Crystal and Molecular Structure Studies of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate and Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate
Abstract
:1. Introduction
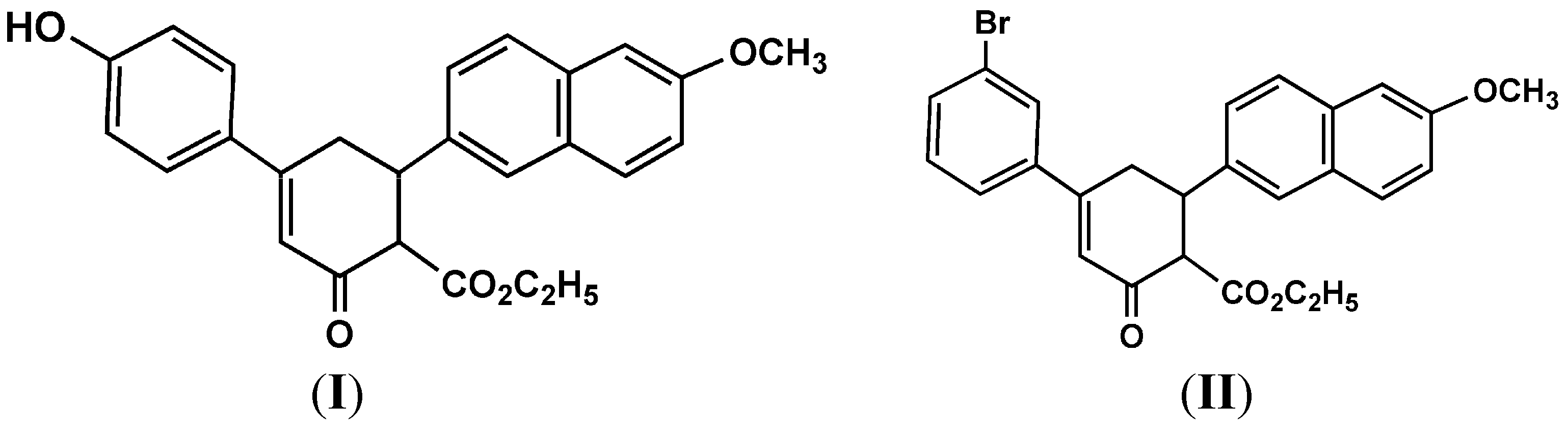
2. Results and Discussion
| D–H...A | d (D–H) | d (H...A) | d (D...A) | < (DHA) |
|---|---|---|---|---|
| O1A—H1A…O5A #1 | 0.84 | 2.44 | 3.219(7) | 154(1) |
| 1B—H1B…O5B #2 | 0.84 | 2.30 | 3.135(2) | 177(1) |
| O2B—H2B1…O2B | 0.84 | 2.55 | 3.36(3) | 162(1) |
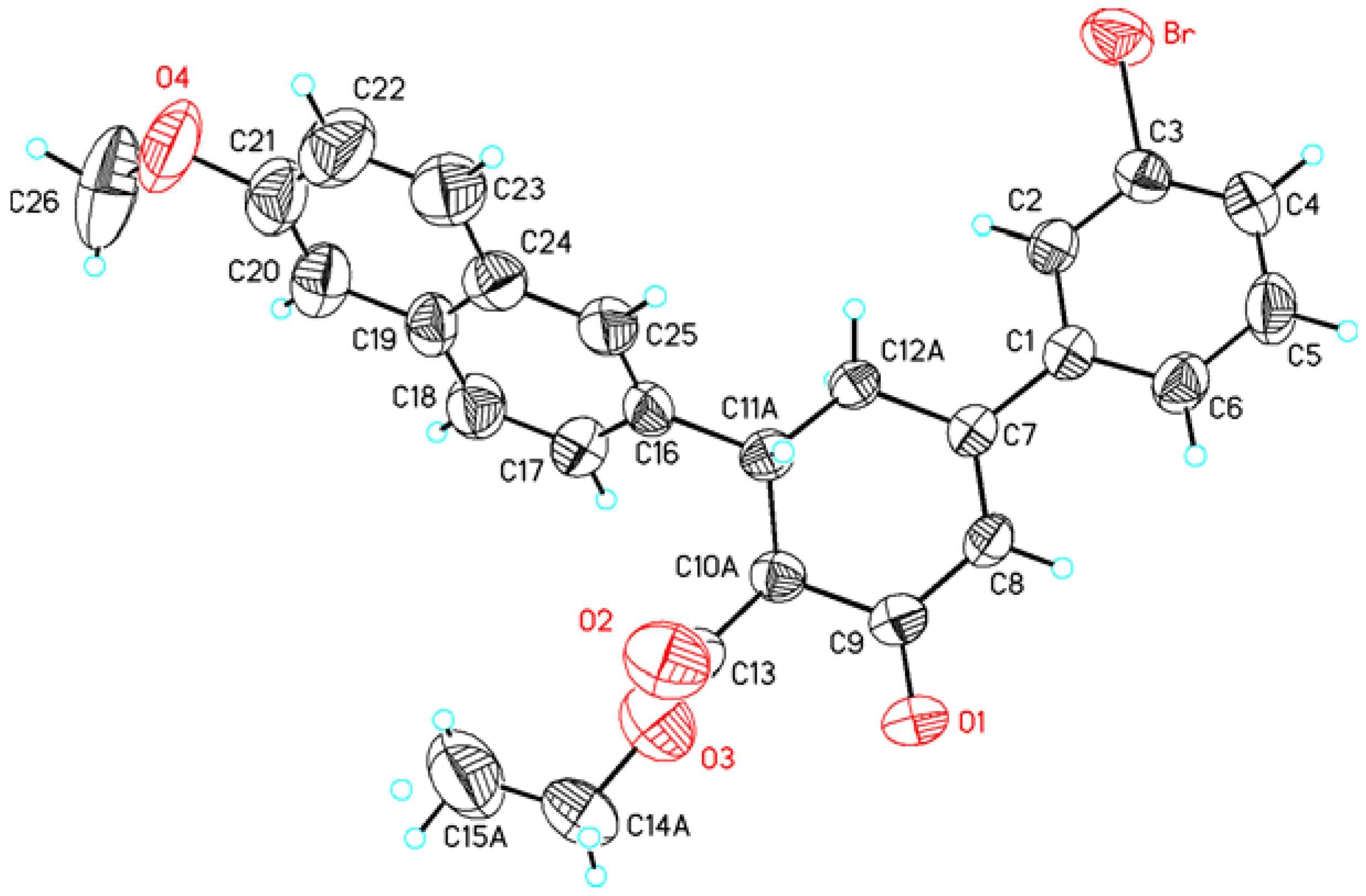
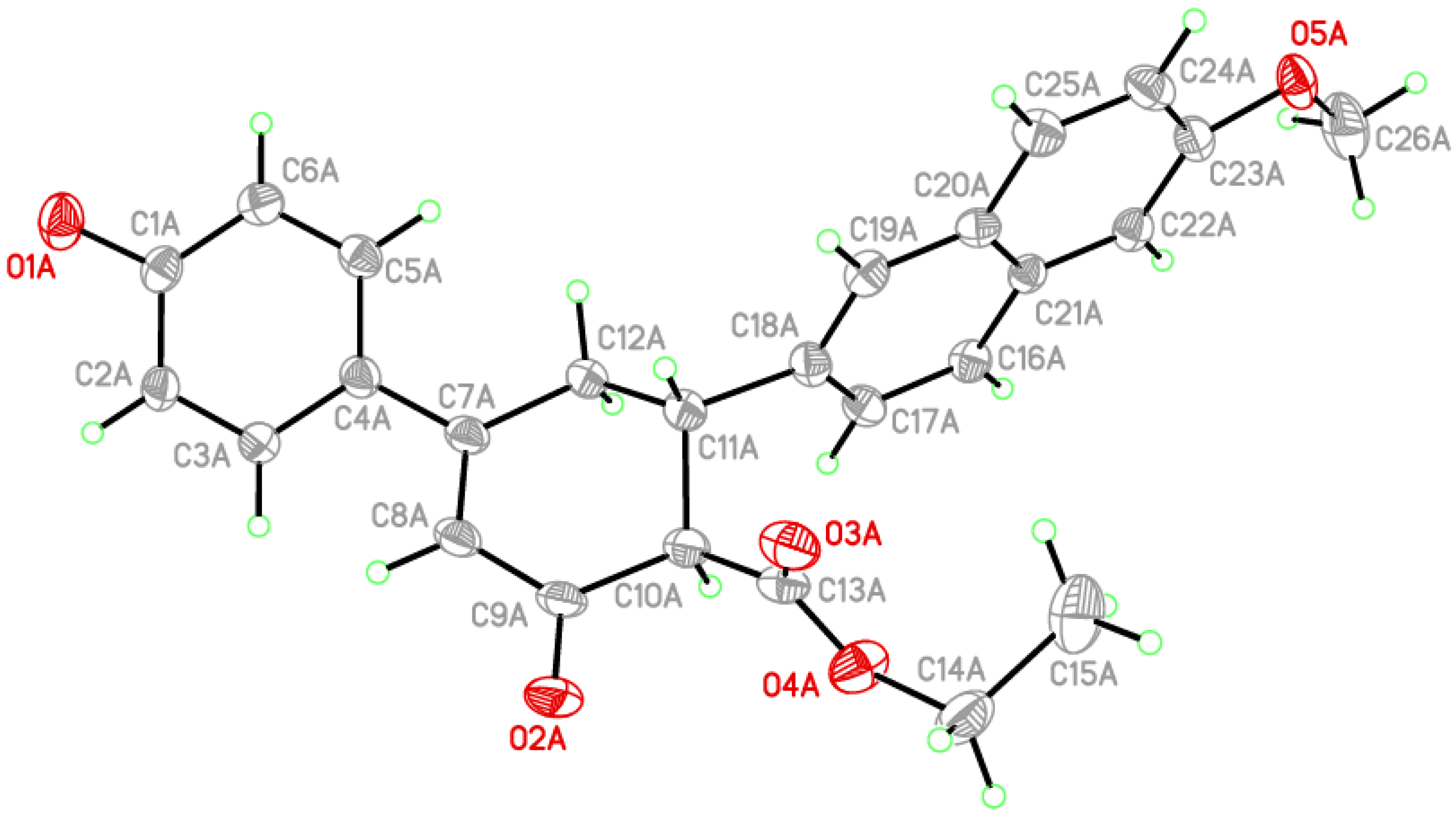
| Atoms (I) | Distance (I) | Atoms (II) | Distance (II) |
|---|---|---|---|
| C1A—O1A | 1.365(4) | C3—Br | 1.895(3) |
| C9A—O2A | 1.211(5) | C9—O1 | 1.212(3) |
| C9A—C10A | 1.492(5) | C9—C10A | 1.524(4) |
| C10A—C11A | 1.520(5) | C10A—C11A | 1.523(4) |
| C10A—C13A | 1.525(6) | C10A—C13 | 1.520(5) |
| C13A—O3A | 1.182(8) | C13—O2 | 1.191(4) |
| C13A—O4A | 1.308(7) | C13—O3 | 1.310(4) |
| C14A—O4A | 1.430(6) | C14A—O3 | 1.444(5) |
| C14A—C15A | 1.602(9) | C14A—C15A | 1.440(7) |
| C23A—O5A | 1.386(4) | C21—O4 | 1.384(4) |
| O5A—C26A | 1.422(7) | O4—C26 | 1.432(6) |
| D–H...A | d (D–H) | d (H...A) | d (D...A) | < (DHA) |
|---|---|---|---|---|
| C12B—H12D...O1 #1 | 0.97 | 2.33 | 3.110(4) | 136(2) |
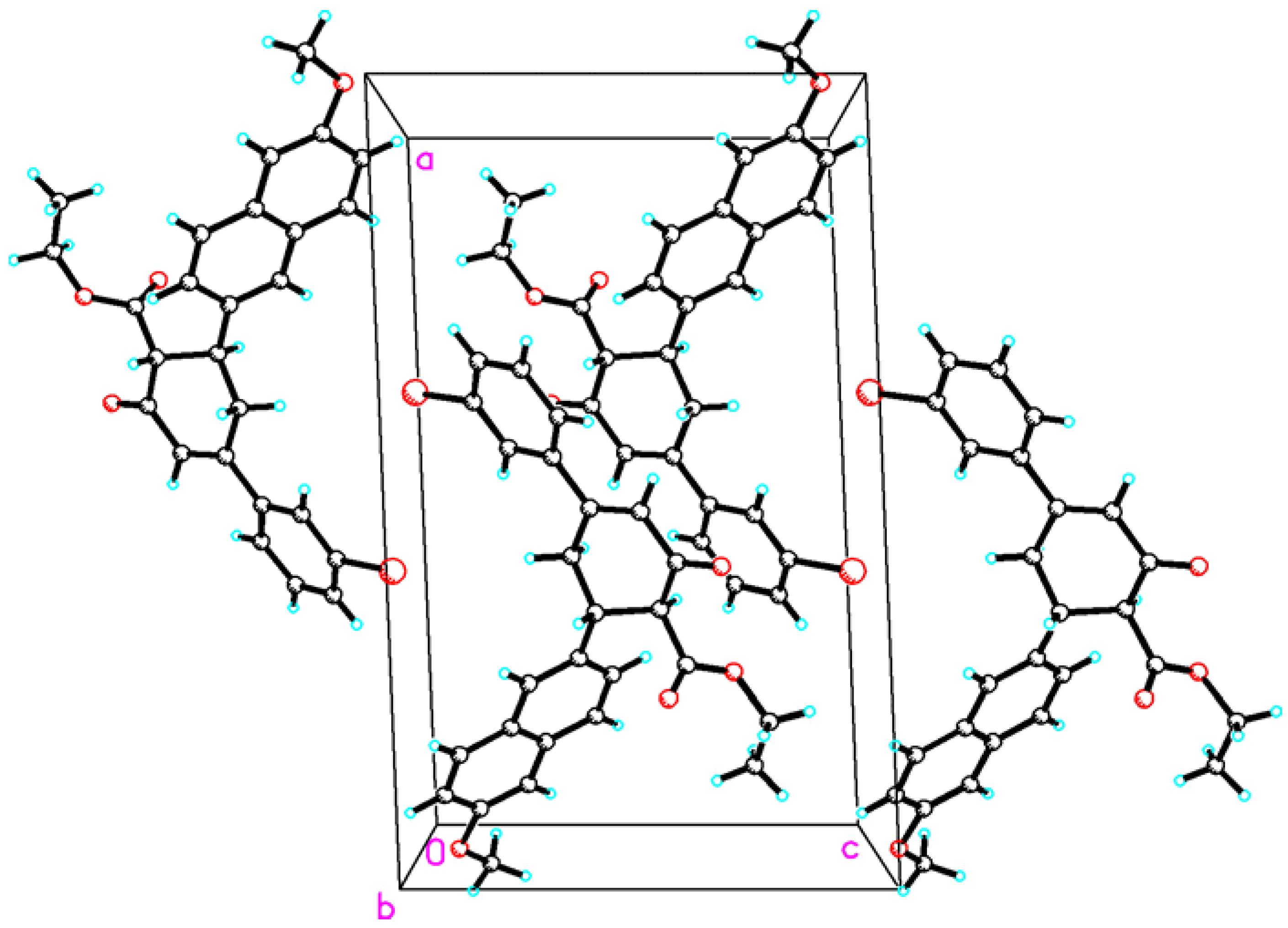
3. Experimental Section
3.1. General
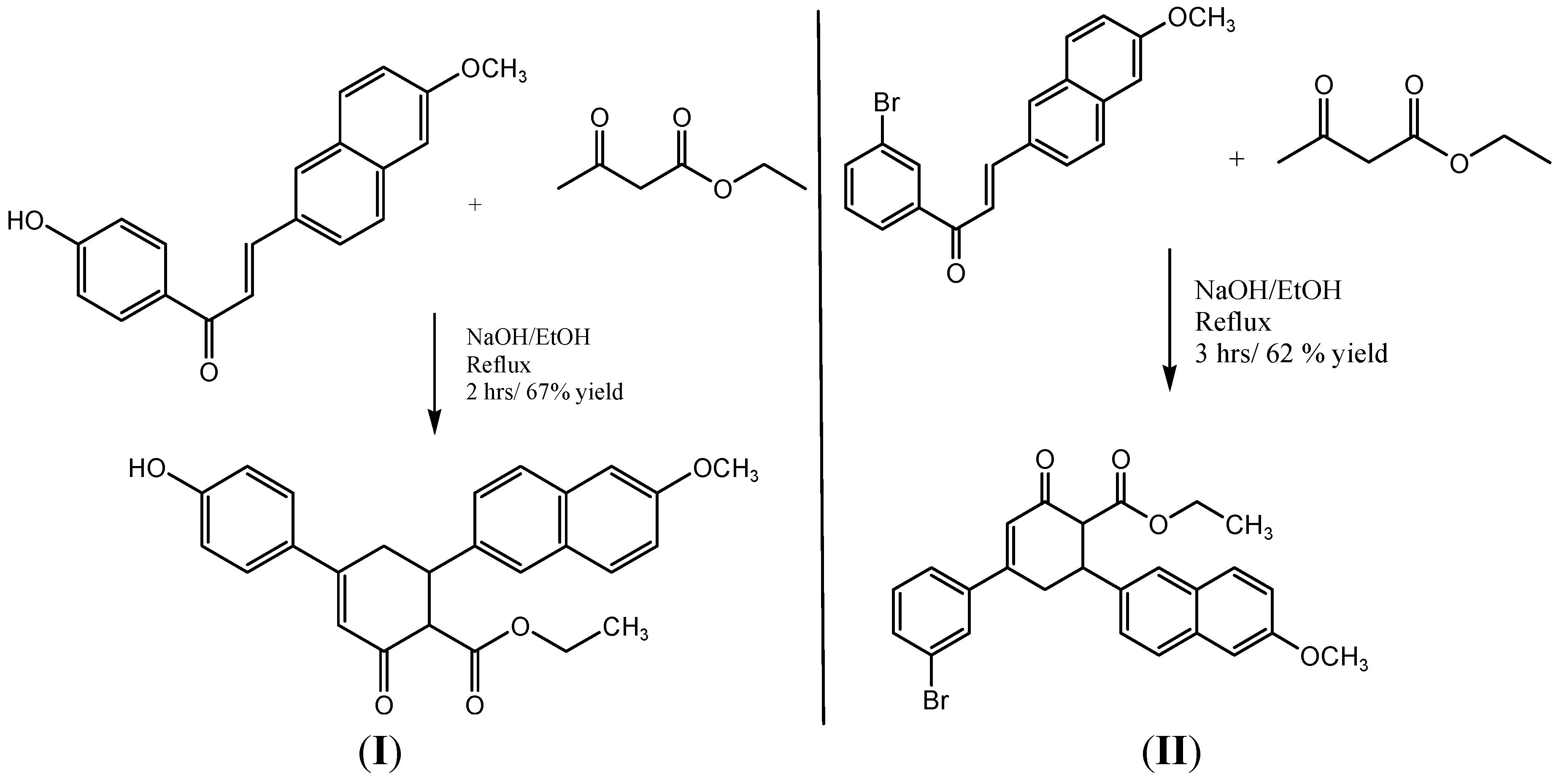
3.2. Synthesis of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate
3.3. Synthesis of Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate
3.4. Data Collection and Refinement
4. Conclusions
Acknowledgements
References
- Dhar, D.N. The Chemistry of Chalcones and Related Compounds; Wiley-Interscience: New York, NY, USA, 1981; pp. 64–70. [Google Scholar]
- Dimmock, J.R.; Elias, D.W.; Beazely, M.A.; Kandepu, N.M. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125–1150. [Google Scholar]
- House, H.O. Modern Synthetic Reactions, 2nd ed; W.A. Benjamin: Menlo Park, CA, USA, 1972; pp. 492–595. [Google Scholar]
- Tabba, H.D.; Yousef, N.M.; Al-arab, M.M. Michael-Michael aldol reaction of chalcones with cyanoacetylurea and cyanoacetylpiperidine. Collect. Czech. Chem. Commun. 1995, 60, 594–604. [Google Scholar] [CrossRef]
- Padmavathi, V.; Sharmila, K.; Somashekara, R.A.; Bhaskar, R.D. Reactivity of 3,5-diarylcyclohexanones—synthesis of spirocyclohexanes. Indian J. Chem. 2001, 40B, 11–14. [Google Scholar]
- Padmavathi, V.; Sharmila, K.; Padmaja, A.; Bhaskar, R.D. An efficient synthesis of 6,8-diarylcarbazoles via Fischer indole cyclizations. Heterocycl. Commun. 1999, 5, 451–456. [Google Scholar] [CrossRef]
- Padmavathi, V.; Mohana, R.B.J.; Balaiah, A.; Venugopal, R.K.; Bhaskar, R.D. Synthesis of some fused pyrazoles and isoxazoles. Molecules 2000, 5, 1281–1286. [Google Scholar] [CrossRef]
- Fischer, A.; Yathirajan, H.S.; Ashalatha, B.V.; Narayana, B.; Sarojini, B.K. (8RS, 9SR)-Ethyl 4-(3-bromothien-2-yl)-6-(2-furyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2007, E63, o254–o255. [Google Scholar]
- Fischer, A.; Yathirajan, H.S.; Ashalatha, B.V.; Narayana, B.; Sarojini, B.K. Ethyl 4-(3-bromo-2-thienyl)-2-oxo-6-phenylcyclohex-3-ene-1-carboxylate. Acta Cryst. 2008, E64, o560. [Google Scholar]
- Yao, L.Y.; Zhang, C.; Wang, B.S. (R, S)-Methyl 3-methyl-5-oxo-1-phenylcyclohex-3-ene-1-carboxylate. Acta Cryst. 2006, E62, o2768–o2769. [Google Scholar]
- Fischer, A.; Swamy, M.T.; Narayana, B.; Yathirajan, H.S. Rac-ethyl 3-(3-bromo-2-thienyl)-2-oxo-6-(4-propoxyphenyl)cyclohex-3-ene-1-carboxylate. Acta Cryst. 2008, E64, o2152. [Google Scholar]
- Li, H.; Mayekar, A.N.; Narayana, B.; Yathirajan, H.S.; Harrison, W.T.A. Ethyl 6-(6-methoxy-2-naphthyl)-4-(4-methylphenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2009, E65, o1186. [Google Scholar]
- Li, H.; Mayekar, A.N.; Narayana, B.; Yathirajan, H.S.; Harrison, W.T.A. Ethyl 6-(6-methoxy-2-naphthyl)-2-oxo-4-(2-thienyl)cyclohex-3-ene-1-carboxylate. Acta Cryst. 2009, E65, o1533. [Google Scholar]
- Dutkiewicz, G.; Narayana, B.; Veena, K.; Yathirajan, H.S.; Kubicki, M. (1RS, 6SR)-Ethyl 4-(4-chlorophenyl)-6-(4-fluorophenyl)-2-oxocyclohex-3-ene-1-carboxylate toluene hemisolvate. Acta Cryst. 2011, E67, o334–o335. [Google Scholar]
- Dutkiewicz, G.; Narayana, B.; Veena, K.; Yathirajan, H.S.; Kubicki, M. (1RS, 6SR)-Ethyl 4-(2,4-dichlorophenyl)-6-(4-fluorophenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2011, E67, o445–o446. [Google Scholar]
- Dutkiewicz, G.; Narayana, B.; Veena, K.; Yathirajan, H.S.; Kubicki, M. (1RS, 6SR)-Ethyl 4,6-bis(4-fluorophenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2011, E67, o336. [Google Scholar]
- Fun, H.-K.; Hemamalini, M.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. Methyl 4,6-bis(4-fluorophenyl)-2-oxocyclohex-3-ene-1-carboxylate. Acta Cryst. 2010, E66, o864–o865. [Google Scholar]
- Cremer, D.; Pople, J.A. A general definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Mayekar, A.N.; Li, H.; Yathirajan, H.S.; Narayana, B.; Suchetha Kumari, N. Synthesis, characterization and antimicrobial study of some new cyclohexenone derivatives. Int. J. Chem. 2010, 2, 114–123. [Google Scholar]
- Oxford Diffraction, CrysAlis PRO and CrysAlis RED; Oxford Diffraction Ltd.: Abingdon, Oxfordshire, UK, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaur, M.; Jasinski, J.P.; Butcher, R.J.; Yathirajan, H.S.; Mayekar, A.N.; Narayana, B. Crystal and Molecular Structure Studies of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate and Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate. Crystals 2012, 2, 1239-1247. https://doi.org/10.3390/cryst2031239
Kaur M, Jasinski JP, Butcher RJ, Yathirajan HS, Mayekar AN, Narayana B. Crystal and Molecular Structure Studies of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate and Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate. Crystals. 2012; 2(3):1239-1247. https://doi.org/10.3390/cryst2031239
Chicago/Turabian StyleKaur, Manpreet, Jerry P. Jasinski, Ray J. Butcher, Hemmige S. Yathirajan, Anil N. Mayekar, and Badiadka Narayana. 2012. "Crystal and Molecular Structure Studies of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate and Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate" Crystals 2, no. 3: 1239-1247. https://doi.org/10.3390/cryst2031239
APA StyleKaur, M., Jasinski, J. P., Butcher, R. J., Yathirajan, H. S., Mayekar, A. N., & Narayana, B. (2012). Crystal and Molecular Structure Studies of Ethyl 4-(4-Hydroxyphenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate and Ethyl 4-(3-Bromophenyl)-6-(6-methoxy-2-naphthyl)-2-oxocyclohex-3-ene-1-carboxylate. Crystals, 2(3), 1239-1247. https://doi.org/10.3390/cryst2031239




