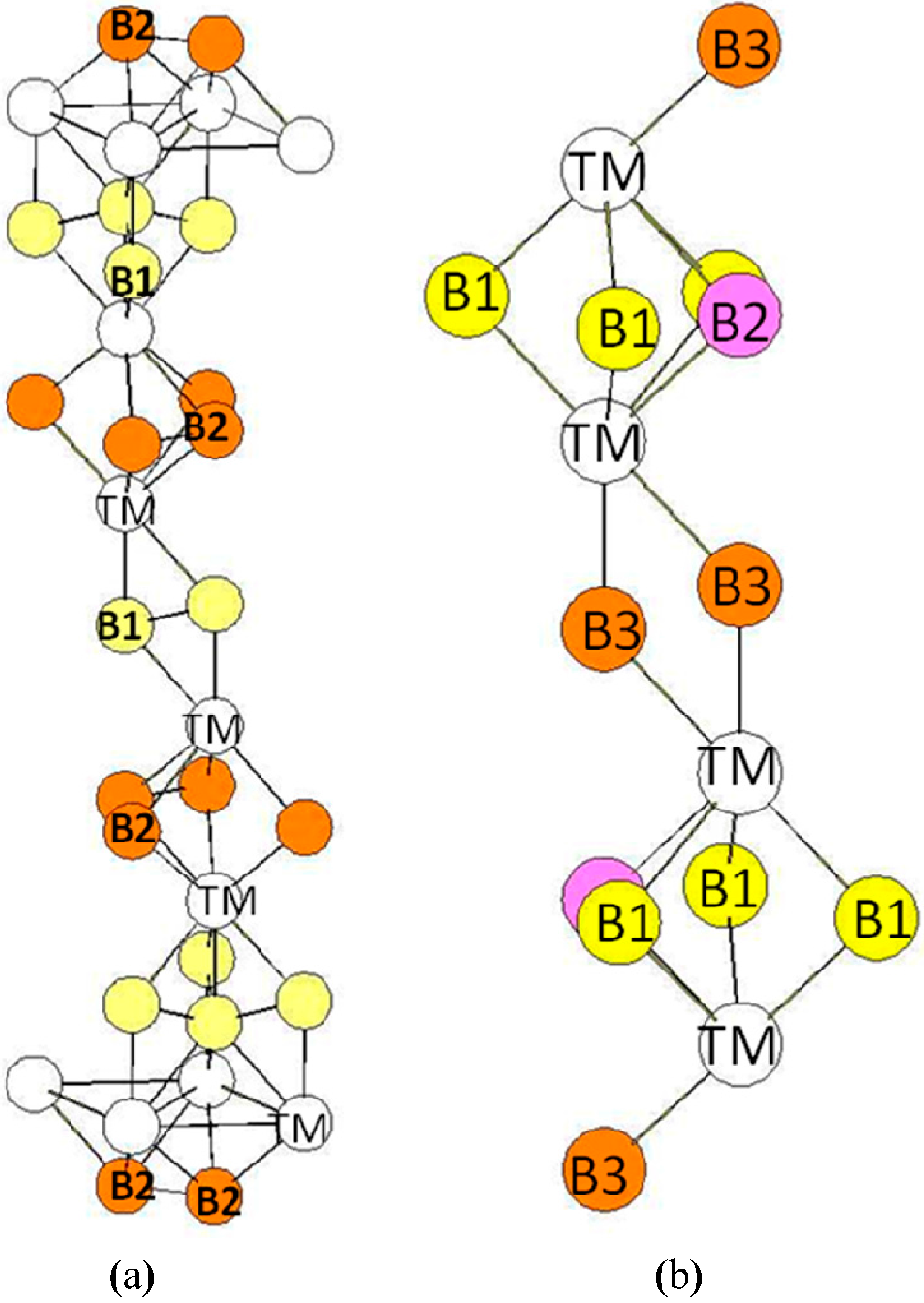
| Atom | Env. | Nb. | Distance(Å) |
|---|---|---|---|
| B (2b) | B (2c) | 3 | 1.743 |
| W (4f) | 6 | 2.382 | |
| B (2c) | B (2b) | 3 | 1.743 |
| W (4f) | 6 | 2.382 | |
| B (4f) | B (4f) | 2 | 1.858 |
| W (4f) | 1 | 2.221 | |
| W (4f) | 3 | 2.352 | |
| W (4f) | B (4f) | 1 | 2.221 |
| B (4f) | 3 | 2.352 | |
| B (2b) | 3 | 2.382 | |
| B (2c) | 3 | 2.382 |
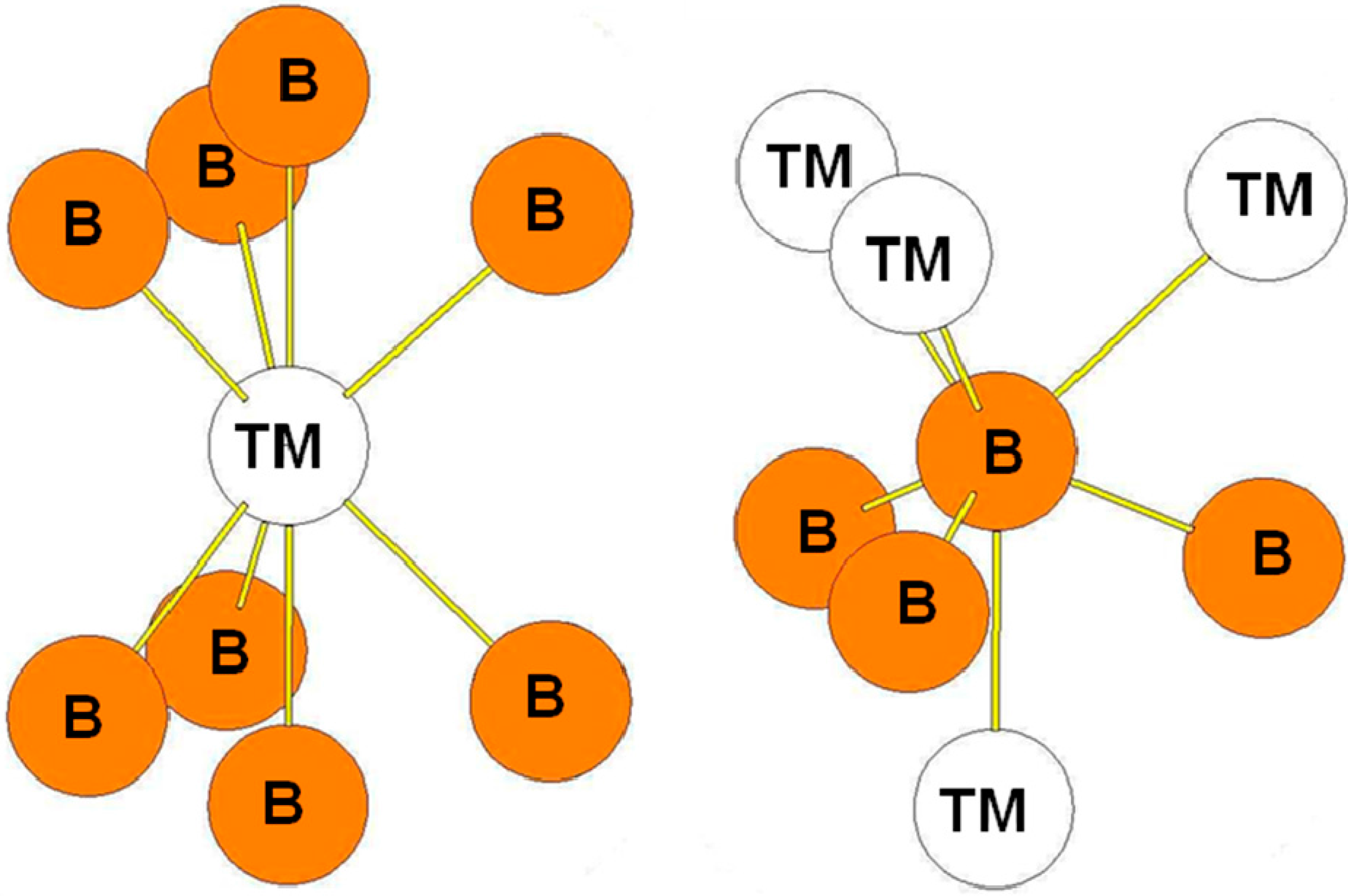
| Atom | Env. | Nb. | Distance(Å) |
|---|---|---|---|
| B | B | 3 | 1.831 |
| Re | 1 | 2.234 | |
| Re | 3 | 2.267 | |
| Re | B | 2 | 2.234 |
| B | 6 | 2.267 |

| Atom | Env. | Nb. | Distance (Å) |
|---|---|---|---|
| B | B | 1 | 1.824 |
| B | 2 | 1.886 | |
| Ru | 1 | 2.168 | |
| Ru | 1 | 2.282 | |
| Ru | 4 | 2.197 | |
| Ru | B | 2 | 2.282 |
| B | 2 | 2.168 | |
| B | 4 | 2.197 |
| Structure | hP3, tI12 | hR6, hP12 | hP6, oP6, hR3 | oP12 |
|---|---|---|---|---|
| CN (TM) | 12 B | 10 B | 8 B | 10 B |
| CN (B) | 6 TM, 3 B | 6 TM, 3B | 4 TM, 3B | 5 TM, 4 B |
| CN(B) | -- | 4 TM, 3B | -- | -- |
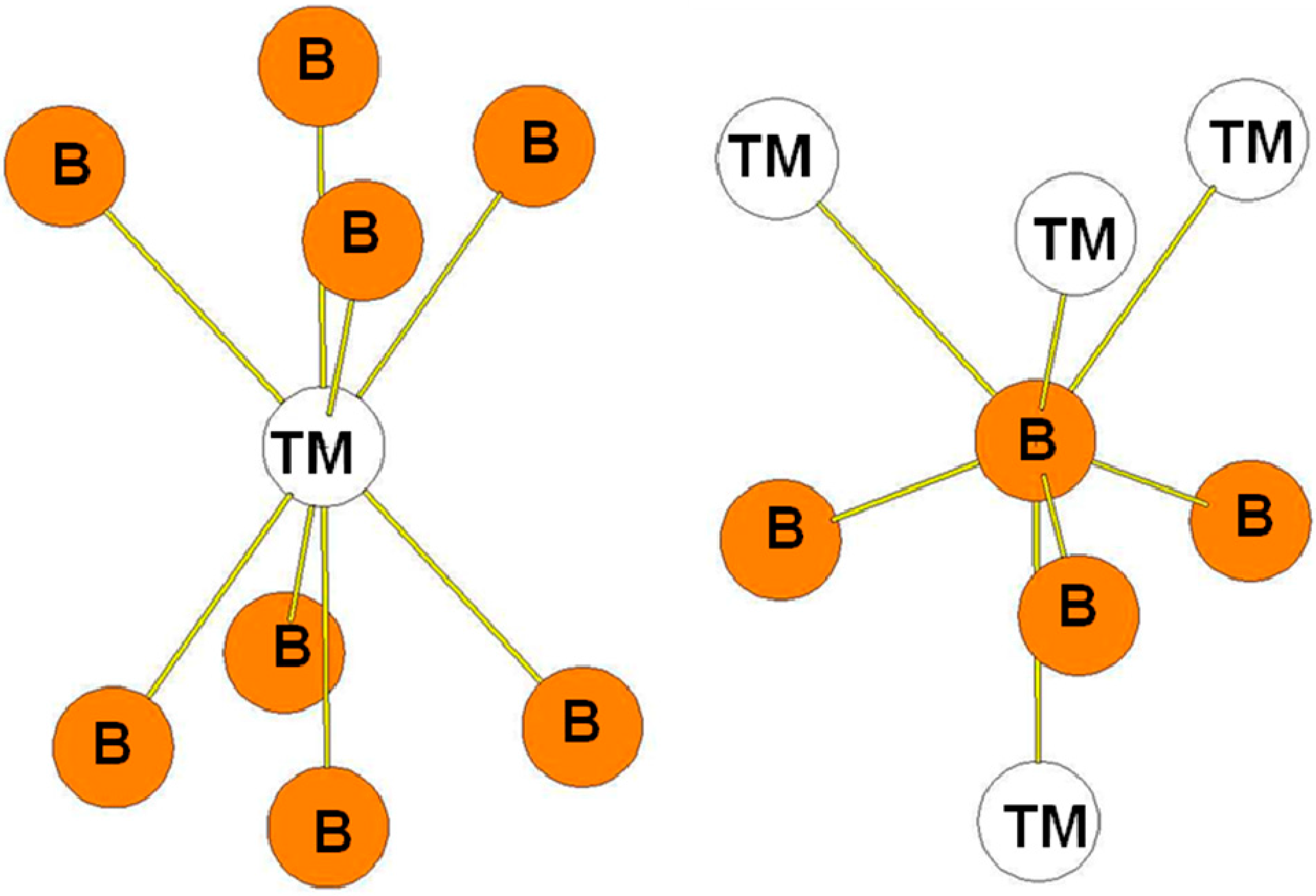
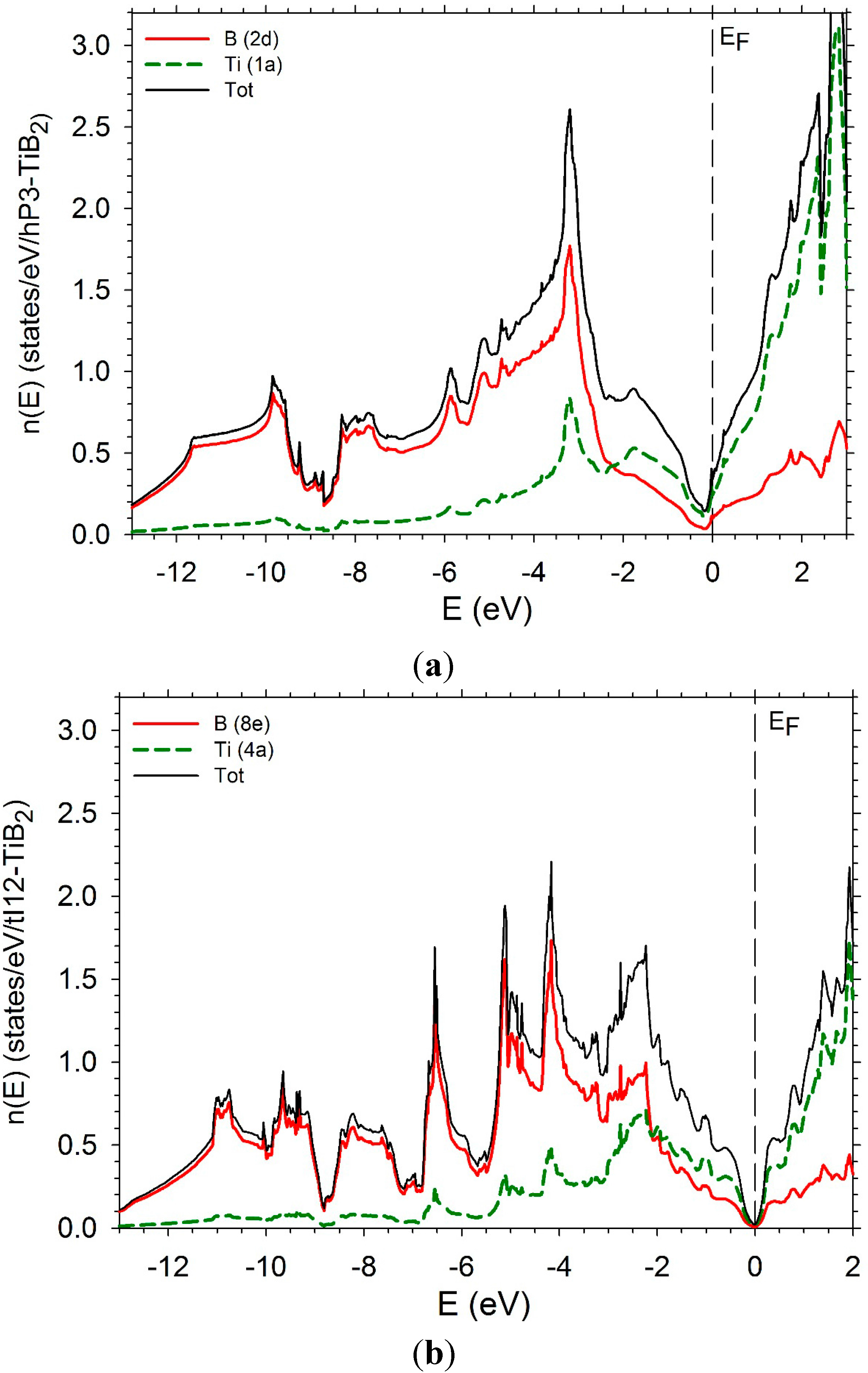

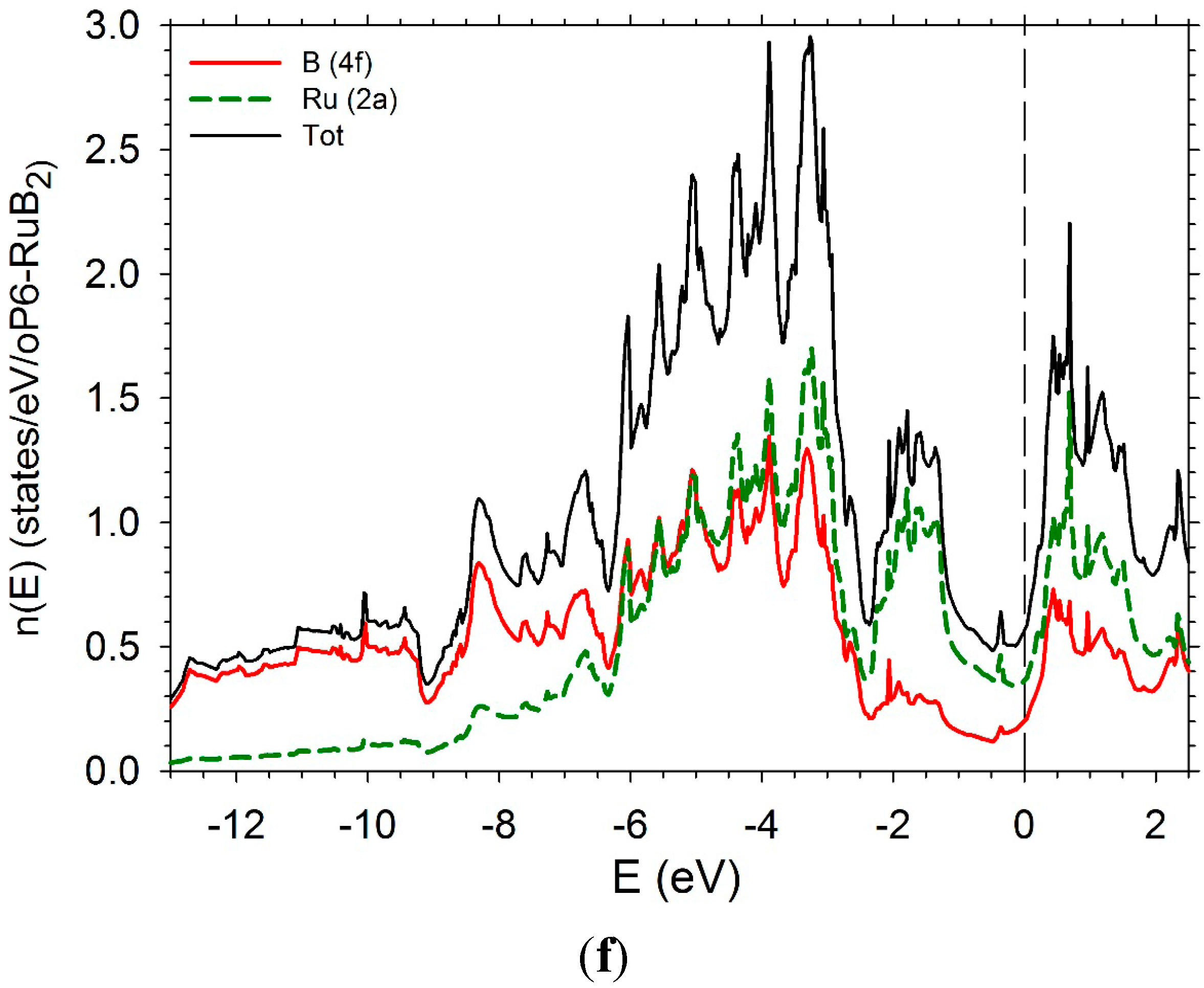
5. Conclusions
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Ivanovskii, A.L. Mechanical and electronic properties of diborides of transition 3D-5D metals from first principles: Toward search of novel ultra-incompressible and superhard materials. Progr. Mater. Sci. 2012, 57, 184–228. [Google Scholar] [CrossRef]
- Ivanovskii, A.L. Hardness of hexagonal AlB2-like diborides of s, p and d metals from semi-empirical estimations. Int. J. Refract. Metals Hard Mater. 2013, 36, 179–182. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wang, H.; Zhang, X.; Litaka, T.; Ma, Y. First-Principles prediction on the high-pressure structures of transition metal diborides (TMB2, TM = Sc, Ti, Y, Zr). Inorg. Chem. 2010, 49, 6859–6864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, H.; Wei, Q.; Wang, H. Pressure-induced phase transition and mechanical properties of molybdenum diboride: First principles calculations. J. Appl. Phys. 2012, 112, 013522. [Google Scholar] [CrossRef]
- Waskowska, A.; Lyaschenko, A.; Gerward, L.; Olsen, J.S.; Babu, K.R.; Vaitheeswaran, G.; Kanchana, V.; Svane, A.; Filipov, V.B.; Levchenko, G. Thermoelastic properties of ScB2, TiB2, YB4 and HoB4: Experimental and theoretical studies. Acta Mater. 2011, 59, 4886–4894. [Google Scholar] [CrossRef]
- Aviles, M.A.; Cordoba, J.M.; Sayagués, M.J.; Gotor, F.J. Mechanochemical synthesis of Ti1-xZrxB2 and Ti1-xHfxB2 solid solutions. Ceram. Int. 2011, 37, 1895–1904. [Google Scholar] [CrossRef]
- Frotscher, M.; Klein, W.; Bauer, J.; Fang, C.-M.; Halet, J.-F.; Senyshyn, A.; Baehtz, C.; Albert, B. M2B5 or M2B4 ? A reinvestigation of the Mo/B and W/B system. Z. Anorg. Allg. Chem. 2007, 633, 2626–2630. [Google Scholar] [CrossRef]
- Kodess, B.N.; Butman, L.A.; Sambueva, S.R. Refinement of Mo2B5 structural type.
- Leithe Jasper, A.; Klesnar, H.P.; Rogl, P. Reinvestigation of isothermal sections in M(M = Mo, W)-Fe-B ternary systems at 1323 K. Nippon Kinzoku Gakkaishi 2000, 64, 154–162. [Google Scholar]
- Frotscher, M.; Hölzel, M.; Albert, B. Crystal structures of the metal diborides ReB2, RuB2, and OsB2. Z. Anorg. Allg. Chem. 2010, 636, 1783–1786. [Google Scholar] [CrossRef]
- Zogal, O.J.; Fojud, Z.; Herzig, P.; Pietraszko, A.; Lyashchenko, A.B.; Jurga, S.; Paderno, V.N. Crystal structure, electric field gradient, and electronic charge densities in ReB2: A single crystal X-ray, 11B nuclear magnetic resonance, and first-principles study. J. Appl. Phys. 2009, 106, 033514. [Google Scholar] [CrossRef]
- Singh, Y.; Niazi, A.; Vannette, M.D.; Prozorov, R.; Johnston, D.C. Superconducting and normal-state properties of the layered boride OsB2. Phys. Rev. B. 2007, 76, 1–15. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data—Crystal Structure Database for Inorganic Compounds, Release 2014/15; ASM International: Materials Park, OH, USA, 2014. [Google Scholar]
- Van Der Geest, A.G.; Kolmogorov, A.N. Stability of 41 metal-boron systems at 0 GPa and 30 GPa from first principles. Calphad 2014, 46, 184–204. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Ravi, C.; Asokamani, R. Electronic structure, bonding, and ground-state properties of AlB2-type transition-metal diborides. Phys. Rev. B 2001, 63, 045115. [Google Scholar] [CrossRef]
- Oguchi, T. Cohesion in AlB2-type diborides: A first-principles study. J. Phys. Soc. Jpn. 2002, 71, 1495–1500. [Google Scholar] [CrossRef]
- Xu, X.; Fu, K.; Yu, M.; Lu, Z.; Zhang, X.; Liu, G.; Tang, C. The thermodynamic, electronic and elastic properties of the early-transition-metal diborides with AlB2-type structure: A density functional theory study. J. Alloys Compd. 2014, 607, 198–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Feng, Z.; Li, Z. General Trends in Electronic Structure, Stability, Chemical Bonding and Mechanical Properties of Ultrahigh Temperature Ceramics TMB2 (TM = transition metal). J. Mater. Sci. Technol. 2015, 31, 285–294. [Google Scholar] [CrossRef]
- Liang, Y.; Zhong, Z.; Zhang, W. A thermodynamic criterion for designing superhard transition-metal borides with ultimate boron content. Comput. Mater. Sci. 2013, 68, 222–228. [Google Scholar] [CrossRef]
- Pallas, A.; Larsson, K. Structure determination of the 4D metal diborides: A quantum mechanical study. J. Phys. Chem. B 2006, 110, 5367–5371. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiang, J.Z. Elastic and electronic properties of low compressible 4D transition metal diborides: First principles calculations. Solid State Commun. 2010, 150, 2093–2096. [Google Scholar] [CrossRef]
- Ying, C.; Zhao, E.; Lin, L.; Hou, Q. Structural determination and physical properties of 4D transitional metal diborides by first-principles calculations. Modern Phys. Lett. B 2014, 28, 1450213. [Google Scholar] [CrossRef]
- Hao, X.; Wu, Z.; Xu, Y.; Zhou, D.; Liu, X.; Meng, J. Trends in elasticity and electronic structure of 5D transition metal diborides: First-principles calculations. J. Phys. Condens. Matter 2007, 19, 196212. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.-J. Mechanical and electronic properties of 5D transition metal diborides MB2 (M = Re, W, Os, Ru). J. Appl. Phys. 2009, 105, 083539. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Fu, C.L.; Krcmar, M.; Painter, G.S. Electronic and structural origin of ultraincompressibility of 5D transition-Metal diborides MB2 (M = W, Re, Os). Phys. Rev. Lett. 2008, 100, 196403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-K.; Zhao, E.-J.; Wu, Z.-J. First-principles calculations of structural thermodynamic and mechanical properties of 5D transitional metal diborides. Wuli Xuebao/Acta Phys. Sinica 2013, 62, 046201. [Google Scholar]
- Zhong, M.-M.; Kuang, X.-Y.; Wang, Z.-H.; Shao, P.; Ding, L.-P.; Huang, X.-F. Phase stability, physical properties, and hardness of transition-metal diborides MB2 (M = Tc, W, Re, and Os): First-principles investigations. J. Phys. Chem. C 2013, 117, 10643–10652. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals an semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1998, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, S.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Methfessel, M.; Paxton, A.T. High precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone Integrations. Phys. Rev. B 1976, 135, 5188–5192. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE Data for Pure Elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Y.; Arroyave, R.; Liu, Z.-K. PhaseStability in α- and β-Rhombohedral Boron. Phys. Rev. B 2007, 75, 092101. [Google Scholar] [CrossRef]
- Van Setten, M.J.; Uijttewaal, M.A.; de Wijs, G.A.; de Groot, R.A. Thermodynamic Stability of Boron. The Role of Defects and Zero Point Motion. J. Am. Chem. Soc. 2007, 129, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Colinet, C.; Tedenac, J.-C. Enthalpies of formation and electronic densities of states of vanadium borides. J. Phase Equilib. Diffus. 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Kolmogorov, A.N.; Shah, S.; Margine, E.R.; Bialon, A.F.; Hammerschmidt, T.; Drautz, R. New superconducting and semiconducting Fe-B compounds predicted with an Ab initio evolutionary search. Phys. Rev. Lett. 2010, 105, 217003. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Wang, W.-L.; Hu, L.; Wei, B.-B. First-principle calculations of structural, elastic, and thermodynamic properties of Fe-B compounds. Intermetallics 2014, 46, 211–221. [Google Scholar] [CrossRef]
- Fan, J.; Bao, K.; Jin, X.; Meng, X.; Duan, D.; Liu, B.; Cui, T. How to get superhard MnB2: A first-principles study. J. Mater. Chem. 2012, 22, 17630–17635. [Google Scholar] [CrossRef]
- Gou, H.; Steinle-Neumann, G.; Bykova, E.; Nakajima, Y.; Miyajima, N.; Li, Y.; Ovsyannikov, S.V.; Dubrovinsky, L.S.; Dubrovinskaia, N. Stability of MnB2 with AlB2-type structure revealed by first-principles calculations and experiments. Appl. Phys. Lett. 2013, 102, 061906. [Google Scholar] [CrossRef]
- Topor, L.; Kleppa, O.J. Enthalpies of Formation of First-Row Transition-Metal Diborides by a New Calorimetric Method. J. Chem. Thermodyn. 1985, 17, 1003–1016. [Google Scholar] [CrossRef]
- Lowell, C.E.; Williams, W.S. High-temperature calorimeter for the determination of heats of formation of refractory compounds. Rev. Sci. Instrum. 1961, 32, 1120–1123. [Google Scholar] [CrossRef]
- Huber, E.J., Jr. The Heat of Formation of Titanium Diboride. J. Chem. Eng. Data 1966, 11, 430–431. [Google Scholar] [CrossRef]
- Akhachinskij, V.V.; Chirin, N.A. Proceedings of Symposium on Thermodynamics of Nuclear Materials; Volume 2. International Atomic Energy Agency (IAEA): Vienna, Austria, 1975; pp. 467–472.
- Kirpichev, E.P.; Rubtsov, Yu.I.; Sorokina, T.V.; Prokudina, V.K. Standard enthalpy of formation of transition metal borides. Russian J. Phys. Chem. 1979, 53, 1128–1130. [Google Scholar]
- Yurick, T.J.; Spear, K.E. Thermodynamics of TiB2 from Ti–B–N studies. In Thermodynamics of Nuclear Materials; International Atomic Energy Agency (IAEA): Vienna, Australia, 1980; pp. 73–79. [Google Scholar]
- Jain, A.; Pankajavallia, R.; Anthonysamy, S.; Ananthasivana, K.; Babua, R.; Ganesana, V.; Gupta, G.S. Determination of the thermodynamic stability of TiB2. J. Alloys Compd. 2010, 491, 747–752. [Google Scholar] [CrossRef]
- Spear, K.E.; Schäfer, H.; Gilles, P.W. Thermodynamics of Vanadium Borides. In High Temperature Technology; Butterworths: London, UK, 1969; pp. 201–212. [Google Scholar]
- Topor, L.; Kleppa, O.J. Molar enthalpy of formation of CrB2 by high-temperature calorimetry. J. Chem. Thermodyn. 1985, 17, 109–116. [Google Scholar] [CrossRef]
- Kleppa, O.J.; Sato, S. New applications of high-temperature solution calorimetry III. Enthalpies of formation of Mn2B, MnB, and MnB2. J. Chem. Thermodyn. 1982, 14, 133–143. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Standard enthalpies of formation of some refractory borides of 4D and 5D elements from high-temperature direct synthesis calorimetry. J. Chem. Phys. 1993, 90, 349–354. [Google Scholar]
- Leitnaker, J.M.; Bowman, M.G.; Gilles, P.W. High-Temperature Evaporation and Thermodynamic Properties of Zirconium Diboride. J. Chem. Phys. 1962, 36, 350–358. [Google Scholar] [CrossRef]
- Huber, E.J., Jr.; Head, E.L.; Holley, C.E., Jr. The Heats of Formation of Zirconium Diboride and Dioxide. J. Phys. Chem. 1964, 68, 3040–3042. [Google Scholar] [CrossRef]
- Trulson, O.C.; Goldstein, H.W. Mass Spectrometric Study of Zirconium Diboride. J. Phys. Chem. 1965, 69, 2531–2536. [Google Scholar] [CrossRef]
- Johnson, G.K.; Greenberg, E.; Margrave, J.L.; Hubbard, W.N. Fluorine bomb calorimetry. Enthalpies of formation of the diborides of ziconium and hafnium. J. Chem. Eng. Data 1967, 12, 137–141. [Google Scholar] [CrossRef]
- Samsonov, G.V. Heats of formation of borides of some transition metals. Zhur. Fiz. Khim. 1956, 30, 2057–2060. [Google Scholar]
- Reznitskii, L.A. Determination of the heat of formation of the NbB1.82 from the NbB2 phase. Russian J. Phys. Chem. 1967, 41, 612–614. [Google Scholar]
- Johnson, G.K.; Greenberg, E.; Margrave, J.L.; Hubbard, W.N. Fluorine bomb calorimetry. Enthalpies of formation of the diborides of niobium and tantalum. J. Chem. Eng. Data 1967, 12, 597–600. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Standard enthalpies of formation of NbB2, MoB, and ReB2 by high-temperature direct synthesis calorimetry. Met. Trans. A 1993, 24, 947–950. [Google Scholar] [CrossRef]
- McClaire, L.A. Thermodynamic and Kinetic Studies for a Refractory Materials Program; Technical Documentary Report No. ASD-TDR-62–204, Part III; Wright Patterson Air Force Base: Dayton, OH, USA, 1964. [Google Scholar]
- Meschel, S.V.; Kleppa, O.J. Enthalpies of formation of refractory borides of 5D elements by high temperature direct synthesis calorimetry I. IrB1.35 and OsB2.5. J. Alloys Compd. 1991, 177, 159–166. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. Thermochemistry of alloys of transition metals and lanthanide metals with some IIIB and IVB elements in the periodic table. J. Alloys Compd. 2001, 321, 183–200. [Google Scholar] [CrossRef]
- Ren, F.; Wang, Y.; Lo, V.C. Pressure induced structural phase transition of OsB2: First-principles calculations. J. Solid State Chem. 2010, 183, 915–919. [Google Scholar] [CrossRef]
- Xie, Z.; Graule, M.; Orlovskaya, N.; Payzant, E.A.; Cullen, D.A.; Blair, R.G. Novel high pressure hexagonal OsB2 by mechanochemistry. J. Solid State Chem. 2014, 215, 16–21. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colinet, C.; Tedenac, J.-C. Enthalpies of Formation of Transition Metal Diborides: A First Principles Study. Crystals 2015, 5, 562-582. https://doi.org/10.3390/cryst5040562
Colinet C, Tedenac J-C. Enthalpies of Formation of Transition Metal Diborides: A First Principles Study. Crystals. 2015; 5(4):562-582. https://doi.org/10.3390/cryst5040562
Chicago/Turabian StyleColinet, Catherine, and Jean-Claude Tedenac. 2015. "Enthalpies of Formation of Transition Metal Diborides: A First Principles Study" Crystals 5, no. 4: 562-582. https://doi.org/10.3390/cryst5040562
APA StyleColinet, C., & Tedenac, J.-C. (2015). Enthalpies of Formation of Transition Metal Diborides: A First Principles Study. Crystals, 5(4), 562-582. https://doi.org/10.3390/cryst5040562





