3.1. Dendrite Growth Theory
Crystal growth in undercooled melts leads to heating up the solid–liquid interface due to the release of the heat of crystallization. As a consequence, a negative temperature gradient will be established in front of the interface since the undercooled melt acts as a heat sink. This will destabilize the initially planar interface. Due to limited solubility of the solute in the solid phase of alloys, compared to the liquid phase, solute will pile up in front of the interface. The resulting concentration gradient will reinforce, in addition to the negative temperature gradient, the instability of the solidification front. Eventually, the morphological destabilization of an initially planar interface will lead to dendrite growth [
14]. Dendrites consist of the main stem and side-branches, which grow into the melt.
An extended model of sharp interface theory is applied to describe the growth dynamics of dendrites as a function of undercooling [
15,
16]. Accordingly, the total undercooling measured in the experiment is expressed as the sum of various individual contributions:
where ∆
Tt is the thermal undercooling; ∆
Tr is the curvature undercooling; ∆
Tn is the undercooling due to the shift of the equilibrium slope of the liquidus
mE to its velocity dependent value
mV; ∆
Tk is the kinetic undercooling; and ∆
Tc is the constitutional undercooling. The thermal undercooling ∆
Tt =
Ti −
T∞ with
Ti the temperature at the tip of the dendrite and
T∞ the temperature of the undercooled melt far from the interface is expressed by:
∆
Thyp is the hypercooling; ∆
Hf is the heat of fusion;
is the specific heat of the liquid;
Iv(
Pet) =
Pet·exp(
Pet)·
E1 is the Ivantsov function for heat diffusion with
Pet = (
VR)/2
a the thermal Peclet number;
V is the velocity of the tip of the dendrite;
R is the radius of curvature at the tip of the dendrite; and
a is the thermal diffusivity;
E1 denotes the first exponential integral function. Due to the strong curvature of the dendrite tip, a reduction of the melting temperature, due to the Gibbs-Thomson effect, has to be taken into account by the curvature undercooling ∆
Tr = TL − Ti with
TL the liquidus temperature and
Ti the temperature at the tip:
where Γ = σ/∆
Sf (σ: interface energy, ∆
Sf the entropy of fusion) is the capillary constant (Gibbs-Thomson parameter), ε
s is the parameter of anisotropy of the interface energy, and θ is the angle between the normal to the interface and the direction of growth along the growth-axis. ∆
Tn takes into account the change of liquidus line, due to deviations from equilibrium at large dendrite growth velocities, and is expressed by:
mE is the slope of liquidus line of the equilibrium phase diagram and
mV is the slope of the liquidus line in the kinetic phase diagram at nominal composition
co.
The kinetic undercooling ∆
Tk is given by:
where µ is the kinetic growth coefficient for growth of the dendrite tip,
is the parameter of anisotropy for the growth kinetics [
17] and is determined by atomistic simulations [
18]. The kinetic undercooling is controlled by the atomic attachment kinetics at the solid–liquid interface that can differ essentially for specific atomic bonding conditions and structural peculiarities. In non-congruently melting alloys, chemical mass transport by segregation has to be considered. The constitutional undercooling in alloys with solidification interval is given by:
with
Pec = (
VR)/2
D the Péclet number of mass diffusion with
D the diffusion coefficient,
Iv(
Pec) =
Pec·exp(
Pec)·
E1 the Ivantsov function for mass diffusion, and
k(
V) is the velocity dependent partition coefficient. Under the conditions of rapid solidification, for the range of growth velocity
V <
VD (where
VD is the atomic diffusive speed in the bulk liquid), the liquidus slope is described by [
19]:
with
kE the partition coefficient of the equilibrium phase diagram. The solute partitioning as a function of growth velocity is described by the non-equilibrium partition coefficient
kV, which becomes dependent on the growth velocity
V for the case of rapid solidification [
20]:
with
VDi the interface diffusion velocity obtained by dividing the diffusion coefficient in the solid–liquid interface by the interatomic spacing. The diffusion coefficient in the interface is smaller compared with the bulk diffusion coefficient [
21]. Equation (1) describes the relation of undercooling in terms of the Péclet numbers,
i.e., as a function of the product
V·R. For unique determination of the growth velocity
V and tip radius
R as a function of undercooling, ∆
T one needs a second equation for the tip radius
R, which comes from stability analysis:
ξ
t and ξ
c are the stability functions depending on the thermal and the chemical Péclet number. They are given by:
and are defined by the stiffness ε = 15ε
c for a crystal with cubic symmetry and with the anisotropy ε
c of the interface energy. The parameters σ
o,
a1, and
a2 are obtained by fitting to experimental data, or from an asymptotic analysis, as described in [
22].
Since we are dealing with solidification of electromagnetically levitated drops, forced convection, induced by the strong alternating electromagnetic fields needed to levitate the drop, has to be taken into account. Accordingly, the thermal undercooling ∆
Tt =
Ti −
T∞ is expressed by [
23]:
where
is the flow thermal Péclet number, with
Uo the velocity of the uniformly forced flow far from the dendrite tip. We estimate the fluid flow velocity from the energy balance between the electromagnetic field, the gravitational field, and the viscous dissipation:
where g is the modulus of vector of the gravity acceleration; ρ is the mass density; η is the dynamic viscosity of the liquid phase; δ is the skin depth;
Ro is the radius of the sample; and
Bo is the time averaged value of the magnetic field inside the levitation coil. Using typical parameters of a metallic system and regarding the boundary conditions of electromagnetic levitation experiments, typical fluid flow velocities in liquid metallic drops are determined, ranging in the order of magnitude of several tenths of centimeters per second. This is in agreement with magneto-hydrodynamic simulations and experimental observations [
24].
In case of forced convection inside the melt, the stability parameter σ* becomes dependent on the fluid flow velocity
Uo. It is given by:
where σ
o is a constant;
Re = UoR/η is the Reynolds number. The function χ(
Re) can be found in [
25]. For computation of the stability parameter σ* we choose the results of phase-field modeling [
26] with
= 1.675 for the 3D upstream fluid flow imposed on the scale of a freely growing dendrite. Thus, from the two main Equations (1) and (9), the velocity
V and the tip radius
R of the dendrite can be calculated as a function of the initial undercooling ∆
T.
3.2. Disorder Trapping in Al50Ni50
Disorder trapping occurs during rapid crystallization of undercooled melts of intermetallics with superlattice structure. In intermetallics, crystal growth is very sluggish at small undercoolings. The atomic attachment of atoms from the liquid to the solid needs short-range atomic diffusion, as atoms have to sort themselves out to find their proper lattice place in the superlattice structure. If undercooling increases the non-equilibrium effect of disorder trapping leads to the solidification of a metastable disordered structure.
Measurements of the dendrite growth velocity of intermetallic phases exhibit a steep rise in the growth velocity
versus undercooling relation at a critical undercooling ∆
T*. This change of the dendrite growth kinetics has been attributed to a transition from ordered to disordered growth of superlattice structures [
27,
28,
29]. However, for Al
50Ni
50 diffraction experiments on the as-solidified samples at ambient temperatures failed to prove a disordered superlattice structure [
29]. This result was explained by transformations of primarily solidified disordered structures to stable ordered phases during the post-recalescence and the post-solidification period. It was shown that metastable disordered phases transform to the ordered state on a rather short time scale [
30]. Transmission electron microscopy on rapidly solidified Al-Ni intermetallic alloys reveal antiphase domains, which indicate the occurrence of disorder trapping during crystallization of drop tube processed melts [
31] and rapid laser surface resolidification of Al-Ni intermetallic phases [
32]. During pulsed laser melting studies on Ni
3Al, a disordered fcc phase has been quenched in although an ordered L1
2 phase is stable up to the melting temperature, providing indirect evidence of disorder trapping during non-equilibrium solidification [
33]. Nevertheless, these studies provide no direct experimental link between the occurrence of disorder trapping and the growth velocity–undercooling relationship.
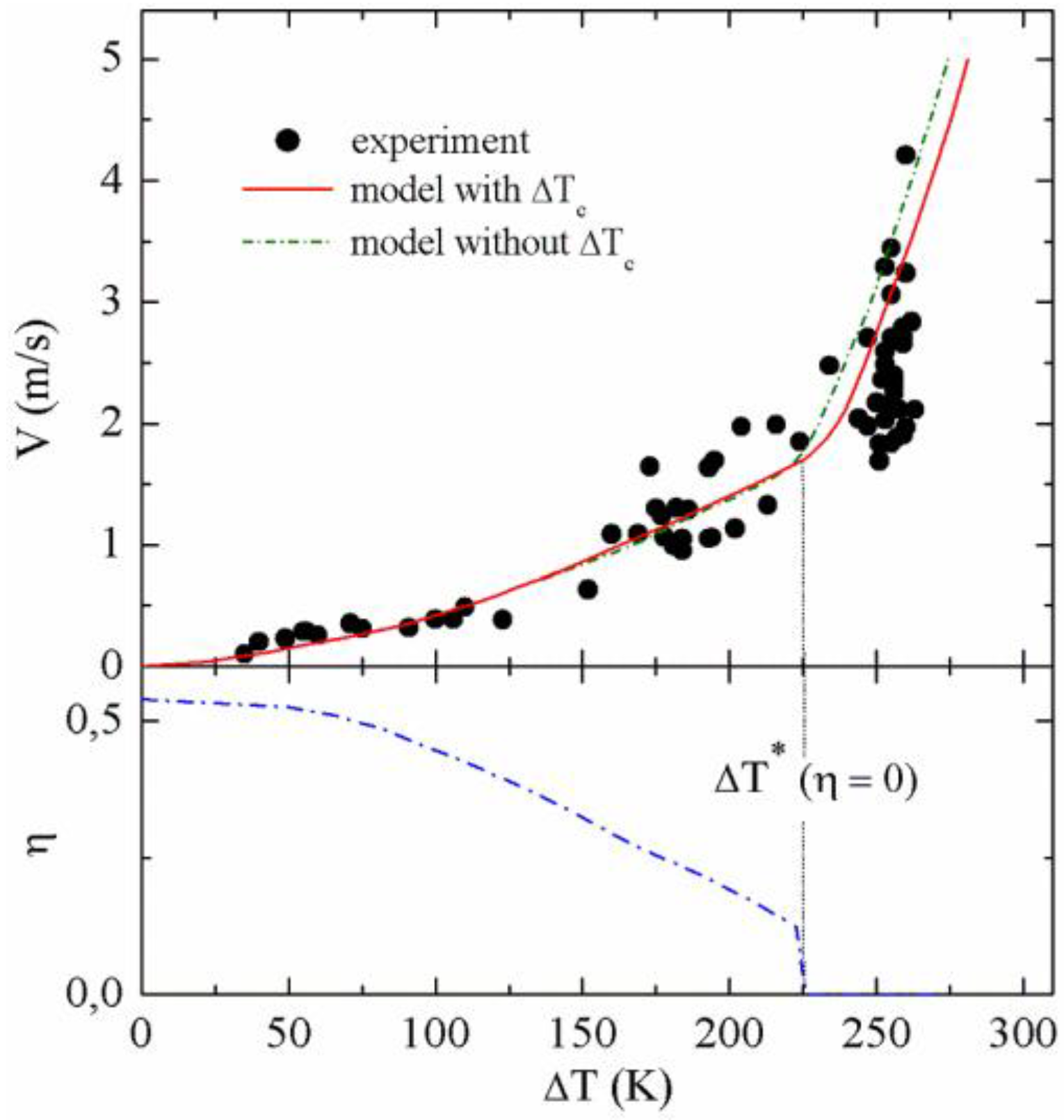
Figure 1 shows the results of measurements of dendrite growth velocity as a function of undercooling for the intermetallic Al
50Ni
50 alloy. The measured growth velocities continuously increase with undercooling. If the undercooling exceeds a value of ∆
T* ≈ 250 K, a steep rise of
V is observed. The intermetallic Al
50Ni
50 alloy melts congruently. Hence, mass transport by mass redistribution and, consequently, constitutional effects can be neglected, therefore, the constitutional undercooling ∆
Tc ≈ 0. Due to the large curvature radius of thermal dendrites, the curvature undercooling can be equally neglected. Therefore, the thermal undercooling and the kinetic undercooling control the dendrite growth kinetics of the intermetallic Al
50Ni
50 compound.
Figure 1.
(Top) Dendrite growth velocity V as a function of undercooling ∆T of Al50Ni50 alloy measured (full circles) and computed with (solid line) and without (dashed-dotted line) constitutional undercooling ∆Tc, assuming effects due to the shift of the congruent melting point in the kinetic phase diagram. If ∆Tc = 0 the temperature characteristics of V(∆T) does not change with the exception that the sharp increase of V sets in at a critical undercooling, being about 25 K smaller (dashed-dotted line); (Bottom) The order parameter η(V) is shown as a function of undercooling as inferred from the analysis of the experimental results.
Figure 1.
(Top) Dendrite growth velocity V as a function of undercooling ∆T of Al50Ni50 alloy measured (full circles) and computed with (solid line) and without (dashed-dotted line) constitutional undercooling ∆Tc, assuming effects due to the shift of the congruent melting point in the kinetic phase diagram. If ∆Tc = 0 the temperature characteristics of V(∆T) does not change with the exception that the sharp increase of V sets in at a critical undercooling, being about 25 K smaller (dashed-dotted line); (Bottom) The order parameter η(V) is shown as a function of undercooling as inferred from the analysis of the experimental results.
The results of the measured dendrite growth velocities are analyzed within the sharp interface model. In addition to the system of equations given by this model, the non-equilibrium effect of disorder trapping has to be introduced in this concept. In order to do so, we combine the sharp interface theory with a model of disorder trapping, as developed by Boettinger and Aziz [
34] that has been extended by Assadi and Greer [
35]. This approach bases on the thermodynamic description in which the Gibbs free energy of the liquid,
GL, is expressed by a regular solution model and that of the solid intermetallic phase,
GS, is expressed as a function of the order parameter, η. η is defined by the difference of the fractions of atoms located in the correct and the wrong places within the superlattice of the ordered B2 structure. The link between non-equilibrium thermodynamics and crystal growth is established by three kinetic equations. One of these equations is the growth equation by Wilson and Frenkel:
with Δ
GLS =
GL −
GS. The solidification of the congruently melting intermetallic phase of Al
50Ni
50 requires no long-range diffusion. Collision limited growth for the atomic attachment kinetics of atoms from the liquid to the solid is assumed so that the kinetic prefactor
V0 is approximated to be the velocity of sound
VS. For sorting of the atoms on the different sublattices, however, diffusion within the solid–liquid interface is required, which is governed by the speed of interface diffusion
VDI and by diffusion in the bulk liquid,
VD, which are two to three orders of magnitude smaller than
VS. The balance of the mass fluxes to the different sublattices of the more or less ordered solid phase during crystal growth defines two other kinetic equations [
29,
36]. Apart from thermodynamic and kinetic parameters, the equation system depends on five variables. These are the temperature of the solid–liquid interface
Ti, the composition of the solid,
cs, and of the liquid phase,
cl, the order parameter η, and the growth velocity
V. For a given
V and at a fixed nominal composition of the liquid,
cl, the other three variables,
cs,
Ti and η can be determined by numerically solving the equation system. Hence, the model provides a description for the velocity dependence of the order parameter η(
V). Moreover, by linking
cl,
cs, and
Ti, it allows for calculating a metastable phase diagram in which the liquidus temperature line depends on the velocity
V, thus,
TL(
V). From this kinetic phase diagram, the kinetic undercooling ∆
TK (difference between local equilibrium liquidus and velocity dependent liquidus temperature),
kV and
mV are directly inferred. More details of the computations are given in [
37].
The results of the computations of dendrite growth velocity as a function of undercooling are given in the upper part of
Figure 1 (solid line). It is evident that the predictions of the extended sharp interface model are in reasonable agreement with the experimental results over the entire range of undercooling accessible by application of the electromagnetic levitation technique.
At large undercoolings, the model reproduces the sharp increase of
V at ∆
T*. Small constitutional effects by the slight shift of the congruent melting point in the kinetic phase diagram are taken into account in the present calculations. If these constitutional effects are neglected, the critical undercooling at which
V steeply rises is slightly shifted to lower undercoolings (
cf. dashed-dotted line in
Figure 1). The variation of the order parameter η with undercooling as predicted by the model of disorder trapping [
27,
37] is shown in the lower part of
Figure 1. It continuously decreases with increasing undercooling and drops suddenly to zero at an undercooling at which disorder trapping sets in as indicated by the sharp increase of dendrite growth velocity in the upper part of
Figure 1. Even for small velocities, the order parameter is considerably smaller than unity because some degree of disorder is favorable at elevated temperatures due to the entropic term in the Gibbs free energy.
These experiments give the direct relation of dendrite growth velocity and disorder trapping at large undercoolings. However, investigations of solid samples solidified at large undercoolings do not reveal a disordered B2 structure. This surprising result finds an explanation by
in situ Energy-Dispersive X-ray Diffraction (EDXD) on levitation undercooled Al
50Ni
50 melts using synchrotron radiation at the European Synchrotron Radiation Facility Grenoble.
Figure 2 shows diffraction spectra recorded during rapid solidification of two Al
50Ni
50 alloy undercooled less than 225 K (left) and undercooled more than 225 K. The inserts display temperature–time profiles during which X-ray diffraction spectra are recorded: in the undercooled liquid state (A), during recalescence (B), and during post-recalescence period (C). The spectra A reflect the characteristic feature of a liquid with a halo at low diffraction angle. The spectra B give the superposition of the spectrum of the liquid with primarily crystallized phase. There is an important difference of the spectra B on the left hand and on the right hand side. While in the spectrum of the sample undercooled less than 225 K a peak appears that is ascribed to the superlattice structure of B2 (β) phase this peak is missing in spectrum B of the sample undercooled more than 225 K. This gives direct evidence that the B2 phase is primarily solidifying in disordered superlattice structure in the sample undercooled more than the critical undercooling ∆
T* = 225 K as inferred from the growth velocity
versus undercooling measurements as depicted in
Figure 1.
Figure 2.
Energy Dispersive X-ray Diffraction (EDXD) spectra recorded on levitation undercooled Al
50Ni
50 alloy using synchrotron radiation at the European Synchrotron Radiation Facility (ESRF) Grenoble. The inserts give the temperature time profiles with the time periods during which an EDXD spectrum is recorded, in the undercooled melt (A), during recalescence (B), and during post-recalescence period (C). The left spectra (
a) are taken on a sample undercooled less than 225 K while the right spectrum (
b) is recorded on a sample undercooled more than 225 K. It is obvious that the spectra B differ. While, in the B spectrum on the left hand side, the diffraction peak of the ordered B2 (β) phase (denoted by β 100 ÜS) is clearly detected it is missing in the B spectrum on the right hand side. This result gives direct evidence that disorder trapping leads to the formation of a primarily solidified disordered superlattice structure provided that the sample is undercooled more than the critical undercooling ∆
T* = 225 K that has been determined by the measurements of the dendrite growth velocity
V as a function of undercooling ∆
T (
cf.
Figure 1).
Figure 2.
Energy Dispersive X-ray Diffraction (EDXD) spectra recorded on levitation undercooled Al
50Ni
50 alloy using synchrotron radiation at the European Synchrotron Radiation Facility (ESRF) Grenoble. The inserts give the temperature time profiles with the time periods during which an EDXD spectrum is recorded, in the undercooled melt (A), during recalescence (B), and during post-recalescence period (C). The left spectra (
a) are taken on a sample undercooled less than 225 K while the right spectrum (
b) is recorded on a sample undercooled more than 225 K. It is obvious that the spectra B differ. While, in the B spectrum on the left hand side, the diffraction peak of the ordered B2 (β) phase (denoted by β 100 ÜS) is clearly detected it is missing in the B spectrum on the right hand side. This result gives direct evidence that disorder trapping leads to the formation of a primarily solidified disordered superlattice structure provided that the sample is undercooled more than the critical undercooling ∆
T* = 225 K that has been determined by the measurements of the dendrite growth velocity
V as a function of undercooling ∆
T (
cf.
Figure 1).
![]()
However, the diffraction peak of the ordered B2 (β) phase reappears in spectrum C on the right hand side of
Figure 2. Obviously, the disordered B2 phase is ordering during post-recalescence period. This can be understood taking into account the small cooling rate and the short diffusion time, respectively. The cooling rate in the present experiments is small in the order of 1–10 K/s. On the other hand, ordering of a disordered superlattice structure needs only short-range diffusion and hence small diffusion time. Accordingly, this explains why the disordered B2 phase cannot be detected in the microstructure analysis of samples undercooled more than 225 K. It is concluded that the analysis of as-solidified microstructures does not tell the entire truth of primarily solidification processes.
3.3. Convection and Dendrite Growth
So far, experiments of dendrite growth velocities have been presented which give evidence for various effects of non-equilibrium solidification at large dendrite growth velocities. At moderate and small growth velocities, there will be an influence of convection in heat and mass transport that controls the dendrite growth kinetics. In electromagnetic levitation experiments, strong stirring of the melt by the induced eddy currents leads to forced convection. The fluid flow velocity
U estimated for such experiments are ranging up to 0.6 m/s. Therefore, one expects an influence of forced convection in the dendrite growth velocity range
V ≤
U [
24,
38].
Al
50Ni
50 was chosen for the investigations on growth kinetics under the conditions of forced convection on Earth and small convection in reduced gravity [
39]. This alloy melts congruently and forms an intermetallic B2 β-phase under equilibrium conditions. Crystallization of ordered superlattice structures requires short-range atomic diffusion at the solid–liquid interface. This leads to sluggish growth dynamics, at least at small and intermediate undercoolings (
V: 0.1–0.5 m/s) [
40]. These growth velocities are directly comparable to the speed of fluid flow in levitated metallic melts. Fluid flow motion inside the liquid drop changes the growth dynamics.
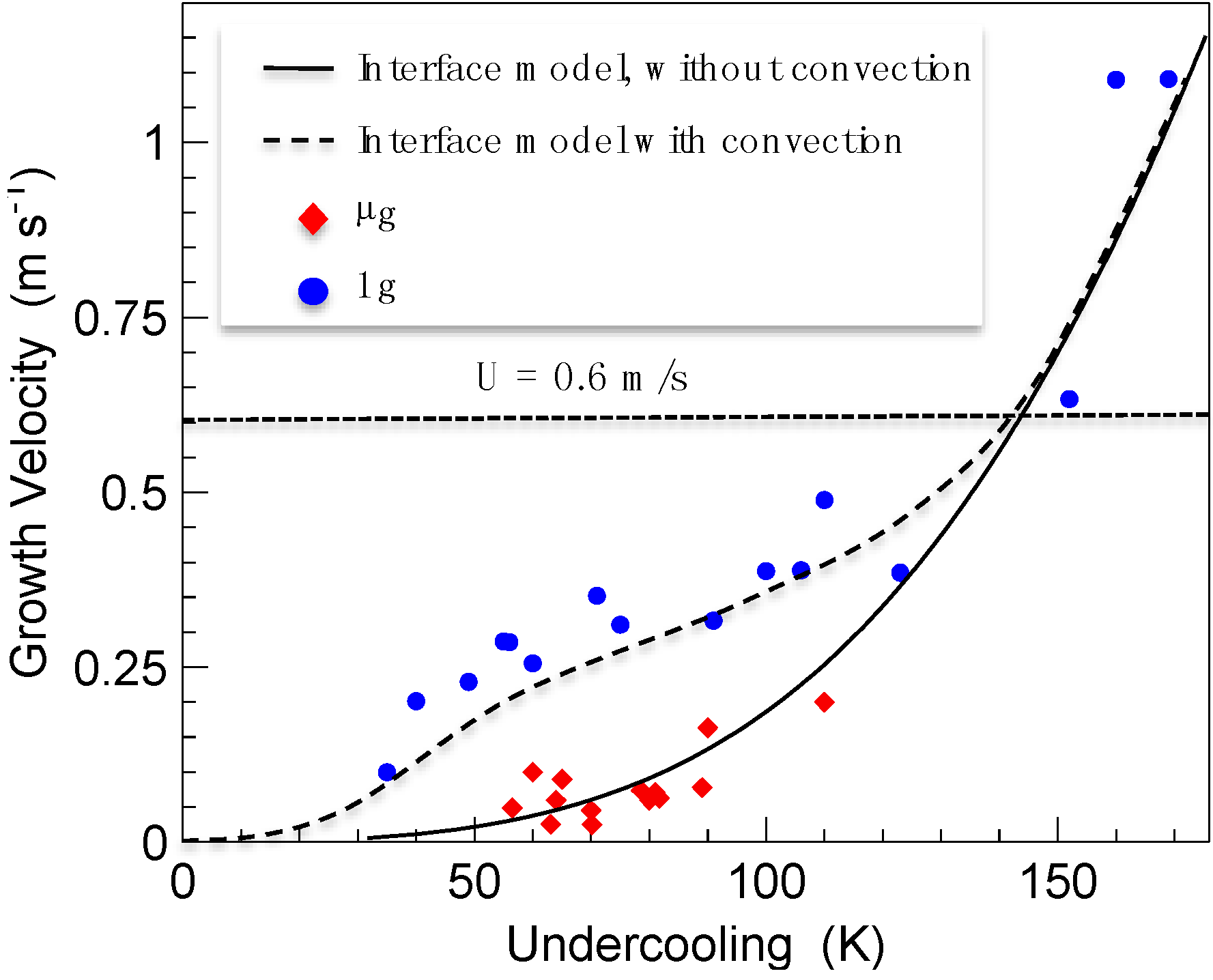
Figure 3.
Dendrite growth velocity of B2 β-phase of Al
50Ni
50 alloy as a function of undercooling measured under terrestrial conditions (circles) and in reduced gravity (diamonds). The solid line represents the prediction of dendrite growth theory without convection and the dashed line with convection.
U denotes the speed of fluid flow inside an electromagnetically levitated droplet as estimated by magneto-hydrodynamic simulations [
24].
Figure 3.
Dendrite growth velocity of B2 β-phase of Al
50Ni
50 alloy as a function of undercooling measured under terrestrial conditions (circles) and in reduced gravity (diamonds). The solid line represents the prediction of dendrite growth theory without convection and the dashed line with convection.
U denotes the speed of fluid flow inside an electromagnetically levitated droplet as estimated by magneto-hydrodynamic simulations [
24].
This effect will be reduced if the liquid drops are processed in a reduced gravity environment since convection is much less pronounced.
Figure 3 shows the results of measurements of dendrite growth velocity as a function of undercooling for Al
50Ni
50 alloy, both under terrestrial conditions (circles) and in reduced gravity (diamonds). All growth velocities measured in reduced gravity are significantly smaller than those determined under terrestrial conditions in the growth velocity range
V < U. At growth velocities exceeding the fluid flow velocity
V >
U ≈ 0.6 m/s, data of dendrite growth velocity from terrestrial and from reduced gravity experiments coincide. The results of sharp interface modeling neglecting the influence of fluid flow are depicted in
Figure 3 (solid line). It describes the experimental results obtained in reduced gravity. The sharp interface model is able to reproduce the experimental results in the regime
V <
U, if a fluid flow velocity of
U ≈ 1.2 m/s, is assumed for the calculations within the frame of the sharp interface theory (
cf. dashed line in
Figure 3). At growth velocities
V > 0.6 m/s, the computed relation of
V =
f(∆
T), without and with convection, converge to one line since, in this region, the dynamics of solidification is mainly limited by thermal diffusivity.
3.4. Microstructure Development in Ni2B
Convection does not only influence the dendrite growth kinetics but also affects microstructure evolution. A particular interesting finding is observed in measurements of the dendrite growth velocity as a function of undercooling of intermetallic Ni2B alloy. This alloy system is characterized by a dimensionless entropy of fusion ∆Sf/R ≈ 2 (∆Sf: entropy of fusion, R: gas constant). According to Jackson’s rule, such a value of the reduced entropy of fusion ranges between ∆Sf/R = 1 and ∆Sf/R = 3. ∆Sf/R = 1 is known for metallic systems of more or less isotropic bonding. These systems show a rough interface on the atomic scale and dendritic microstructures on a mesoscopic scale. On the other hand, ∆Sf/R = 3 is known for systems with strong anisotropic bonding as present in covalent systems. These systems show a smooth interface on an atomic scale and facetted microstructures on a mesoscopic scale. Similar to other intermetallic systems growth kinetics is sluggish. Therefore, one would expect that convection affects both the growth kinetics and microstructure evolution.
We have investigated the solidification of undercooled melt of Ni
2B alloy under different conditions of convection. Different techniques were applied to measure the dendrite growth kinetics as a function of undercooling all of them creating various levels of convection. These are electromagnetic levitation on Earth, forced convection, electromagnetic levitation in reduced gravity, reduced forced convection, melt fluxing technique, natural convection only, melt fluxing in a strong external magnetic field, reduced natural convection, and eventually electrostatic levitation on small samples with almost no convection [
41].
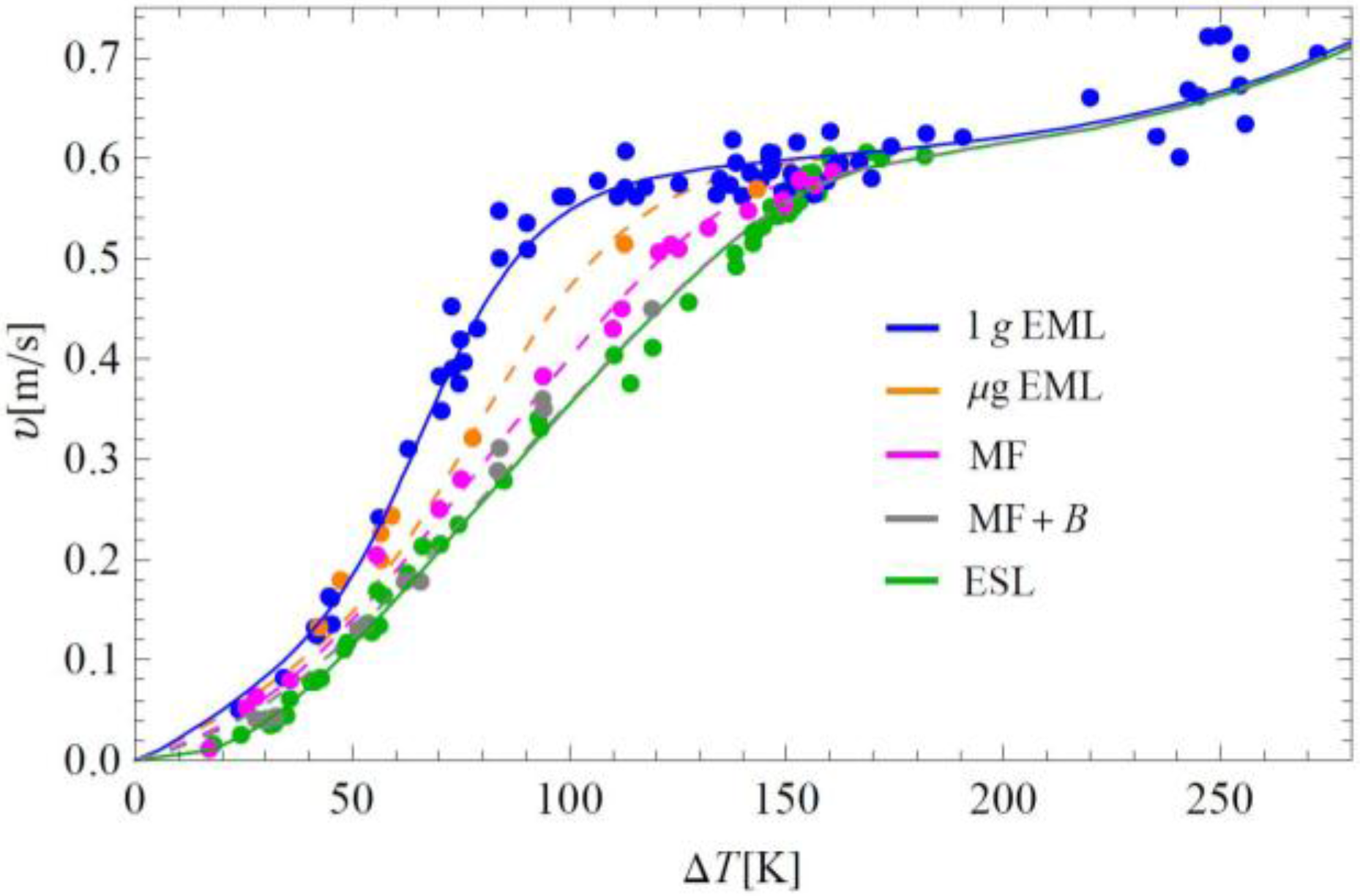
The Ni
2B dendrite growth velocity along the (111) normal directions as a function of undercooling measured under different convective flow conditions is presented in
Figure 4. The error bars result from uncertainties in the fitting procedure, e.g., from samples not being perfectly spherical and/or partially hidden by the levitation coil in EML. The growth velocities measured up to ∆
Tmax = 272 K are well below 1 m/s and are thus comparable with or even less than the expected fluid flow velocities present in 1 g EML. The growth velocity
V(∆
T) is found to increase monotonically. As can be seen in
Figure 4, for undercoolings 40 K < ∆
T < 150 K, the growth velocity increases with the fluid flow velocity. The lowest growth velocities are obtained by ESL, followed by the melt fluxing (MF) and µg EML, whereas the highest values are obtained by 1 g EML. Indeed, as soon as convection may play a not negligible role in solidification kinetics, we observed the increase of dendrite growth velocity. This is in close agreement with the predicted order of the flow velocities in the various experiment techniques. In the presence of an external static magnetic field of 1.2 Tesla, the growth velocities obtained by MF are slightly shifted to lower values, which, within the limit of accuracy, overlap with the ESL values. Deviations may be due to the influence of residual flow that is not completely stabilized by the magnetic field. Interestingly, the results obtained by µg EML are quite close to the velocities measured under the condition of natural convention in MF.
Figure 4.
Dendrite growth velocities as a function of undercooling of Ni2B alloy for various fluid flow velocities: ESL: u = 0.00 m/s, MF + B: u = 0.01 m/s, MF: u = 0.05 m/s, µg EML: u = 0.18 m/s, and 1 g EML: u = 0.25 m/s, respectively.
Figure 4.
Dendrite growth velocities as a function of undercooling of Ni2B alloy for various fluid flow velocities: ESL: u = 0.00 m/s, MF + B: u = 0.01 m/s, MF: u = 0.05 m/s, µg EML: u = 0.18 m/s, and 1 g EML: u = 0.25 m/s, respectively.
For ∆T < 40 K as well as ∆T ≥ 150 K, the data coincide within the uncertainty of the measurements. This is physically reasonable since, on the one hand, the growth velocity must vanish for ∆T = 0 K and, on the other hand, the influence of convection is likely to become less pronounced in the high-velocity region. The difference in the growth velocities in the medium undercooling range is due to an apparent change in the slope of the 1 g EML V(∆T) data measured under the condition of forced convection. This results in a significant gap of roughly 60% at ∆T ≈ 100 K between the ESL and 1 g EML data of growth velocities, which may be attributed to an enhanced heat and mass transfer due to electromagnetically induced convection.
For the further analysis, we apply the sharp interface model taking into account heat transport by convection similar to the case of the Al
50Ni
50 alloy. However, surprisingly, this does not lead to a reproduction of the experimental results. In addition, taking into account small shifts in the concentration from the stoichiometric composition of Ni
2B alloy, which may occur during the processing the samples at high temperatures due to evaporation is not satisfactory [
42].
Growth in Ni
2B is predominantly governed by the kinetic contribution to the total undercooling. The kinetic undercooling is controlled by atomic attachment kinetics at the solid–liquid interface. It depends on the interface morphology. In general, it can be categorized either as atomically smooth (faceted) or atomically diffuse (rough). In the first case, the solid–liquid interface is thin, in the order of one atomic layer, while, in the second case, the interface is rather diffuse over several atomic layers. According to Jackson, the atomic arrangement at the interface depends mainly on the entropy of fusion ∆
Sf [
43]. If the dimensionless entropy ∆
Sf/R
G < 2 (R
G: gas constant), a rough interface will be favored, while, for ∆
Sf/R
G > 2, a smooth interface will be preferentially formed [
44]. Pure metals are often characterized by ∆
Sf ≈ R
G and are predicted to have a rough interface. However, many intermetallic compounds show high entropy of fusion due to strong chemical bonding and, consequently, a smooth faceted interface will be formed. Faceted interfaces have inherently a low accommodation factor
f < 1 in contrast to
f = 1 for metals. This means not each atomic jump from the liquid to the solid will be successful. In such a case, the interface undercooling as given in Equation (5) can be written as [
45]
where the kinetic exponent
n is determined from experiments. The 1 g EML results can be reproduced much more accurately by setting
n < 1. For pure faceted spiral growth,
n = 0.5 [
46]. It is found that the increase in growth velocity, as observed in 1 g EML, is only partly due to the influence of electromagnetically driven flow on the thermal and solute concentration fields in front of the solid–liquid interface but can be mainly attributed to the substantial change in growth kinetics caused by a convection induced transition from dendrites to more faceted solidification structures. The parameters used for fitting the experimental results are collected in
Table 1.
Table 1.
Best fit parameters used to calculate the Ni
2B growth velocities as shown in
Figure 4. The kinetic growth coefficient µ is obtained for
f·vs = 4.25 m/s.
Table 1.
Best fit parameters used to calculate the Ni2B growth velocities as shown in Figure 4. The kinetic growth coefficient µ is obtained for f·vs = 4.25 m/s.
| Experiment | Stability Parameter * | Fluid Flow Velocity u (m/s) | Kinetic Exponent n |
|---|
| 1 g EML | 5.0 × 10−5 | 0.25 | 0.85 |
| µg EML | 7.0 × 10−5 | 0.18 | 0.93 |
| MF | 9.0 × 10−5 | 0.05 | 0.98 |
| MF + B | 1.0 × 10−4 | 0.01 | 1.00 |
| ESL | 1.0 × 10−4 | 0.00 | 1.00 |
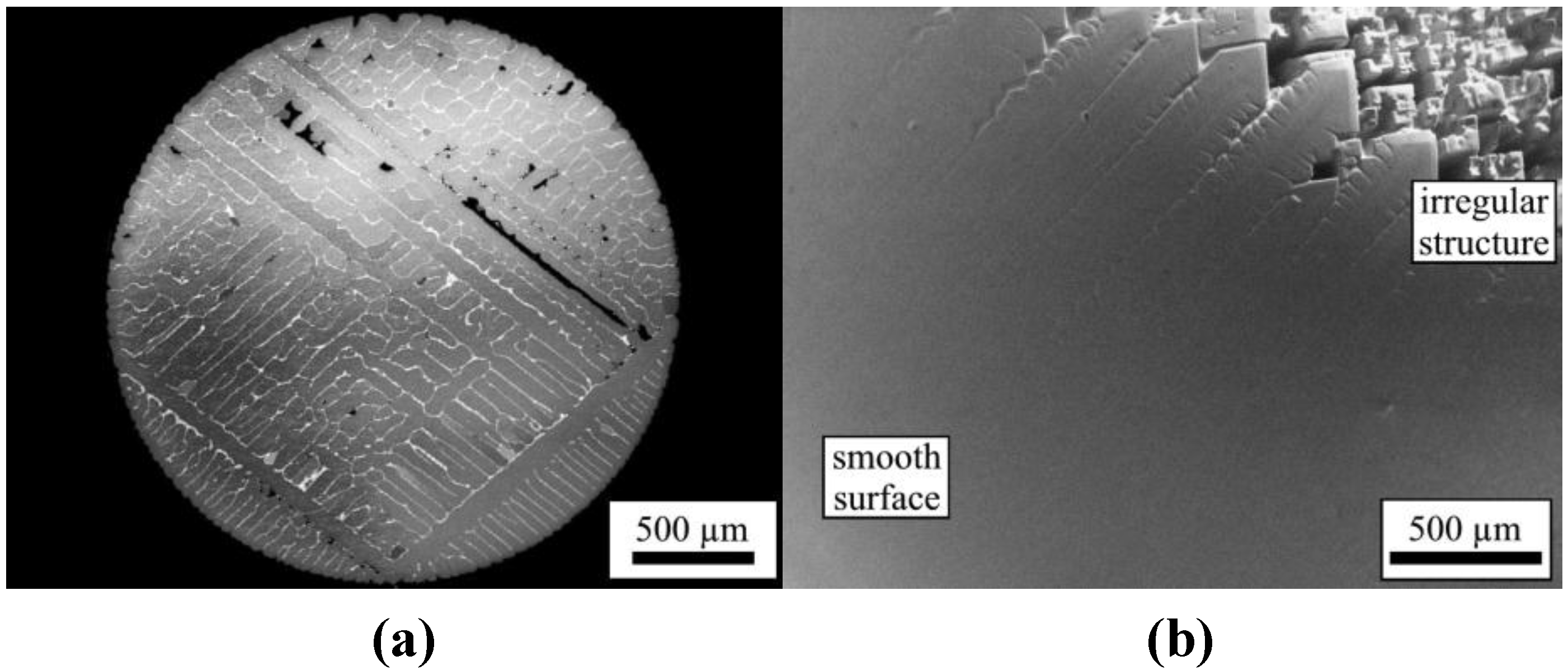
This change is supported by investigating the microstructures of samples solidified upon undercooling in ESL and EML.
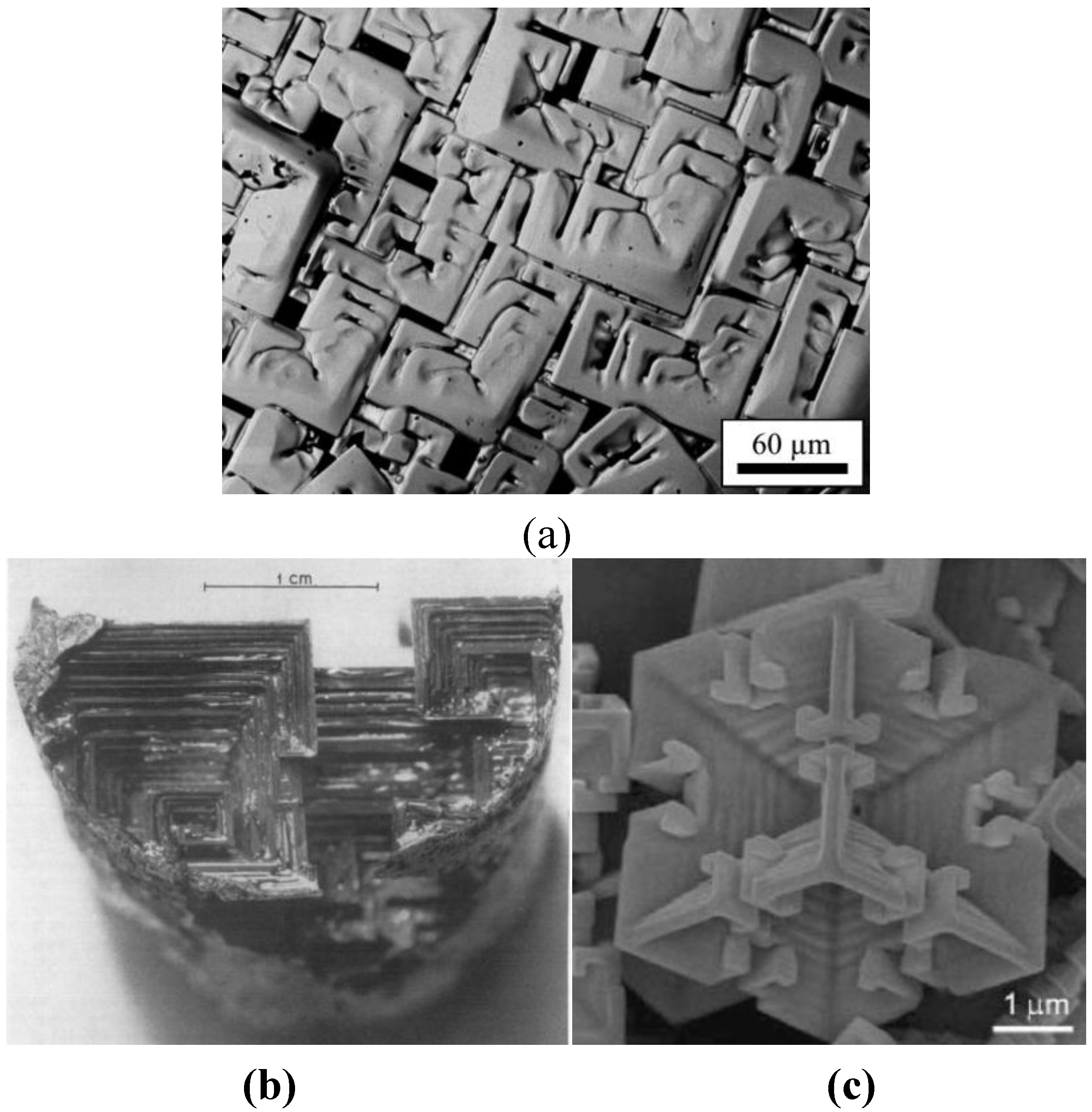
Figure 5a displays the microstructure of a sample solidified under the condition of no convection (ESL), while
Figure 5b gives the structure of a sample solidified under the conditions of forced convection (EML) [
47]. Samples processed in ESL exhibit the regular dendritic pattern. In contrast, the smooth structure of samples processed in EML shows a transition to irregular rod-shaped structures. Apparently, the internal structure resembles the well-known morphology of so-called Hopper crystals, which are rarely found for metallic materials but are often observed in non-metallic systems. The structure found in Ni
2B processed in EML under the conditions of forced convection is compared to Bi and PbTe hopper crystals in
Figure 6.
A hopper crystal is usually formed due to a disparity of growth rates,
i.e., the crystal edges are growing more rapidly than the crystal faces. This is a typical example of faceted growth on a more local level [
48]. Hopper crystals have been reported for non-metallic forsterite (Mg
2SiO
4) [
49] and PbS single crystals [
50]. Faceted growth is expected in systems of high entropy of fusion ∆
Sf, as e.g., in Bi, ∆
Sf = 2.4R
G. This is larger than ∆
Sf = 2R
G as in the present case of Ni
2B. Obviously, forced convection induces faceting of the solid–liquid interface in systems, which show otherwise growth of a rough solid–liquid interface of metallic systems.
Figure 5.
(a) Structure of a sample solidified without convection (ESL); (b) Structure of a sample solidified with forced convection (EML).
Figure 5.
(a) Structure of a sample solidified without convection (ESL); (b) Structure of a sample solidified with forced convection (EML).
Figure 6.
(
a) The structure of the Ni
2B–rod like morphology solidified under the conditions of forced convection (EML); (
b) hopper crystals found in Bi [
48], and (
c) PbTe [
42].
Figure 6.
(
a) The structure of the Ni
2B–rod like morphology solidified under the conditions of forced convection (EML); (
b) hopper crystals found in Bi [
48], and (
c) PbTe [
42].
3.5. Dendrite Growth of Cu50Zr50
So far, the majority of the measured velocity–undercooling (
V – ∆
T) relations in metallic systems show a monotonous increase of
V with ∆
T. In this case, the energetics controls the growth [
51]. In glass-forming systems, however, the mobility of the atomic movement rapidly decreases if ∆
T is approaching ∆
Tg =
Tl −
Tg with
Tl the liquidus and
Tg the glass transition temperature. In the temperature range at large undercoolings where the temperature of the undercooled liquid approaches the glass transition temperature
Tg, the steeply decreasing diffusion coefficient eventually overcomes the acceleration of the interface by the thermodynamic driving force for crystallization. The latter one is given by the Gibbs free energy difference ∆
G = Gl −
Gs with
Gl and
Gs the Gibbs free energy of liquid and solid, respectively. This leads to a maximum in the
V − ∆
T relation. This was experimentally observed in a great variety of non-metallic glass-forming systems, such as
o-terphenyl [
52], tri-
α-naphthylbenzene [
53], Li
2O-2SiO
2 [
54], and MgO-CaO-2SiO
2 [
55]. However, so far, there is only one work that reports a maximum in the
V-∆
T relation measured for the Cu
50Zr
50 glass-forming alloy [
56].
The results of the measurements of
V as a function of ∆
T are shown in
Figure 7. The squares give the experimental data. Taking the values of the melting temperature and the glass temperature of Cu
50Zr
50, the difference between
Tl = 1209 K and
Tg = 670 K, is determined as ∆
Tg = 539 K. This corresponds to a relative glass temperature
Tg/Tl = 0.56 [
57]. Such a high value is indicative for an excellent glass forming ability [
58]. A maximum in the
V – ∆
T relation is experimentally observed. It indicates that at undercoolings less than the undercooling of the maximum growth velocity, dendrite growth is controlled by the thermal transport, while at undercoolings larger than the undercooling of the maximum growth velocity, dendrite growth is governed by atomic diffusion. The maximum undercooling achieved in the experiment is approaching the temperature range above the glass temperature where the rapidly decreasing diffusion coefficient progressively influences the atomic attachment kinetics and thus the mobility of the solidification front.
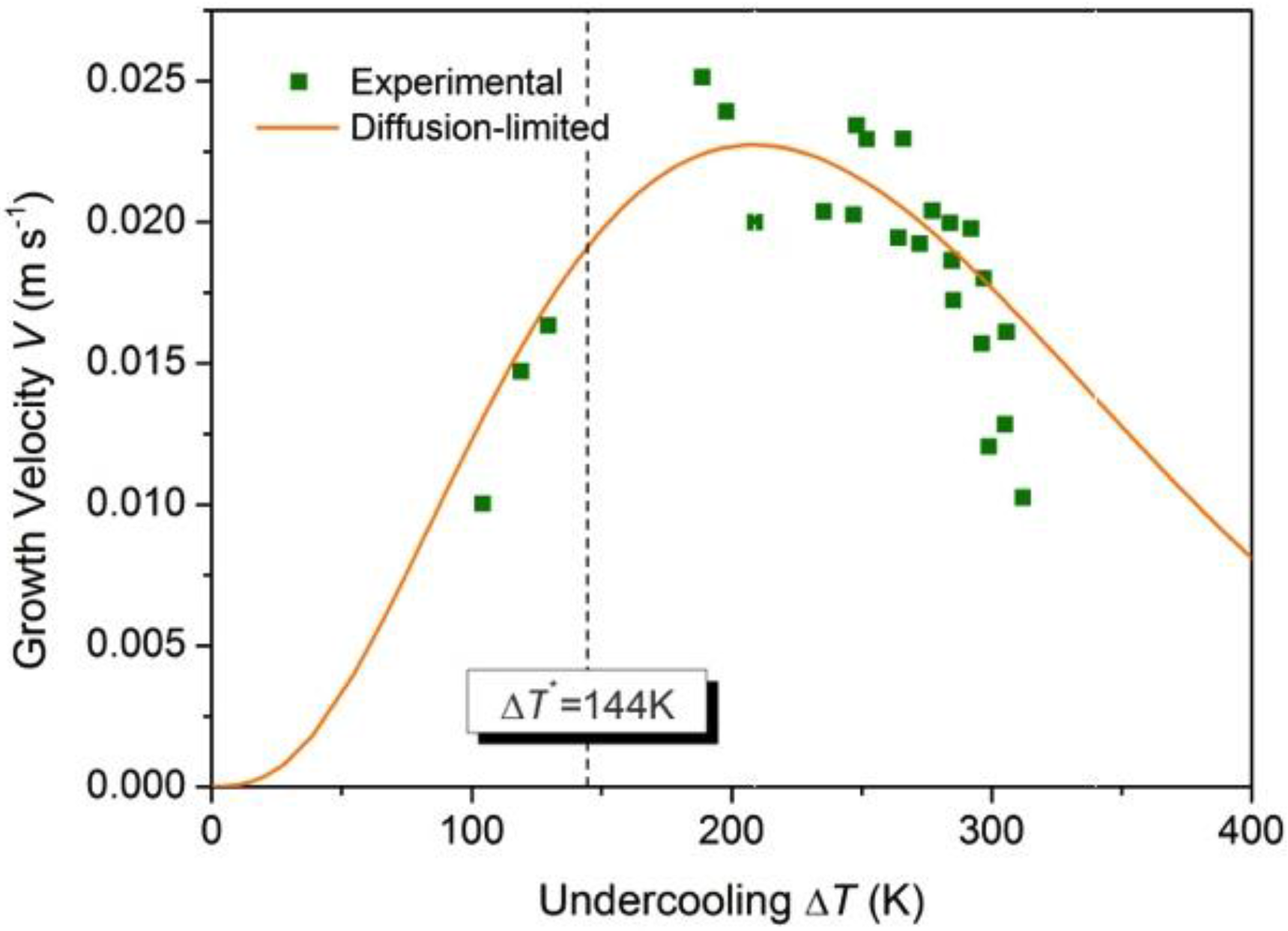
Figure 7.
Measured growth velocity V as a function of undercooling ∆T (squares). There is a specific undercooling: At ∆T* = 144 K the thermal undercooling ∆Tt equals to the kinetic undercooling ∆Tk, Solid line gives the prediction of dendrite growth theory assuming diffusion-limited growth and taking into account a temperature dependent diffusion coefficient (see the text).
Figure 7.
Measured growth velocity V as a function of undercooling ∆T (squares). There is a specific undercooling: At ∆T* = 144 K the thermal undercooling ∆Tt equals to the kinetic undercooling ∆Tk, Solid line gives the prediction of dendrite growth theory assuming diffusion-limited growth and taking into account a temperature dependent diffusion coefficient (see the text).
The experimental results are analyzed within the sharp interface model, as described in
Section 3.1. Taking into account the dependence of the diffusion coefficient on the temperature extends this model. The Cu
50Zr
50 is an intermetallic compound, which melts congruently. Therefore, constitutional contributions to the undercooling can be excluded similar as in the case of Al
50Ni
50 compound discussed in a previous chapter. In addition, the curvature undercooling is neglected because this contribution is small for thermal dendrites with their large curvature radius at the tip. Therefore, the total undercooling is approximated by ∆
T ≈ ∆TT + ∆TK. The kinetic undercooling ∆
TK is controlled by the atomic attachment kinetics at the solid–liquid interface. In the case of an intermetallic compound, such as Cu
50Zr
50, and even more because of the good glass-forming ability of this alloy, the atomic attachment kinetics will be diffusion controlled. In this case the prefactor
Vo in Equation (13) shall correspond to the atomic diffusive speed,
Vd. Equation (13) is then rewritten as
with
where
Dl(
T) is the temperature dependent diffusion coefficient in the liquid and
Qd is the activation energy for diffusion. This is the case when ordering in the liquid [
59,
60] is necessary for crystallization [
61]. The activation energy of crystallization in a number of metals and alloys is the same as for diffusion [
62]. Obviously, the diffusion-limited crystallization mode prevails even in pure metals at large undercoolings, e.g., it seems thermally-limited for Ag at low Δ
T [
63] but is actually diffusion-limited on the whole for Δ
T up to ∆
Tg [
64]. According to Aziz and Boettinger [
27], the pre-factor C in Equation (15) is defined as
with λ the interatomic spacing and
f a geometrical factor of order unity. If
is given as the average lattice spacing normal to (100) and (110) surfaces in the MD simulation [
65], the inter-diffusion coefficient
can be determined.
For further analysis, each experimental point is fitted with the dendrite growth model to obtain the upper limit of the growth velocity
V0 at each measured undercooling ∆
T.
V0 is then plotted as a function of 1000/
Ti in a semi-logarithmic diagram, as shown in
Figure 8. It is interesting to see that the evolution of
V0 with
Ti follows the Arrhenius law except for the last three experimental points at high ∆
T. This means that crystallization of Cu
50Zr
50 melt is thermally activated with a prefactor
and an activation energy
. Based upon these results, the dendrite growth velocity
V is calculated as a function of the total undercooling ∆
T. The results of these computations are presented as the solid line in
Figure 7. One can see the experimental results of the dendrite growth velocity are well reproduced. A maximum
V = 0.023 m/s is found at ∆
T = 209 K, which is quite close to the experimental measurement of a maximum
V = 0.025 m/s at ∆
T = 200 K. It is interesting to note that using the temperature dependent viscosity does not lead to a matching of the experiments and the modeling [
56], in contrast to the present work where the temperature dependent diffusion coefficient is used to take into account the mobility of the solid–liquid interface. This may be understood by the fact that the Einstein–Stokes relation does not hold for Zr-based glass forming alloys [
66].
Figure 8.
Arrhenius plot of the upper limit of growth velocity V0 as a function of 1000 times the reciprocal interface temperature 1000/Ti: experimental data (squares); results of the computations (solid line).
Figure 8.
Arrhenius plot of the upper limit of growth velocity V0 as a function of 1000 times the reciprocal interface temperature 1000/Ti: experimental data (squares); results of the computations (solid line).
As to a similar undercooled glass-forming Ni
50Zr
50 alloy from which a stoichiometric compound NiZr is crystallized, the self-diffusion coefficient of Ni
DNi was measured [
67]. The activation energy for the atomic diffusion is determined as
Qd = 0.73 ± 0.03 eV, which is very close to the value inferred from the slope of the computed line in
Figure 7,
Qd = 0.827 eV. If
DNi is extended to low temperatures, there are no large differences between
DNi and the current result inferred from the dendrite growth measurements in undercooled Cu
50Zr
50 alloy. The temperature dependent self-diffusion coefficients of Cu,
DCu and, Zr,
DZr were investigated by MD simulations for Cu
50Zr
50 [
67]. The activation energies, as determined from these results, lead to the activation energies of the atomic self-diffusion for Cu and Zr,
QCu = 0.42 eV and
QZr = 0.44 eV [
65]. Despite potential significant uncertainties due to the difference in the interatomic potentials, the current diffusion coefficient and its activity energy are within the uncertainty of MD simulation results. Thus, it is quite reasonable to conclude that crystallization of undercooled Cu
50Zr
50 alloy is diffusion-limited through the undercooling range where the interface undercooling is dominant. The deviations at high ∆
T (
Figure 7 and
Figure 8) are attributed to two effects. First, the anisotropy effect of kinetic coefficient, which is quite important for selecting the operating state of dendrite [
68], especially at high ∆
T, is not considered in the solvability theory. Second, the diffusion changes from the thermally activated single atom to the collective atomic mechanism when
and the Arrhenius law cannot hold at very high ∆
T [
69,
70].