Abstract
The crystal and molecular structures of two 2-aminothiophene derivatives, potential allosteric enhancers at the human A1 adenosine receptor, are reported. (2-Amino-4,5,6,7-tetrahydro-1-benzothiophen-3-yl)(phenyl)methanone (1) crystallizes in the orthorhombic space group Pna21 (a = 9.2080(4) Å, b = 14.0485(7) Å, c = 10.3826(6) Å), and (2-amino-5-ethylthiophen-3-yl)(2-chlorophenyl)methanone (2) crystalizes in the monoclinic P21/c space group with unit cell parameters a = 10.6092(8) Å, b = 10.8355(8) Å, c = 11.1346(9) Å, β = 98.643(6)Å. In both molecules the intramolecular N–H···O=C hydrogen bonds close six-membered planar rings and significantly influence the molecular conformation. Intermolecular N–H···O bonds connect the molecules in infinite chains along a in case of 1, and along b in 2; in each case the appropriate unit cell axis is approximately 10 Å long.
1. Introduction
2-Aminothiophene derivatives have been used in a number of applications in pesticides, dyes and pharmaceuticals. The synthesis and properties of these compounds were reviewed in 1999 by Sabinis et al. [1] and more recently by Puterová et al. [2]. In particular, substituted 2-aminothiophenes with alkyl or cycloalkyl substituents in positions 4 and 5 (see Scheme 1), and aroyl group in position 3 are active as allosteric enhancers at the human A1 adenosine receptor [3,4,5]. According to these results, the 2-amino and 3-keto groups are necessary for the biological action, and the substituents at position 4 can further increase the activity.
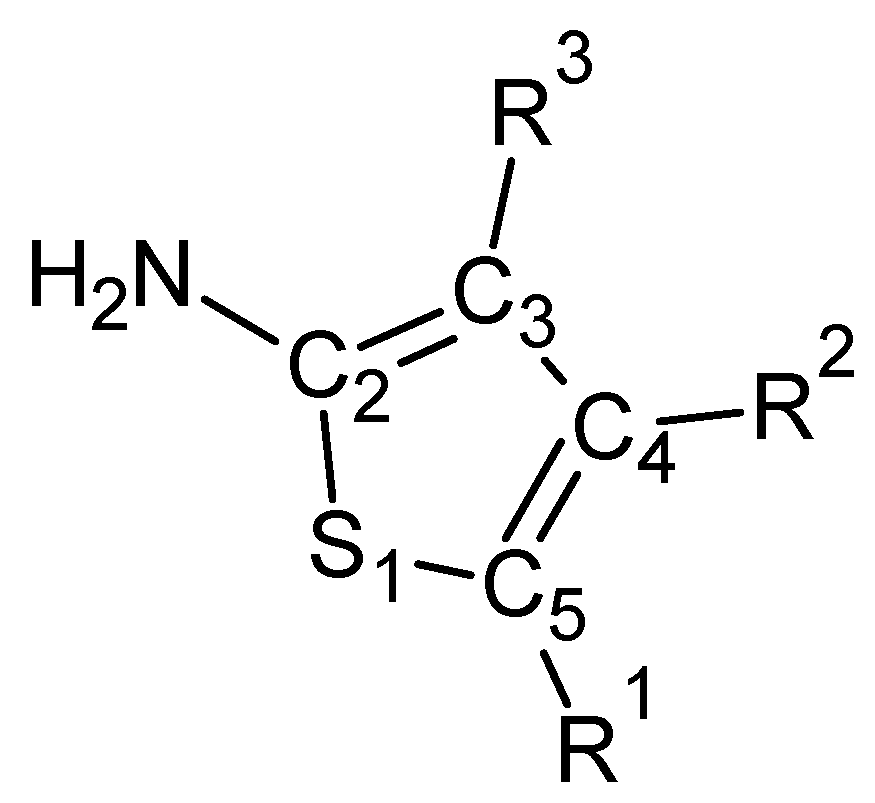
Scheme 1.
A general structural formula of 2-amino-thiophene with the numbering scheme.
Interestingly, in the Cambridge Structural Database [6] there is only one example of a 2-amino-3-aroyl thiophene, (2-amino-7-(trifluoromethyl)-8H-indeno[2,1-b]thiophen-3-yl)(phenyl)methanone [7]. As expected from the first reports of the activity of 2-amino-3-benzoyl thiophenes [8], the intramolecular N–H···O hydrogen bond creates an additional ring, roughly coplanar with the thiophene ring. Here, we present the results of the X-ray crystal structure analysis of two more compounds of this family (Scheme 2), namely (2-amino-4,5,6,7-tetrahydro-1-benzothiophen-3-yl)(phenyl)methanone (1) and (2-amino-5-ethylthiophen-3-yl)(2-chlorophenyl)methanone (2).
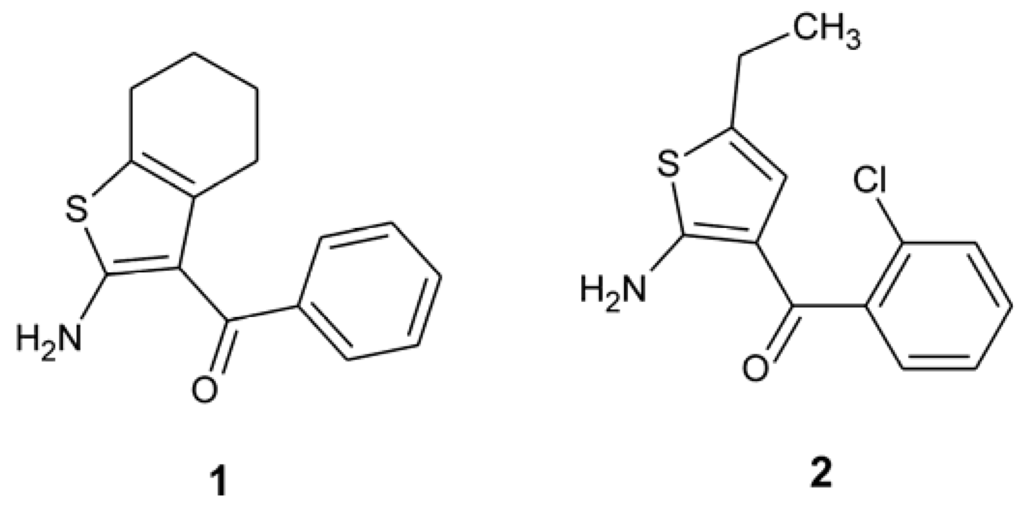
Scheme 2.
The compounds 1 and 2.
2. Results and Discussion
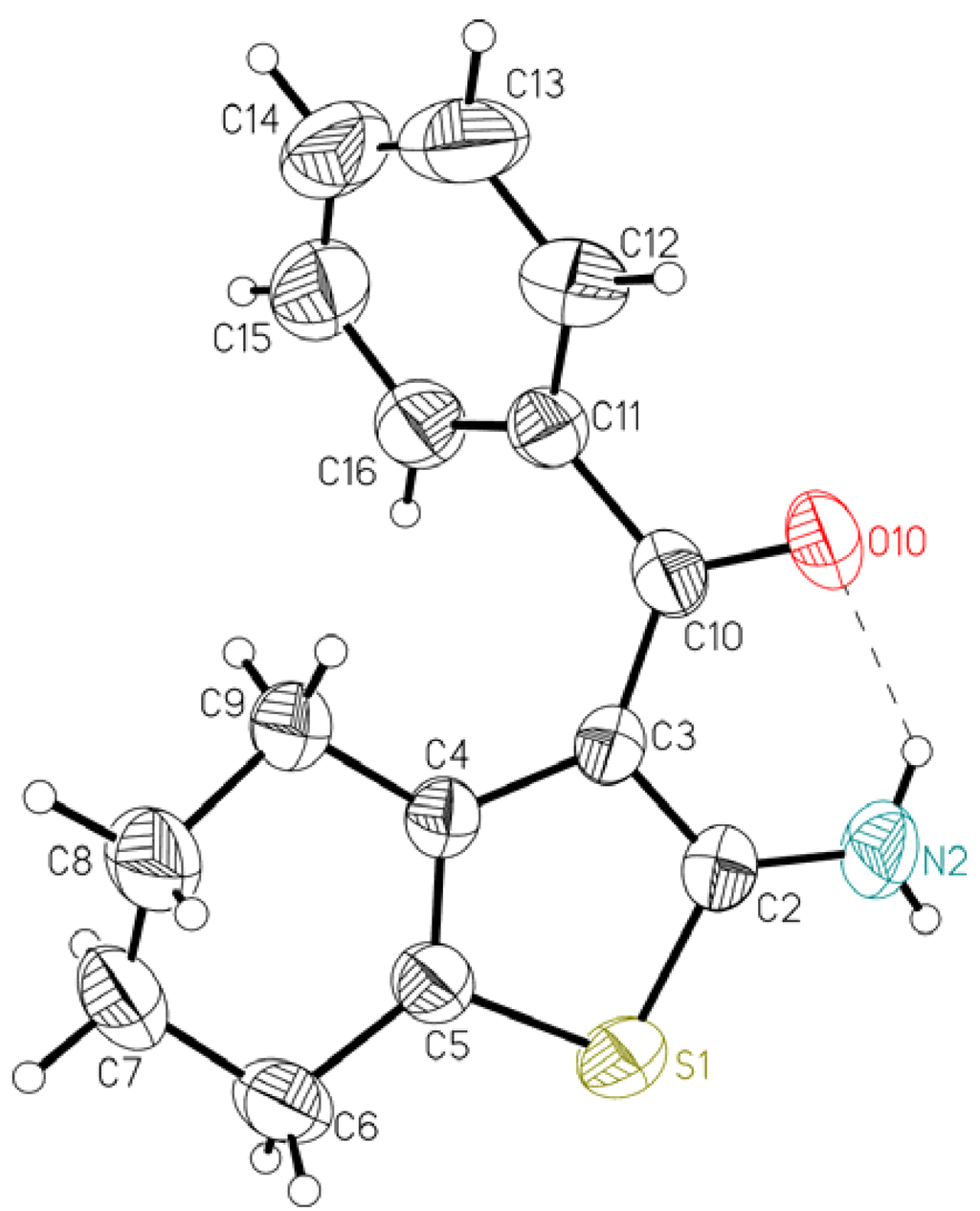
Figure 1.
Ellipsoid representation of molecule 1 together with the atom labeling scheme [9]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are depicted as spheres with arbitrary radii. The intramolecular hydrogen bond is drawn as a dashed line.
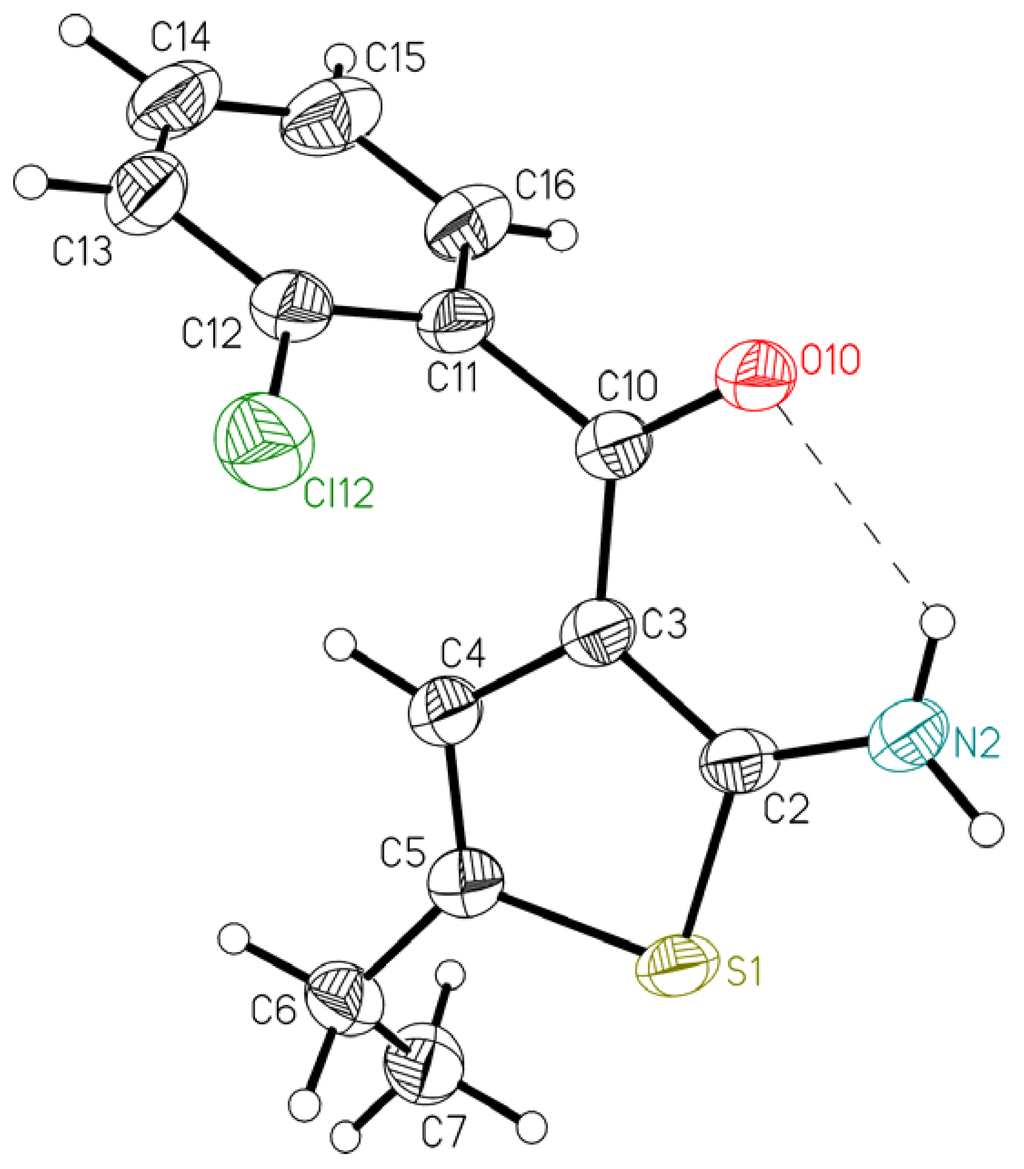
Figure 2.
Ellipsoid representation of molecule 2 together with the atom labeling scheme [9]. The ellipsoids are drawn at 50% probability level, hydrogen atoms are depicted as spheres with arbitrary radii. The intramolecular hydrogen bond is drawn as a dashed line.
Table 1 lists some relevant geometrical features. The overall conformation of the molecules can be described by dihedral angles between the approximately planar fragments: thiophene ring (A), C–C(=O)–C bridge (B) and phenyl ring (C). The presence of the fused ring (tetrahydro) in 1 influences the shape of the molecule; in this case the C3–C10–C11 bridge makes significant dihedral angles with both thiophene and phenyl ring plane (20.02(12)° and 46.47(11)°, respectively). The twist between the ring planes is 57.77(8)°. On the other hand, in 2 the central bridge is almost coplanar with the thiophene ring plane (dihedral angle is only 2.31(12)° and the largest deviation from the plane through all 9 atoms, including N2, C10, O10 and C11, is 0.0163(9) Å), but this plane is practically perpendicular to the phenyl ring (81.67(5)°). The non-aromatic six membered ring in 1 assumes almost ideal half-chair conformation, the asymmetry parameter [10], which describes the deviation from the ideal symmetry (in this case C2) is as small as 1.63°.

Table 1.
Relevant geometrical features (Å, °) with esd’s in parentheses.
| 1 | 2 | |
|---|---|---|
| S1–C2 | 1.722(3) | 1.7311(15) |
| S1–C5 | 1.745(3) | 1.7611(16) |
| C2–N2 | 1.339(3) | 1.332(2) |
| C2–C3 | 1.405(3) | 1.402(2) |
| C3–C4 | 1.457(3) | 1.444(2) |
| C4–C5 | 1.342(3) | 1.343(2) |
| C10–O10 | 1.242(3) | 1.2485(19) |
| C3–C10 | 1.435(3) | 1.421(2) |
| C10–C11 | 1.499(4) | 1.509(2) |
| C2–S1–C5 | 91.76(12) | 92.30(7) |
| C3–C2–N2 | 127.5(2) | 127.10(14) |
| S1–C2–N2 | 120.9(2) | 121.98(12) |
| C2–C3–C10 | 119.0(2) | 122.28(14) |
| C4–C3–C10 | 129.9(2) | 126.24(14) |
| C3–C10–C11 | 121.2(2) | 117.33(13) |
| C3–C10–O10 | 121.3(3) | 124.13(13) |
| C11–C10–O10 | 117.4(2) | 118.51(13) |
| C12–C11–C16 | 118.9(3) | 118.41(15) |
| S1–C2–C3–C10 | −176.6(2) | −178.96(12) |
| C2–C3–C10–O10 | −17.5(4) | −0.5(3) |
| C4–C3–C10–O10 | 162.3(3) | 179.09(16) |
| C2–C3–C10–C11 | 159.4(2) | 177.67(14) |
| C4–C3–C10–C11 | −20.8(4) | −2.7(2) |
| C3–C10–C11–C12 | 137.6(3) | 82.15(19) |
| C3–C10–C11–C16 | −46.9(4) | −96.86(18) |
The intramolecular N–H···O hydrogen bond (cf. Table 2) in both molecules closes an almost planar six-membered ring. Such hydrogen bonds exist in virtually all examples from the CSD; in 29 structures with thiophene ring and amino (NHR or NH2) group ortho to the R–C=O group the only example without such bond is one end of tetraethyl 2,2'-(2,3-dihydrothieno[3,4-b][1,4]dioxine-5,7-diylbis(methylylidenenitrilo))bis(5-aminothiophene-3,4-dicarboxylate) tetrahydrofuran solvate [11]. Although solid-state findings do not always translate into non-crystalline environments, the supposition that the presence of such a bond is important for the biological activity [8] seems to be obvious.

Table 2.
Hydrogen bond data (Å, °).
| D | H | A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|---|---|
| 1 | ||||||
| N2 | H2A | O10 | 0.85(3) | 2.03(3) | 2.667(4) | 131(3) |
| N2 | H2B | O10 i | 0.85(3) | 1.99(3) | 2.832(3) | 173(3) |
| 2 | ||||||
| N2 | H2A | O10 | 0.87(2) | 2.16(2) | 2.7785(19) | 127.8(17) |
| N2 | H2B | O10 ii | 0.87(3) | 2.02(3) | 2.8750(19) | 169(2) |
| C4 | H4 | Cl12 iii | 0.98(2) | 2.97(2) | 3.8384(17) | 149.0(16) |
| C6 | H6A | Cl12 iv | 1.01(3) | 2.89(2) | 3.8187(19) | 153.0(18) |
| C7 | H7B | Cl12 v | 0.99(3) | 2.95(3) | 3.8454(19) | 152(2) |
Symmetry codes: i ½ + x,5/2 − y,z; ii 1 − x,1/2 + y,1/2 − z; iii x,3/2 − y,−1/2 + z; iv 1 − x,1/2 + y,1/2 − z; v 1 − x,2 − y,−z.
Interestingly, this hydrogen-bonded ring is almost ideally planar in 2 (largest deviation 0.016(10) Å, dihedral angle with thiophene ring plane 0.7(5)°), while the deviations are significant in 1 (appropriate values are 0.112(8) Å and 4.1(9)°).
In both molecules the second hydrogen atom from the NH2 group is involved in a relatively short and linear intermolecular hydrogen bond with the O10 atom. These bonds connect molecules in infinite chains along x in 1 (Figure 3) and along y in 2 (Figure 4).
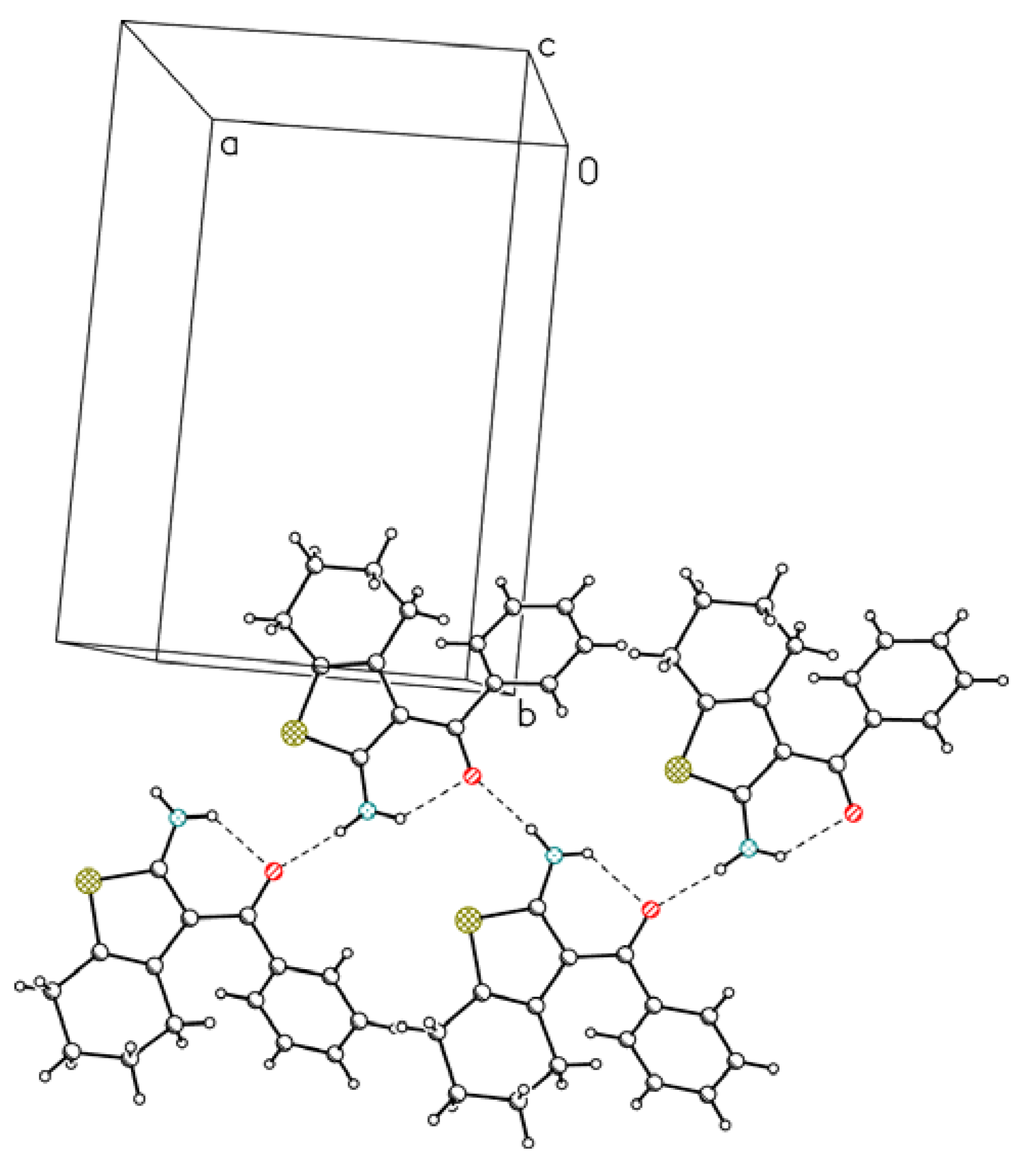
Figure 3.
Hydrogen-bond chain of molecule 1 [12]. Hydrogen bonds are depicted as dashed lines.
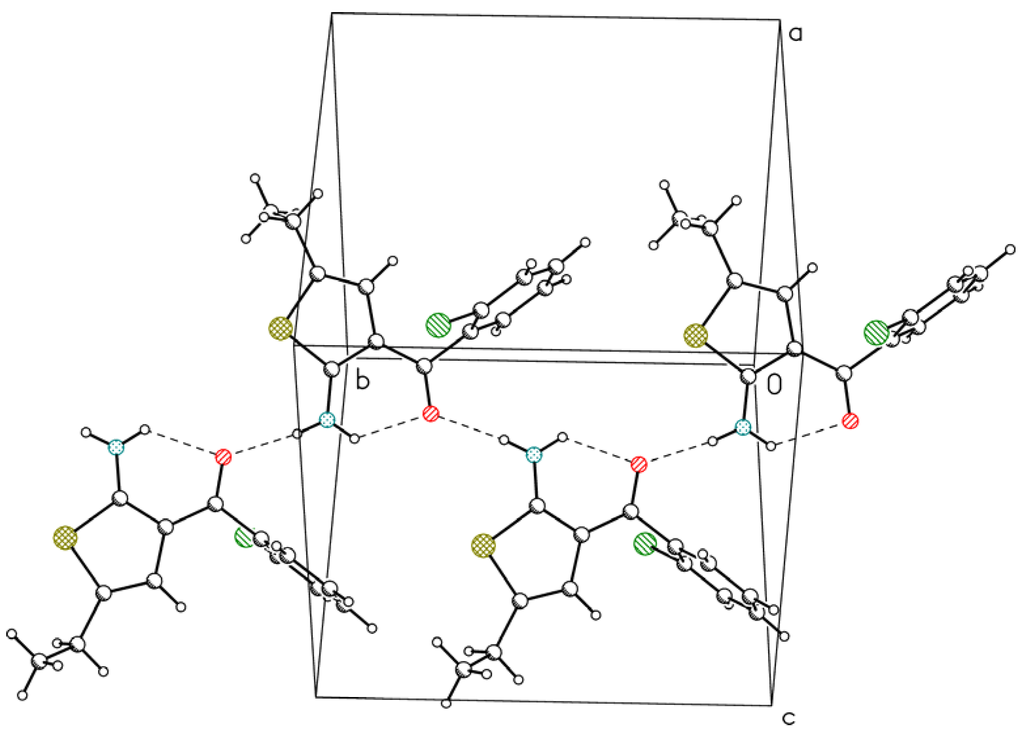
Figure 4.
Hydrogen-bond chain of molecule 2 [12]. Hydrogen bonds are depicted as dashed lines.
In 2, the chains are connected by a number of weak but directional C–H···Cl interactions (Figure 5); in contrast 1 is a rare example of a structure without any short intermolecular contacts other than well-defined, “classical” hydrogen bonds.
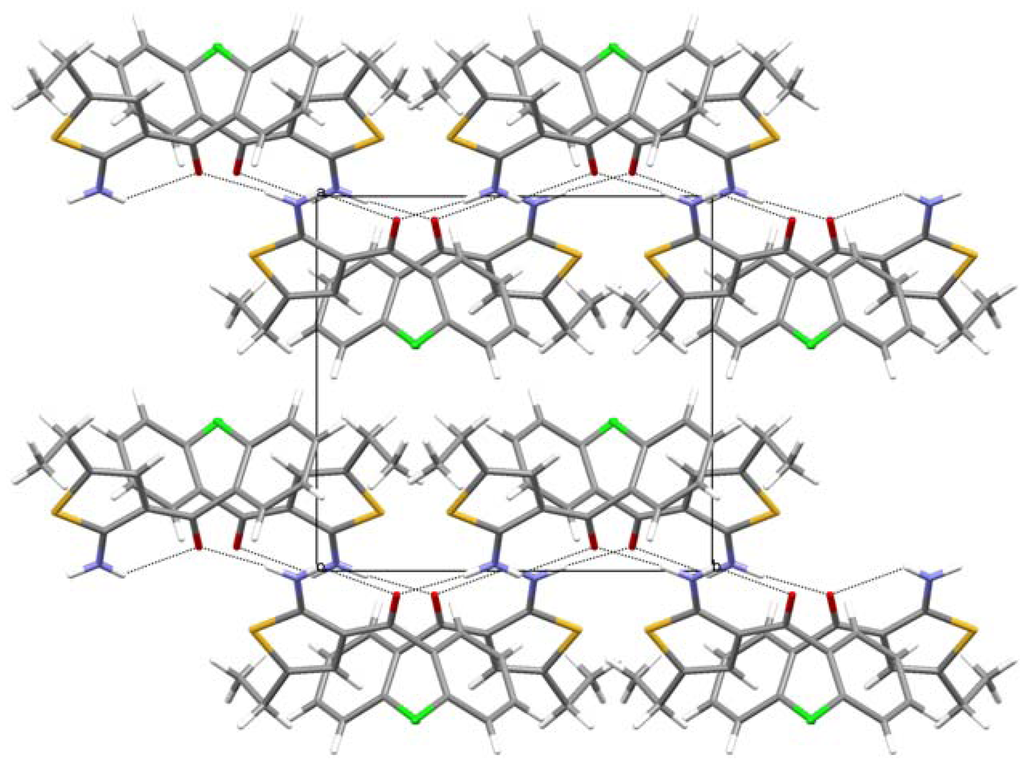
Figure 5.
The crystal packing of 2 as seen along direction [12]. N–H···O hydrogen bonds and C–H···Cl contacts are shown as dashed lines.
In the case of 2, the structures from the room temperature and 100 K data can be compared, and it turns out that the unit cell b parameter (i.e., along the hydrogen-bonded chain) does almost not change with temperature and the shrinking of the unit cell volume is almost exclusively caused by the shortening of the c unit cell parameter, the direction without any significant intermolecular interactions.
3. Experimental Section
The title compounds were obtained as a gift samples from R. L. Fine Chem., Bengaluru, India. Melting points: 373 K for 1, 368 K for 2.
The crystals appropriate for X-ray data collection were grown from 2-butanone solutions by slow evaporation. X-ray diffraction data for 1 and 2 were collected at room temperature using the ω-scan technique on an Agilent Technologies four-circle diffractometer equipped with Eos CCD-detector [13] using graphite-monochromatized MoKα radiation (λ = 0.71073 Å), and additionally for 2—due to the relatively low quality of room-temperature data—at 130(1) K on an Agilent SuperNova four-circle diffractometer equipped with Atlas CCD-detector [13] using mirror-monochromatized CuKα radiation from high-flux micro-focus source (λ = 1.54178 Å). The data were corrected for Lorentz-polarization effects as well as for absorption [13]. Accurate unit-cell parameters were determined by a least-squares fit of 1187 (1), 1335 (2, rt) and 3736 (2, 130 K) reflections of highest intensity, chosen from the whole experiment. The calculations were mainly performed with the WinGX program system [14]. The structures were solved with SIR92 [15] and refined with the full-matrix least-squares procedure on F2 by SHELXL97 [9]. Scattering factors incorporated in SHELXL97 were used. The function Σw(|Fo|2 − |Fc|2)2 was minimized, with w−1 = [σ2(Fo)2 + (A·P)2 + B·P], where P = [Max (Fo2, 0) + 2Fc2]/3. The final values of A and B are listed in Table 1. All non-hydrogen atoms were refined anisotropically, all hydrogen atoms in 2 (130 K) and amino hydrogens in 1 were found in difference Fourier maps and isotropically refined; other hydrogen atoms in 1 were placed in calculated positions and were refined as ‘riding’ on their parent atoms; the Uiso’s of hydrogen atoms were set as 1.2 times the Ueq value of the appropriate carrier atom. Relevant crystal data are listed in Table 3, together with refinement details.

Table 3.
Crystal data and refinement details.
| Compound | 1 | 2 |
|---|---|---|
| Formula | C15H15NOS | C13H12ClNOS |
| Formula weight | 257.34 | 265.75 |
| Crystal system | orthorhombic | monoclinic |
| Space group | Pna21 | P21/c |
| a (Å) | 9.2080(4) | 10.6092(8) |
| b (Å) | 14.0485(7) | 10.8355(8) |
| c (Å) | 10.3826(6) | 11.1346(9) |
| β (º) | 90 | 98.643(6) |
| V (Å3) | 1343.08(12) | 1265.45(17) |
| Z | 4 | 4 |
| Dx (g cm−3) | 1.72 | 1.40 |
| F(000) | 544 | 552 |
| μ (mm−1) | 0.23 | 4.07 |
| Crystal size (mm) | 0.3 × 0.15 × 0.15 | 0.35 × 0.2 × 0.1 |
| Θ range (°) | 3.29-28.18 | 4.21-73.59 |
| hkl range | −11 ± h ± 11 | −11 ± h ± 13 |
| −7 ± k ± 17 | −10 ± k ± 13 | |
| −8 ± l ± 13 | −13 ± l ± 13 | |
| Reflections: | ||
| collected | 3556 | 4715 |
| unique (Rint) | 1984 (0.021) | 2476 (0.0145) |
| with I > 2σ(I) | 1720 | 2400 |
| Number of parameters | 171 | 202 |
| Weighting scheme: | ||
| A | 0.0481 | 0.0624 |
| B | 0.1426 | 0.396 |
| R(F) [I > 2σ(I)] | 0.037 | 0.035 |
| wR(F2) [I > 2σ(I)] | 0.087 | 0.099 |
| R(F) [all data] | 0.045 | 0.036 |
| wR(F2) [all data] | 0.092 | 0.099 |
| Goodness of fit | 1.04 | 1.07 |
| max/min Δρ (e Å−3) | 0.14/−0.24 | 0.35/−0.31 |
Crystallographic data (excluding structure factors) for the structural analysis has been deposited with the Cambridge Crystallographic Data Centre, Nos. CCDC 867592 (1), 867593 (2, room temperature) and 867594 (2, 130(1) K). Copies of this information may be obtained free of charge from: The Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. Fax: +44(1223)336-033, e-mail: deposit@ccdc.cam.ac.uk, or www: www.ccdc.cam.ac.uk.
4. Conclusions
The crystal structures of two 2-amino-3-aroyl thiophenes, potential allosteric enhancers at the human A1 adenosine receptor, were determined by means of X-ray diffraction. In both molecules, intramolecular N–H···O hydrogen bonds close approximately planar six-membered rings, roughly (in case of 1) or almost perfectly (2) coplanar with the thiophene ring plane. The intermolecular N–H···O hydrogen bonds connect molecules in infinite chains along the approximately 10 Å long unit cell axis.
Acknowledgments
ASD thanks the University of Mysore for research facilities. We thank R. L. Fine Chem., Bengaluru, for the gift samples of the title compounds.
Conflict of Interest
The authors declare no conflict of interest.
References
- Sabnis, R.W.; Rangnekar, D.W.; Sonawane, N.D. 2-Aminothiophenes by the Gewald Reaction. J. Heterocycl. Chem. 1999, 36, 333–345. [Google Scholar] [CrossRef]
- Puterová, Z.; Krutošiková, A.; Végh, D. Gewald reaction: Synthesis, properties and applications of substituted 2-aminothiophenes. Arkivoc 2010, 209–246. [Google Scholar]
- Cannito, A.; Perrisin, M.; Luu-Duc, C.; Huguer, F.; Gaultier, C.; Narcisse, G. Synthèse et propriétés pharmacologiques de quelques thiéno[2,3-d]pyrimidin-4-one 2-thiones. Eur. J. Med. Chem. 1990, 25, 635–639. [Google Scholar]
- Nikolakopoulos, G.; Figler, H.; Linden, J.; Scammells, P. 2-Aminothiophene-3-carboxylates and carboxamides as adenosine A1 receptor allosteric enhancers. Bioorg. Med. Chem. 2006, 14, 2358–2365. [Google Scholar]
- Lütjens, H.; Zickgraf, A.; Figler, H.; Linden, J.; Olsson, R.A.; Scammells, P.J. 2-Amino-3-benzoylthiophene allosteric enhancers of A1 adenosine agonist binding: new 3-, 4-, and 5-modifications. J. Med. Chem. 2005, 46, 1870–1877. [Google Scholar]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. ActaCrystallogr. B 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Aurelio, L.; Valant, C.; Flynn, B.L.; Sexton, P.M.; White, J.M.; Christopoulos, A.; Scammells, P.J. Effects of conformation restriction of 2-amino-3-benzoylthiophenes on A1 adenosine receptor modulation. J. Med. Chem. 2010, 53, 6550–6559. [Google Scholar]
- Bruns, R.F.; Fergus, J.H.; Coughenour, L.L.; Courtland, G.G.; Pugsley, T.A.; Dodd, J.H.; Tinney, F.J. Structure-activity relationships for enhancement of adenosine A1 receptor binding by 2-amino-3-benzoylthiophenes. Mol. Pharmacol. 1990, 38, 950–958. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Duax, W.L.; Norton, D.A. Atlas of Steroid Structure; Plenum: NewYork, NY, USA, 1975; Volume 1, pp. 16–22. [Google Scholar]
- Bolduc, A.; Dufresne, S.; Skene, W.G. EDOT-containing azomethine: An easily prepared electrochromically active material with tuneable colours. J. Mater. Chem. 2010, 20, 4820–4826. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar]
- CrysAlisPro Setup, Version 1.171.35.15 (release 3 August 2011 CrysAlis171.NET); Oxford Diffraction: Palo Alto, CA, USA, 2010.
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).