Abstract
The crystal structures of 2,4,5-tribromo-1-(prop-2-ynyl)imidazole and seven new 1,3-dialkyl-2,4,5-tribromoimidazolium salts (R1 = propenyl, propynyl, dibromopropenyl; R2 = Me, Et) with halogen-containing anions (tetrafluoroborate, hexafluorophosphate, triflimide) were determined. The structures revealed halogen...halogen and anion...π interactions. Contacts of the type Br...Br, Br...F, Br...O, Br...N, F...F, H...Br, H...F, F...π and O...π were identified. Specific interactions were quantified by Hirshfeld surface analysis.
1. Introduction
2,4,5-Tribromoimidazole [CARN 2034-22-2], first described in 1877 [1], is readily prepared by bromination of imidazole [2,3,4,5,6,7]. It has been found in nature [8] and is an effective fire-retardant agent [9]. It has been used as starting material for the synthesis of quaternary salts as high-density ionic liquids [10]. Halogenated imidazoles exhibit insecticidal [11], parasiticidal [12], acaricidal [13], and herbicidal [14] activity. Only few crystal structures of partially brominated imidazole [15] and quaternary imidazolium salts [16,17,18] have been reported. A cobalt complex of 2,4,5-tribromoimidazole has been published [19]. To our knowledge, no other crystal structures of 2,4,5-tribromoimidazole derivatives are known to date. Here we present the structures of eight new compounds of this kind. The quaternary salts are intended for application as non-volatile fire retardants. The olefinic substituents allow the preparation of new materials by polymerization.
2. Results and Discussion
The synthesis (Figure 1) of the propenyl and propynyl derivatives1 and 2 was carried out by known methods. The salts 3–6 were prepared by quaternization of 1 and 2. Compound 7 was obtained by ion metathesis from 4, and compounds 8 and 9 by bromination of 6. The choice of fluorine-containing anions such as tetrafluoroborate, hexafluorophosphate, and bis(trifluoromethanesulfonyl)imide (“triflimide”) provided an opportunity to study non-covalent hetero-halogen, homo-halogen, hydrogen-halogen, and other interactions. Crystal data and structure refinement details are summarized in Table 1. Charge transfer complexes of halogens and electron-donor molecules have been the subject of the 1970 Nobel Prize lecture by Odd Hassel [20]. Today it is known that halogen atoms can act as electron-acceptors (X-bond donors) and electron donors (X-bond acceptors). Theoretical calculations predicted negative ring and positive end cap domains of halogen atoms due to their polarizability [21], even for fluorine [22]. In fact, hetero halogen–halogen interactions in both directions, i.e., Cl→F and F→Cl, have been observed recently [22]. As expected, a classic Br...N interaction was found in 2 (Table 2). Some typical examples of this interaction, displayed by brominated N-heterocycles, are found in the Cambridge Structural Database (CSD) [23,24,25,26].
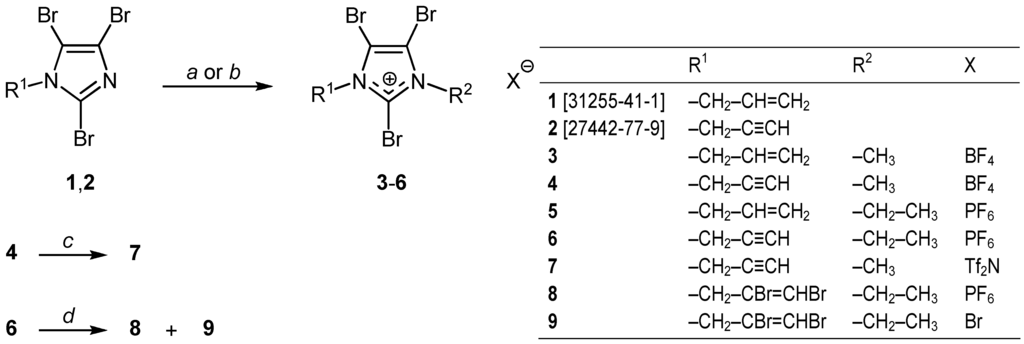
Figure 1.
Synthesis of 2,4,5-tribromoimidazolium salts. Reagents and solvents: a: (CH3)3O+BF4−/CH2Cl2; b: (C2H5)3O+PF6−/CH2Cl2; c: Li+Tf2N−/MeOH; d: Br2/CH2Cl2.
The role of fluorine, which exhibits the lowest polarizability and highest electronegativity of the halogens, in crystal structures of organic compounds has been reviewed [27]. After some controversy, it has been concluded that short C–H...F contacts between oppositely charged molecules are genuine interionic hydrogen bonds [28]. Non-Linear allylic C–H...F interactions [28] were observed in compound 8. Short interactions between covalent halogen atoms (Br...F and Br...Br) [21] were observed in compounds 3–9. Classic H...Br− and Br...Br− bonds were found in the bromide 9. Similar Br...Br− interactions have been observed in the structures of 2-bromoimidazolium bromides [16,18,29,30,31], a related Br...F contact in a 2-bromoimidazolium hexafluorophosphate [30], and a typical Br...Br interaction for example in pentabromoferrocene [32].

Table 1.
Crystal data and structure refinement details.
| Compound | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|
| CCDC no. | 881953 | 881952 | 881955 | 881951 | 881954 | 881956 | 881957 | 881958 |
| Chemical formula | C6H3Br3N2 | C7H8Br3N2·BF4 | C7H6Br3N2·BF4 | C8H10Br3N2·F6P | C8H8Br3N2·F6P | C7H6Br3N2·C2F6NO4S2 | C8H8Br5N2·F6P | C8H8Br5N2·Br |
| Mr | 342.83 | 446.69 | 444.68 | 518.88 | 516.86 | 638.02 | 676.68 | 611.62 |
| Crystal size/mm3 | 0.48 × 0.08 × 0.08 | 0.28 × 0.24 × 0.20 | 0.27 × 0.24 × 0.17 | 0.32 × 0.20 × 0.02 | 0.16 × 0.12 × 0.01 | 0.24 × 0.24 × 0.02 | 0.28 × 0.20 × 0.08 | 0.32 × 0.08 × 0.08 |
| Crystal system | Orthorhombic | Tetragonal | Tetragonal | Monoclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | Pbc21 | I41/a | I41/a | P21/n | P 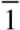 | P 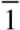 | P 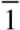 | P 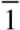 |
| a/Å | 7.1109(2) | 23.6138(3) | 23.4674(4) | 7.5434(3) | 8.2368(5) | 7.5438(4) | 7.6729(4) | 7.3200(7) |
| b/Å | 7.8014(2) | 23.6138(3) | 23.4674(4) | 15.5493(7) | 13.4522(8) | 8.6311(5) | 7.8858(5) | 11.5867(12) |
| c/Å | 15.6397(5) | 18.8371(4) | 18.6531(4) | 12.7552(4) | 13.6605(6) | 15.9716(7) | 15.8383(7) | 18.1972(16) |
| α/° | 90 | 90 | 90 | 90 | 92.476(4) | 75.020(4) | 90.001(4) | 87.611(8) |
| β/° | 90 | 90 | 90 | 90.198(3) | 100.965(4) | 87.083(4) | 88.104(4) | 81.121(8) |
| γ/° | 90 | 90 | 90 | 90 | 92.884(5) | 66.580(5) | 62.169(8) | 86.375(8) |
| V/Å3 | 867.61(4) | 10503.8(3) | 10272.6(3) | 1496.11(10) | 1481.96(14) | 920.25(8) | 846.88(9) | 1521.0(3) |
| Z | 4 | 32 | 32 | 4 | 4 | 2 | 2 | 4 |
| Dx/g·cm−3 | 2.63 | 2.26 | 2.30 | 2.30 | 2.32 | 2.30 | 2.65 | 2.67 |
| μ/mm−1 | 13.88 | 9.24 | 9.45 | 8.25 | 11.70 | 6.88 | 12.01 | 15.81 |
| F(000)/e | 632 | 6720 | 6656 | 984 | 976 | 608 | 628 | 1120 |
| sin θmax/λ | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.67 | 0.60 |
| h, k, l range | −8 ≤ h ≤ 8 | −28 ≤ h ≤ 26 | −28 ≤ h ≤ 24 | −9 ≤ h ≤ 8 | −9 ≤ h ≤ 9 | −9 ≤ h ≤ 6 | −9 ≤ h ≤ 10 | −8 ≤ h ≤ 8 |
| −9 ≤ k ≤ 9 | −28 ≤ k ≤ 28 | −28 ≤ k ≤ 23 | −18 ≤ k ≤ 15 | −16 ≤ k ≤ 15 | −10 ≤ k ≤ 9 | −10 ≤ k ≤ 10 | −12 ≤ k ≤ 13 | |
| −18 ≤ l ≤ 15 | −14 ≤ l ≤ 22 | −22 ≤ l ≤ 21 | −15 ≤ l ≤ 15 | −16 ≤ l ≤ 12 | −19 ≤ l ≤ 19 | −20 ≤ l ≤ 14 | −21 ≤ l ≤ 18 | |
| Absorption correction | Multi-scan | Multi-scan | Analytical | Multi-scan | Multi-scan | Gaussian | Multi-scan | Multi-scan |
| Measured reflections | 4789 | 32072 | 31973 | 9203 | 16144 | 5550 | 5619 | 12598 |
| Independent reflections | 1276 | 4811 | 4684 | 2715 | 5289 | 3329 | 3506 | 5551 |
| ( Rint) | (0.031) | (0.052) | (0.068) | (0.066) | (0.038) | (0.040) | (0.023) | (0.052) |
| Observed reflections | 1243 | 3786 | 3838 | 2078 | 4748 | 2302 | 2920 | 4184 |
| [ I ≥ 2 σ ( I )] | ||||||||
| Restraints/parameters | 1/104 | 10/315 | 14/315 | 15/188 | 0/363 | 1/243 | 7/204 | 0/291 |
| R1/wR2 [I ≥ 2σ(I)] | 0.019/0.042 | 0.043/0.097 | 0.044, 0.103 | 0.055/0.121 | 0.039/0.102 | 0.039/0.091 | 0.043/0.102 | 0.040/0.062 |
| R1/wR2 (all data) | 0.021/0.043 | 0.060/0.105 | 0.057, 0.110 | 0.0810.138 | 0.043/0.106 | 0.061/0.095 | 0.057/0.110 | 0.065/0.071 |
| Goodness of fit | 1.09 | 1.04 | 1.07 | 1.04 | 1.03 | 0.94 | 1.07 | 1.00 |
| ∆ ρmax/min/e Å−3 | 0.53/−0.48 | 1.59/−0.76 | 1.96/−1.03 | 1.26/−0.88 | 1.82/−0.79 | 1.01/−0.88 | 1.73/−1.49 | 0.86/−0.77 |

Table 2.
Halogen bonding and hydrogen bonding in 2–9.
| Compound | Interaction | Distance (d/Å) | d − ∑rvdW (Å) | Angle (°) | Symmetry Operator |
|---|---|---|---|---|---|
| 2 | C–Br3...N2 | 3.089(4) | −0.311 | 159.8(2) | 1 + x, y, z |
| 3 | C–Br11...F5 | 2.869(5) | −0.451 | 166.1(2) | x, y, z |
| C–Br31...F7 | 2.869(4) | −0.451 | 168.9(2) | −1/2 + x, y, 1/2 − z | |
| C–Br21...F4 | 2.906(5) | −0.414 | 179.5(2) | 3/4 + y, 3/4 − x, −1/4 + z | |
| C–Br12...F3 | 3.041(6) | −0.279 | 158.1(2) | x, y, z | |
| C–Br22...F8 | 3.090(5) | −0.230 | 172.2(2) | 5/4 + y, 5/4 − x, 1/4 + z | |
| C–Br32...F3 | 3.137(6) | −0.183 | 147.4(2) | 5/4 + y, 5/4 − x, 1/4 + z | |
| 4 | C–Br22...F2 | 2.822(5) | −0.498 | 167.8(2) | 1/4 + y, 1/4 − x, 1/4 + z |
| C–Br12...F5 | 2.826(6) | −0.494 | 166.1(2) | −1/2 + x, y, 3/2 − z | |
| C–Br32...F6 | 2.944(5) | −0.376 | 165.3(2) | x, y, z | |
| C–Br11...F3 | 2.972(7) | −0.348 | 161.7(2) | 1 − x, −y, 1 − z | |
| C–Br21...F7 | 3.094(5) | −0.226 | 167.2(2) | 1/4 − y, −1/4 + x, −1/4 + z | |
| C–Br32...F7 | 3.141(7) | −0.179 | 151.6(2) | x, y, z | |
| C–Br31...F3 | 3.244(7) | −0.076 | 152.2(2) | 1/4 + y, 3/4 − x, 3/4 − z | |
| 5 | C–Br2...F3A | 2.927(10) | −0.393 | 161.5(4) | 1 − x, −y, 2 − z |
| C–Br3...F2 | 2.989(5) | −0.331 | 160.0(3) | 3/2 + x, 1/2 − y, 1/2 + z | |
| 6 | C–Br21...F13 | 3.010(3) | −0.310 | 160.9(1) | 1 − x, 1 − y, 1 − z |
| C–Br31...F22 | 3.060(4) | −0.260 | 161.1(2) | x, y, z | |
| C–Br22...F14 | 3.084(3) | −0.236 | 158.9(1) | x, y, z | |
| C–Br11...F23 | 3.157(5) | −0.163 | 154.9(2) | x, y, 1 + z | |
| C–Br12...F21 | 3.209(6) | −0.111 | 127.0(2) | 1 − x, −y, −z | |
| C–Br32...F15 | 3.255(4) | −0.065 | 171.0(1) | 1 − x, 1 − y, 1 − z | |
| 7 | C–Br3...O3 | 2.943(4) | −0.427 | 167.1(2) | −1 + x, 1 + y, z |
| C–Br2...O3 | 3.201(3) | −0.169 | 152.9(2) | 1 − x, 1 − y, 1 − z | |
| C–H6...O1 | 2.32(5) | −0.402 | 160(5) | −x, 1 − y, 2 − z | |
| C–F3...F3 | 2.700(7) | −0.240 | 163.7(4) | 1 − x, 1 − y, 2 − z | |
| 8 | C–Br3...F2 | 3.017(6) | −0.303 | 173.6(2) | x, 1 + y, z |
| C–Br5...Br5 | 3.517(1) | −0.183 | 143.6(2) | 3 − x, −y, 2 − z | |
| C–Br2...Br2 | 3.575(1) | −0.125 | 129.6(2) | 2 − x, 1 − y, 1 − z | |
| C–H6...F6 | 2.38(3) | −0.290 | 164(4) | x, y, z | |
| C–H6...F2 | 2.50(6) | −0.174 | 140(4) | x, y, z | |
| 9 | C–Br1...Br6 | 3.032(1) | −0.668 | 177.7(2) | 1 − x, −y, −z |
| C–Br7...Br12 | 3.092(1) | −0.608 | 177.6(2) | 1 − x, 1 − y, 1 − z | |
| C–Br8...Br6 | 3.201(1) | −0.499 | 176.6(2) | 1 + x, y, z | |
| C–Br9...Br12 | 3.230(1) | −0.470 | 175.2(2) | 1 − x, −y, 1 − z | |
| C–Br3...Br12 | 3.291(1) | −0.409 | 175.6(2) | x, y, z | |
| C–Br4...Br4 | 3.474(1) | −0.226 | 152.9(2) | 2 − x, −1 − y, −z | |
| C–H8...Br6 | 2.756(1) | −0.294 | 148.3(4) | x, y, z | |
| C–H16...Br12 | 2.812(1) | −0.238 | 155.1(4) | x, y, z |
Lately, attractive anion...π interactions have been recognized as supramolecular forces between anions and electron-deficient heterocyclic ring system [33,34]. We indeed observed interactions of the tetrafluoroborate, hexafluorophosphate, and triflimide anions with the tribromoimidazolium system. The cut-off for anion...π interactions has not been clearly established [33]. We judged such an interaction as existing when at least three interactions with the atoms of a five-membered ring shorter than the sum of van der Waals (vdW) radii were found, as in compounds 3–5 and 7 (Table 3).

Table 3.
Anion...π interactions in 3, 4, 5 and 7.
| Compound | Interaction | Distance (d/Å) | d − ∑rvdW (Å) | Symmetry Operator |
|---|---|---|---|---|
| 3 | C11...F5 | 2.900(7) | −0.270 | 3/4 + y, 5/4 − x, 1/4 − z |
| C21...F5 | 3.084(7) | −0.086 | 3/4 + y, 5/4 − x, 1/4 − z | |
| N21...F5 | 2.935(6) | −0.085 | 3/4 + y, 5/4 − x, 1/4 − z | |
| C31...F5 | 3.137(7) | −0.033 | 3/4 + y, 5/4 − x, 1/4 − z | |
| N11...F5 | 3.025(7) | +0.005 | 3/4 + y, 5/4 − x, 1/4 − z | |
| centroid...F5 | 2.790 | |||
| 4 | C32...F5 | 3.162(9) | −0.008 | 1/4 − y, −1/4 + x, −1/4 + z |
| N22...F5 | 2.957(7) | −0.063 | 1/4 − y, −1/4 + x, −1/4 + z | |
| C12...F5 | 2.940(8) | −0.230 | 1/4 − y, −1/4 + x, −1/4 + z | |
| C22...F5 | 3.109(8) | −0.061 | 1/4 − y, −1/4 + x, −1/4 + z | |
| N12...F5 | 3.073(8) | +0.053 | 1/4 − y, −1/4 + x, −1/4 + z | |
| centroid...F5 | 2.824 | |||
| 5 | C3...F4B | 3.10(1) | –0.068 | 1/2 + x, 1/2 − y, 1/2 + z |
| C1...F4B | 3.11(1) | –0.055 | 1/2 + x, 1/2 − y, 1/2 + z | |
| C2...F4B | 3.13(1) | –0.044 | 1/2 + x, 1/2 − y, 1/2 + z | |
| N1...F4B | 3.10(1) | +0.080 | 1/2 + x, 1/2 − y, 1/2 + z | |
| N2...F4B | 3.16(1) | +0.140 | 1/2 + x, 1/2 − y, 1/2 + z | |
| centroid...F4B | 2.901 | |||
| 7 | C3...O2 | 2.901(6) | −0.319 | x, y, z |
| C2...O2 | 3.054(7) | −0.166 | x, y, z | |
| N1...O2 | 3.041(7) | −0.029 | x, y, z | |
| C1...O2 | 3.255(8) | +0.035 | x, y, z | |
| N2...O2 | 3.278(7) | +0.208 | x, y, z | |
| centroid...O2 | 2.887 |

Table 4.
Percent contributions of selected interactions relative to the whole Hirshfeld surface. Independent cations are termed A and B.
| Interaction | Compound | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3A | 3B | 4A | 4B | 5 | 6A | 6B | 7 | 8 | 9A | 9B | |
| Br...Br | 12.0 | 9.4 | 9.5 | 11.1 | 10.1 | 7.3 | 5.8 | 5.2 | 5.6 | 19.0 | 22.4 | 22.7 |
| Br...H/H...Br | 36.3 | 24.4 | 27.0 | 23.1 | 21.8 | 22.3 | 20.5 | 18.5 | 13.5 | 29.2 | 54.7 | 55.4 |
| Br...C/C...Br | 18.8 | 1.6 | 0.9 | 1.8 | 4.2 | 1.1 | 3.2 | 3.5 | 4.0 | 5.9 | 8.9 | 6.9 |
| Br...N/N...Br | 10.5 | 0.2 | 0.2 | 0.2 | 0.4 | 0 | 0 | 0 | 0.2 | 0 | 1.3 | 1.3 |
| Br...O | - | - | - | - | - | - | - | - | 8.9 | - | - | - |
| Br...F | - | 13.3 | 13.2 | 16.5 | 13.7 | 13.8 | 17.5 | 15.8 | 14.4 | 13.7 | - | - |
| C...H/H...C | 12.9 | 4.0 | 5.1 | 16.6 | 9.9 | 1.7 | 15.2 | 12.4 | 0.7 | 0 | 3.2 | 4.5 |
| C...O | - | - | - | - | - | - | - | - | 6.3 | - | - | - |
| C...F | - | 4.9 | 4.5 | 7.6 | 9.2 | 5.1 | 5.3 | 5.8 | 6.8 | 4.4 | - | - |
| H...F | - | 20.9 | 16.8 | 7.6 | 16.3 | 26.6 | 18.7 | 24.7 | 17.2 | 19.6 | - | - |
| H...H | 1.1 | 19.1 | 20.6 | 5.5 | 11.8 | 20.4 | 11.4 | 11.7 | 4.2 | 6.5 | 9.6 | 9.1 |
| H...O | - | - | - | - | - | - | - | - | 11.9 | - | - | - |
A few imidazolium tetrafluoroborates which also exhibit this type of interaction with short F...centroid distances have been located in the CSD [35,36,37], although not described as such by the respective authors. Likewise, examples of PF6...π [38,39,40] and S=O...π [30,41,42] interactions in imidazolium hexafluorophosphates, triflimides, and triflates should be mentioned.
Commonly accepted van der Waals radii [43,44] were used in this work, although the radii of anionic species may be considered to be larger [33]. Finally, Hirshfeld surface analysis [45,46,47,48] was performed to obtain quantitative insight into the cation–anion interactions (Table 4).
2.1. 2,4,5-Tribromo-1-(prop-2-ynyl)imidazole (2)
The imidazole molecules are arranged in chains in the [1 0 0] direction by short Br...N interactions, as shown in Figure 2. Distance and angle of this directed interaction are in the typical range. Being the only neutral molecule in this study, electrostatic N...H interactions are found by Hirshfeld surface analysis, whereas the ionic compounds show zero interactions of this kind. On the other hand, H...H interactions are negligible in 2 compared to the salt structures 3–9.
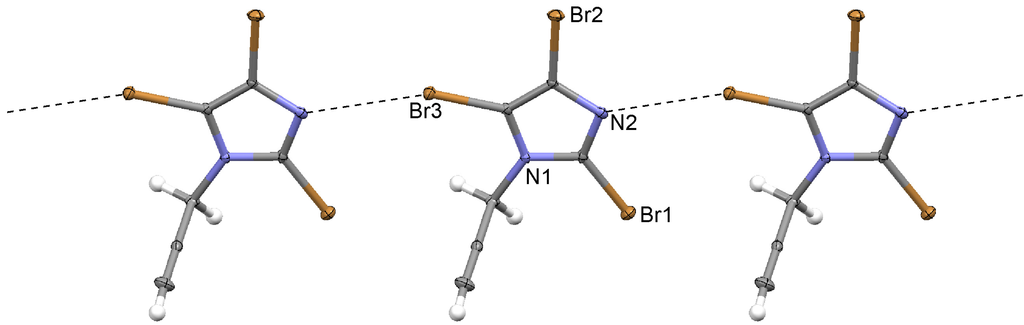
Figure 2.
Chains of molecules of 2 in the direction of the crystallographic a axis.
2.2. 2,4,5-Tribromo-3-methyl-1-(prop-2-enyl)imidazolium Tetrafluoroborate (3)
Two independent ion pairs are found in the asymmetric unit. Short Br...F contacts are clearly detected between the tribromoimidazolium cations and the tetrafluoroborate anions (Figure 3). The C–Br...F angles are nearly linear, exceptions are probably due to packing effects. There are indisputable interactions of the anion with the aromatic π system observed, with a short anion...centroid distance. Evidently, the anion is located on top of the aromatic ring, fixed by four contacts shorter than the sum of van der Waals radii (Table 3). These weak interactions can be overridden by packing effects [49], and it has been remarked that it is still not possible to predict anion...π interactions [49]. Thus, it is not surprising that the π-system of the second cation does not exhibit perceptible interactions with an anion.
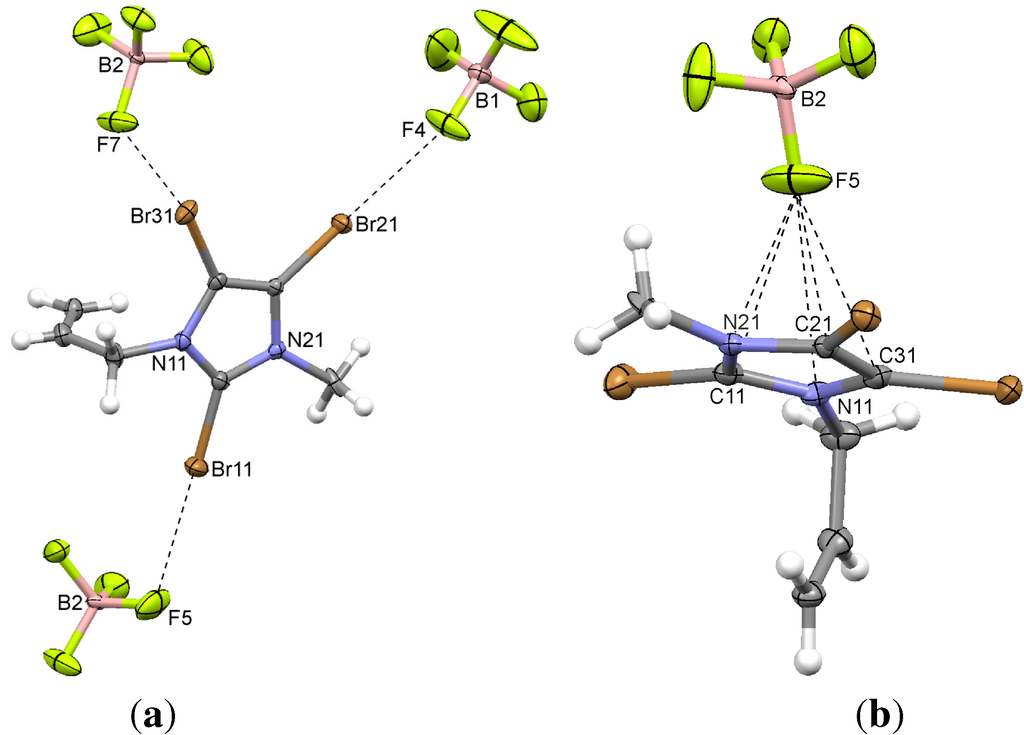
Figure 3.
(a) Typical Br...F interactions; and (b) the anion...πinteractions in the propenyl imidazolium tetrafluoroborate 3. Only one of the two independent cations is shown. The other one exhibits very similar Br...F contacts.
2.3. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Tetrafluoroborate (4)
The unit cell is very similar to that of the previous compound 3. Again, two ion pairs are in the asymmetric unit. The Br...F contacts, with angles between 152 and 168° (Table 2) are slightly different, one bromine atom exhibiting a bifurcated Br...F interaction (Figure 4a). The π-system of one imidazolium cation is involved in four short contacts with a tetrafluoroborate anion (Table 3). In contrast to 3, a surprisingly high contribution of C...H interactions is revealed by Hirshfeld surface analysis which, everything else being equal, can only be attributed to the propynyl substituent.
Figure 4.
(a) Typical Br...F interactions; and (b) the anion...π interactions in the propenyl imidazolium tetrafluoroborate 4. Only one of the two independent cations is shown. The other one exhibits very similar Br...F contacts.
2.4. 2,4,5-Tribromo-3-ethyl-1-(prop-2-enyl)imidazolium Hexafluorophosphate (5)
In this structure, the two alkyl substituents of the imidazolium cation adopt an anti conformation. The pronounced prolate shape of the thermal ellipsoids of some of the fluorine atoms of the PF6 anion indicated the presence of a disorder that was modeled by introducing split positions (A and B with occupancy factors of 0.50) for the F1, F3 and F4 atoms, which were refined isotropically. Since no temperature dependent measurements were performed the question remains whether the disorder is static or dynamic. Two short Br...F contacts with C–Br...F angles around 160° are observed. A skewed anion...π interaction (Figure 5a) is found with a short F...centroid distance (Table 3). Obviously, the anion is not located exactly over the center of the ring, only three contacts shorter than the sum of van der Waals radii are found in this case. The anion is interacting predominantly with the carbon atoms of the heterocyclic ring system.
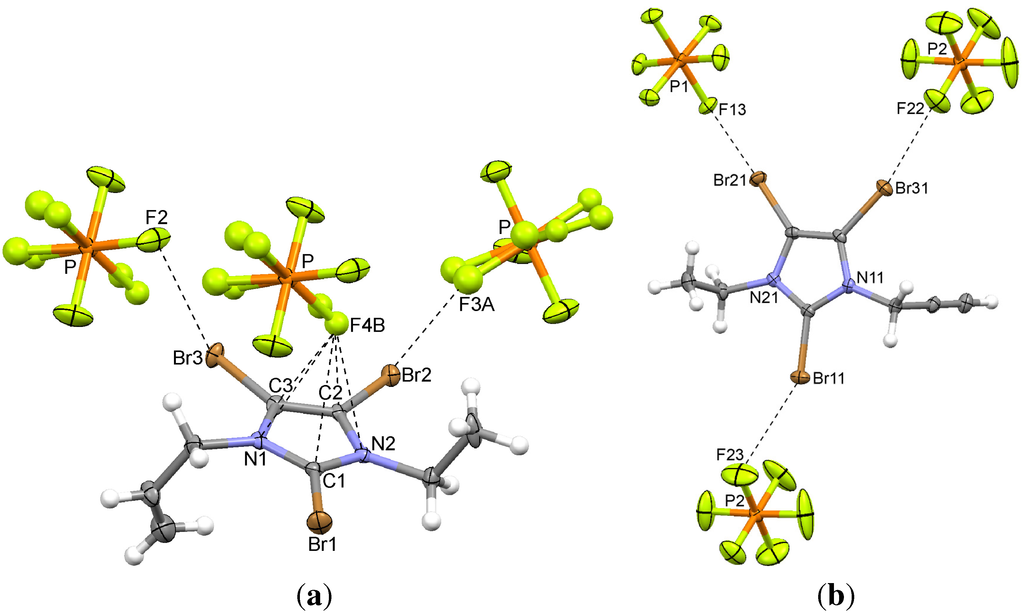
Figure 5.
Interionic interactions in the hexafluorophosphates of (a) the propenyl imidazolium salt 5 (Br...F and anion...π) and (b) the propynyl imidazolium salt 6 (Br...F). Only one of the two independent cations in 6 is shown.
2.5. 2,4,5-Tribromo-3-ethyl-1-(prop-2-ynyl)imidazolium Hexafluorophosphate (6)
Two ion pairs are located in the asymmetric unit. Again, the two alkyl substituents adopt the anti conformation. In this instance, no anion...π interaction is detected, but the typical Br...F contacts (Figure 5b) are found in both ion pairs. The C–Br...F angles range from 155 to 171° (Table 2) which is the typical angular preference [28]. One interaction (C–Br12...F21) exhibits a suspiciously small angle. However, this contact is three percent shorter than the sum of van der Waals radii and cannot be ignored. Once again, a surprisingly high contribution of C...H interactions is revealed by Hirshfeld surface analysis.
2.6. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Triflimide (7)
The triflimide ion adopts the anti conformation (C–S...S–C torsion angle of 170.6°); this conformation is preferred in metal-free triflimide structures [50]. The angle C–C≡C is found to be 175.8°. A “classic” Csp–H...O hydrogen bond between H6 and O1 is observed, thus forming a cyclic dimer (Figure 6). Interestingly, in this structure no Br...Br or Br...F interactions are found, but Br...O contacts to the sulfonyl group, as noticed earlier in related structures [17,51,52], and a F...F contact across a symmetry center. However, an anion...π interaction is observed. This time one partially negatively charged oxygen atom of a sulfonyl group is involved in three contacts shorter than the sum of van der Waals radii. Again, the interacting atom of the anion is shifted from the center of the π system and is located over the rim of the heterocycle, exhibiting short contacts with N1, C2, and C3 of the imidazolium ring.
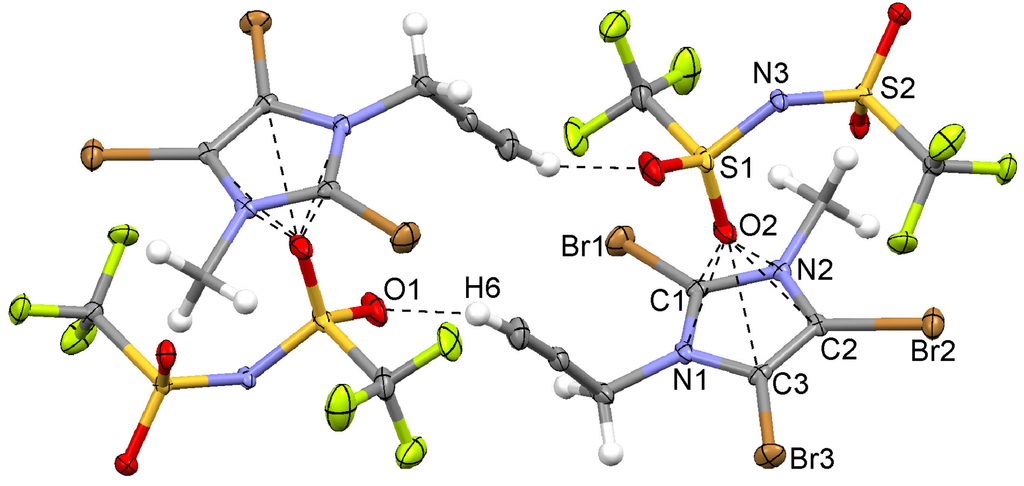
Figure 6.
Classic C–H...O hydrogen bonds forming a cyclic dimer and anion...π interaction in the propynyl imidazolium triflimide 7.
2.7. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Hexafluorophosphate (8)
As before, there are almost linear Br...F contacts between the ions. The structure also exhibits Br2...Br2 interactions in the ring plane, forming layers which are connected by Br5...Br5 contacts to give a three-dimensional network (Figure 7). The Br...Br contacts are both across a symmetry center, deviate from linearity due to packing (Table 2), and are also found prominently in the Hirshfeld surface analysis (Table 4). The allylic C–H...F interactions are also detected in the Hirshfeld analysis.
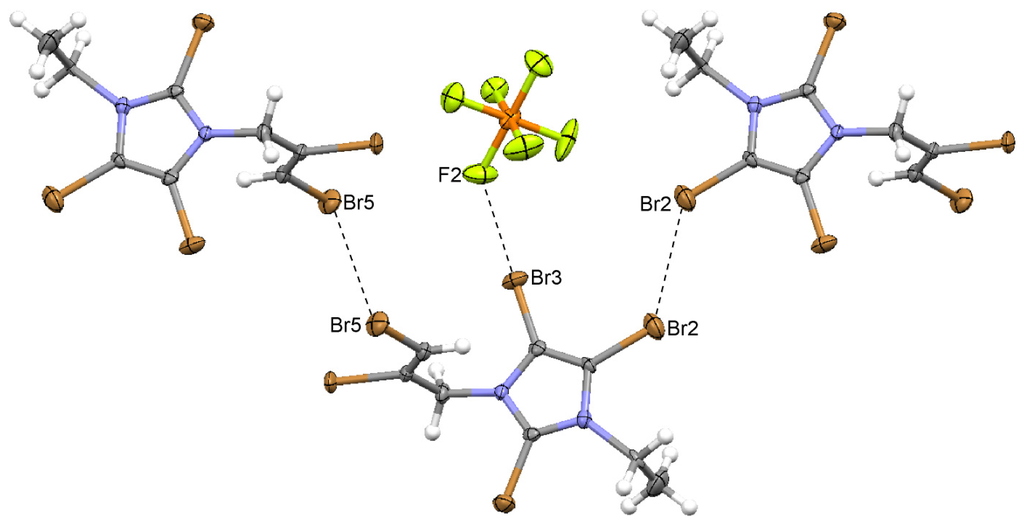
Figure 7.
The halogen...halogen interactions in 2,4,5-tribromo-1-(2,3-dibromoprop-2-enyl)-imidazolium hexafluorophosphate (8).
2.8. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Bromide (9)
Two independent ion pairs are located in the asymmetric unit. The presence of the brominated side chain results in an increased number of Br...Br contacts such as short Br4...Br4 and weak Br1...Br1 and Br9...Br9 contacts across a symmetry center. Strong bromine...bromide contacts exist in the crystal which are significantly shorter than the sum of van der Waals radii, even without taking into account the fact that bromide has a larger van der Waals radius than covalently bound bromine [33]. The C–Br...Br− angles are close to 180°, indicating linearity of the X-bonds. The coordination around the two independent bromide ions is shown in Figure 8. It is not unexpected that, due to the brominated side chain, contacts involving Br atoms are quite dominant in the Hirshfeld surface analysis. According to this analysis, C–H...Br contacts by far represent the majority of interactions, obviously due to the relatively strong Csp2–H...Br− interactions, followed by Br...Br contacts. Some examples of similar situations are found in the Cambridge Structural Database [53,54,55,56].
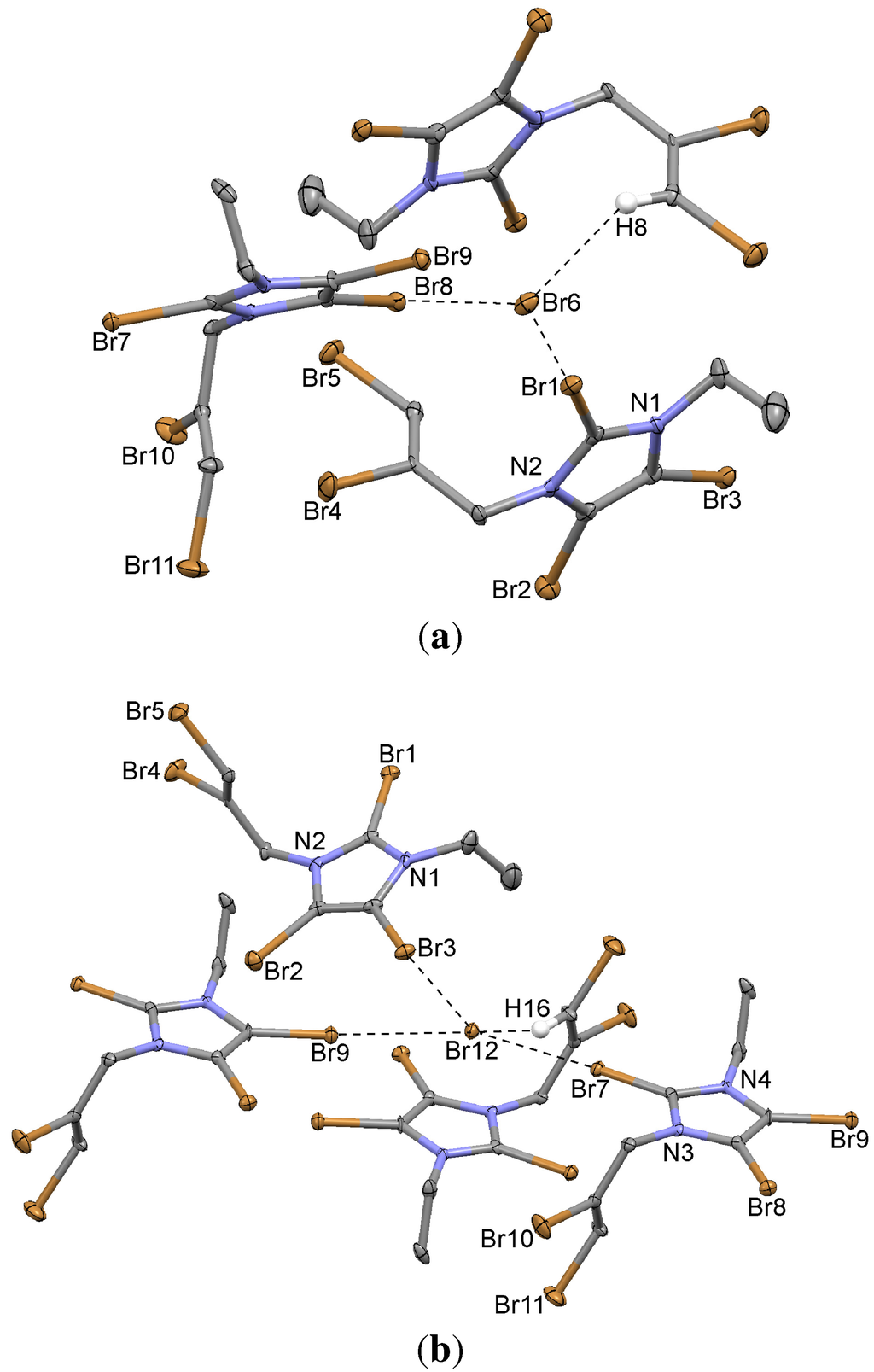
Figure 8.
Bromine...Bromide and hydrogen...bromide coordination of the two independent bromide ions (a) Br6 and (b) Br12 in the structure of 2,4,5-tribromo-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium bromide (9).
3. Experimental Section
Chemicals and solvents were purchased from Sigma-Aldrich and used as received. 2,4,5-Tribromo-1-(prop-2-enyl)imidazole (1) [CARN 31255-41-1] and 2,4,5-tribromo-1-(prop-2-ynyl)imidazole (2) [CARN 27442-77-9] were prepared according to published procedures [12,13,57].
3.1. Data of 2,4,5-Tribromo-1-(Prop-2-Ynyl)Imidazole (2)
m.p. 123 °C. IR (neat): ν 3288 (m), 2129 (w), 1494 (m), 1433 (m), 1410 (m), 1380 (m), 1335 (m), 1214 (m), 1192 (m), 1121 (m), 985 (m), 937 (m), 758 (m), 682 (s), 651 (s)·cm−1. 13C NMR (DMSO-d6, 75 MHz): δ 37.7, 76.4, 76.8, 105.9, 116.0, 118.9 ppm. Single crystals were obtained from MeOH.
3.2. General Procedure for the Alkylation of 1-Alkylimidazoles (3–6)
To a solution of trimethyloxonium tetrafluoroborate or triethyloxonium hexafluorophosphate (16 mmol) in anhydrous CH2Cl2 (5 mL) the respective 2,4,5-tribromo-1-alkylimidazole (1.0 eq.) was added and stirred overnight at room temperature. Et2O was added, and the resulting colorless solid was filtered and dried under reduced pressure. Single crystals were obtained from MeOH.
2,4,5-Tribromo-3-methyl-1-(prop-2-enyl)imidazolium tetrafluoroborate (3): m.p. 179 °C. IR (neat): ν 1553 (w), 1501 (w), 1049 (s), 1035 (s), 946 (w), 669 (w)·cm−1. 13C NMR (DMSO-d6, 75 MHz): δ 37.8, 52.4, 110.8, 112.5, 118.9, 124.5, 129.1 ppm.
2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium tetrafluoroborate (4): m.p. 203 °C. IR (neat): ν 2135 (w), 1554 (w), 1498 (w), 1050 (s), 1035 (s), 816 (w), 679 (w), 652 (w)·cm−1.
2,4,5-Tribromo-3-ethyl-1-(prop-2-enyl)imidazolium hexafluorophosphate (5): m.p. 228 °C (dec). IR (neat): ν 1496 (w), 1122 (w), 936 (w), 831 (s), 669 (w)·cm−1.
2,4,5-Tribromo-3-ethyl-1-(prop-2-ynyl)imidazolium hexafluorophosphate (6): m.p. 232 °C (dec). IR (neat): ν 2135 (w), 1559 (w), 1494 (w), 1118 (m), 1051 (s), 833 (s), 710 (s)·cm−1.
3.3. 2,4,5-Tribromo-3-methyl-1-(prop-2-ynyl)imidazolium Triflimide (7)
A solution of 2,4,5-tribromo-3-methyl-1-(prop-2-ynyl)imidazolium tetrafluoroborate (4) and lithium triflimide (1.05 eq.) in MeOH was stirred for 18 h. After evaporation of the solvent, the residual solid was suspended in H2O. The mixture was extracted twice with CH2Cl2. The organic phase was washed three times with H2O and dried under reduced pressure to give a colorless solid. m.p. 122 °C. IR (neat): ν 2135 (w), 1556 (w), 1500 (w), 1351 (s), 1333 (m), 1190 (s), 1178 (s), 1137 (s), 1116 (m), 1046 (s), 1033 (s), 959 (w), 819 (w), 794 (w), 743 (w), 694 (w), 613 (s)·cm−1. Single crystals were obtained from MeOH.
3.4. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Hexafluorophosphate (8)
Bromine (1.0 eq.) was added to an ice-cooled suspension of 6 in CH2Cl2. The mixture was stirred at room temperature for 18 h, and the colorless precipitate was filtered, washed with CH2Cl2, and dried under reduced pressure. m.p. 206 °C. IR (neat): ν 837 (s), 673 (w)·cm−1. Single crystals were obtained from MeOH.
3.5. 2,4,5-Tribromo-1-(2,3-dibromoprop-2-enyl)-3-ethylimidazolium Bromide (9)
Crystals of this minor byproduct were spotted in the major product 8.
3.6. X-ray Crystal Structure Determination
Intensity data were recorded with an Oxford Diffraction Gemini-R Ultra diffractometer using Mo-Kα radiation at T = 173(2) K. For 6, Cu-Kα radiation was used. Experimental details are summarized in Table 1. Structure solution and refinement was performed with the programs SIR2002 (direct methods) [58] and SHELXL-97 [59]. A Flack parameter of 0.03(2) was obtained for the non-centrosymmetric structure of 2. The crystals of 8 were subject to non-merohedral twinning which was taken into consideration during data reduction, integration and refinement. Visualization of the structures and measurements of distances and angles was performed with the program Mercury [60]. CCDC reference numbers: 881951–881958. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
4. Conclusions
The crystal structures of eight N-alkylated 2,4,5-tribromoimidazole compounds were determined. Halogen interactions and anion...π interactions were detected. Hirshfeld surface analysis (Figure 9) yielded quantitative information about these interactions. These results are supposed to further our understanding of halogen interactions in the solid state.
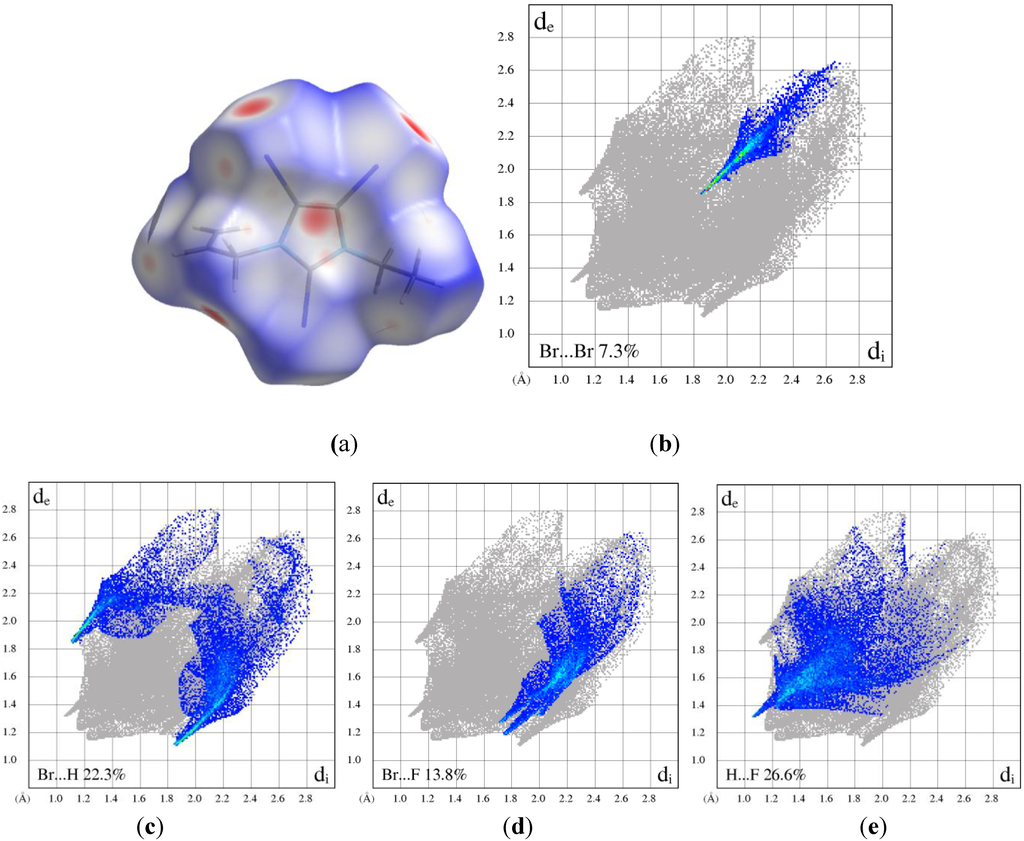
Figure 9.
(a) Normalized Hirshfeld surface of the cation in 5 and associated fingerprint plots highlighting specific interactions; (b) Br...Br; (c) Br...H (reciprocal interactions included); (d) Br...Fand (e) H...F. The full fingerprint appears as a grey shadow.
Acknowledgments
Financial support was provided by the Austrian government, the provinces of Lower Austria, Upper Austria and Carinthia as well as by the Lenzing AG.
References
- Wyss, G. Zur Kenntnis des Glyoxalins. Chem. Ber. 1877, 10, 1365–1375. [Google Scholar]
- Balaban, I.E.; Pyman, F.L. Bromo-Derivatives of Glyoxaline. J. Chem. Soc. Trans. 1922, 121, 947–958. [Google Scholar]
- Chaudhuri, M.K.; Khan, A.T.; Patel, B.K.; Dey, D.; Kharmawophlang, W.; Lakshmiprabha, T.R.; Mandal, G.C. An environmentally benign synthesis of organic ammonium tribromides (OATB) and bromination of selected organic substrates by tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 1998, 39, 8163–8166. [Google Scholar]
- Bahnous, M.; Mouats, C.; Fort, Y.; Gros, P.C. Convenient multi-gram scale synthesis of polybrominated imidazoles building blocks. Tetrahedron Lett. 2006, 47, 1949–1951. [Google Scholar]
- Muathen, H.A. 1,8-Diazabicyclo[5.4.0]undec-7-ene hydrobromide perbromide: A new mild stable brominating agent for aromatic compounds. J. Org. Chem. 1992, 57, 2740–2741. [Google Scholar] [CrossRef]
- Nath, J.; Chaudhuri, M.K. Boric acid catalyzed bromination of a variety of organic substrates: An eco-friendly and practical protocol. Green Chem. Lett. Rev. 2008, 1, 223–230. [Google Scholar] [CrossRef]
- Stensiö, K.-E.; Wahlberg, K.; Wahren, R. Synthesis of brominated imidazoles. Acta Chem. Scand. 1973, 27, 2179–2183. [Google Scholar]
- Benkendorff, K.; Pillai, R.; Bremner, J.B. 2,4,5-Tribromo-1H-imidazole in the egg masses of three muricid molluscs. Nat. Prod. Res. 2004, 18, 427–431. [Google Scholar]
- Schulze, H.; Klein, H.P. Fire-retardant polyurethane composition. US Patent 4,032,484, 28 June 1977. [Google Scholar]
- Ye, C.; Shreeve, J.M. Syntheses of very dense halogenated liquids. J. Org. Chem. 2004, 69, 6511–6513. [Google Scholar]
- Yano, T.; Tomioka, H.; Takada, Y.; Takeda, H.; Hirata, N. New trihaloimidazole derivatives possessing a high insecticidal activity against the pyrethroid-resistant colony of the German cockroach, Blattella germanica (Dictyoptera: Blattellidae). Appl. Ent. Zool. 1991, 26, 469–475. [Google Scholar]
- Martin, H.; Pissiotas, G. Ectoparasiticidal 1-alkynyl-2,4,5-trihaloimidazoles. German Patent DE 1950991A.
- Pissiotas, G. Acaricidal 2,4,5-trihaloimidazoles. German Patent DE 2031400 A.
- Draber, W.; Falbe, J.F.; Buechel, K.H.; Korte, F.W.A.G.K. Plant Growth Control. U.S. Patent 3,501,286, 17 March 1970. [Google Scholar]
- Noland, W.E.; Cole, K.P.; Britton, D. 4,5-Dibromo-1-methyl-1H-imidazole. Acta Crystallogr. 2003, E59, o458–o460. [Google Scholar]
- Mukai, T.; Nishikawa, K. Halogen-Bonded and hydrogen-bonded network structures in crystals of 1-propyl- and 1-butyl-4,5-dibromo-3-methylimidazolium bromides. Chem. Lett. 2009, 38, 402–403. [Google Scholar] [CrossRef]
- Mukai, T.; Nishikawa, K. Syntheses and crystal structures of two ionic liquids with halogen-bonding groups: 4,5-dibromo- and 4,5-diiodo-1-butyl-3-methylimidazolium trifluoromethane-sulfonates. Solid State Sci. 2010, 12, 783–788. [Google Scholar] [CrossRef]
- Serpell, C.J.; Kilah, N.L.; Costa, P.J.; Felix, V.; Beer, P.D. Halogen bond anion templated assembly of an imidazolium pseudorotaxane. Angew. Chem. Int. Ed. 2010, 49, 5322–5326. [Google Scholar]
- Blackman, A.G.; Buckingham, D.A.; Clark, C.R.; Simpson, J. Bromination of imidazole complexes of pentaammine-cobalt(III). Synthesis, structure and reactivity. J. Chem. Soc. Dalton Trans. 1991, 3031–3041. [Google Scholar]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar]
- Awwadi, F.F.; Willett, R.D.; Peterson, K.A.; Twamley, B. The nature of halogen...halogen synthons: Crystallographic and theoretical studies. Chem. Eur. J. 2006, 12, 8952–8960. [Google Scholar] [CrossRef]
- Dikundwar, A.G.; Guru Row, T.N. Evidence for the “amphoteric” nature of fluorine in halogen bonds: An instance of Cl...F contact. Cryst. Growth Des. 2012, 12, 1713–1716. [Google Scholar]
- Kubicki, M. Structural phase transitions, and Br...N and Br...Br interactions in 1-phenyl-2-methyl-4-nitro-5-bromoimidazole. Acta Crystallogr. 2004, B60, 333–342. [Google Scholar]
- Valkonen, J.; Pitkanen, I.; Pajunen, A. Molecular and crystal structure and IR spectrum of 3,5-Dibromo-1,2,4-triazole. Acta Chem. Scand. 1985, A39, 711–716. [Google Scholar]
- Ansell, G.B. Tetrazole studies. Part II. Crystal structure of 5-bromotetrazole. J. Chem. Soc. Perkin Trans. 2 1973, 2036–2038. [Google Scholar] [CrossRef]
- Eißmann, F.; Schindler, D.; Weber, E. X-ray crystal structures of halogen containing nucleobase derivatives in unsolvated and DMSO solvated forms. Struct. Chem. 2010, 21, 245–254. [Google Scholar] [CrossRef]
- Chopra, D.; Guru Row, T.N. Role of organic fluorine in crystal engineering. CrystEngComm. 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- D’Oria, E.; Novoa, J.J. On the hydrogen bond nature of the C-H...F interactions in molecular crystals. An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations. CrystEngComm 2008, 10, 423–436. [Google Scholar]
- Cole, M.L.; Jones, C.; Junk, P.C. Studies of the reactivity of N-heterocyclic carbenes with halogen and halide sources. New J. Chem. 2002, 26, 1296–1303. [Google Scholar] [CrossRef]
- Laus, G.; Schwärzler, A.; Schuster, P.; Bentivoglio, G.; Hummel, M.; Wurst, K.; Kahlenberg, V.; Lörting, T.; Schütz, J.; Peringer, P.; et al. N,N'-Di(alkyloxy)imidazolium salts: New patent-free ionic liquids and NHC precatalysts. Z. Naturforsch. 2007, B62, 295–308. [Google Scholar]
- Kuhn, N.; Abu-Rayyan, A.; Eichele, K.; Schwarz, S.; Steimann, M. Weak interionic interactions in 2-bromoimidazolium derivatives. Inorg. Chim. Acta 2004, 357, 1799–1804. [Google Scholar] [CrossRef]
- Sünkel, K.; Bernhartzeder, S. Coordination chemistry of perhalogenated cyclopentadienes and alkynes. XXVIII new high-yield synthesis of monobromoferrocene and simplified procedure for the synthesis of pentabromoferrocene. Molecular structures of 1,2,3-tribromoferrocene and 1,2,3,4,5-pentabromoferrocene. J. Organomet. Chem. 2011, 696, 1536–1540. [Google Scholar]
- Estarellas, C.; Bauza, A.; Frontera, A.; Quiñonero, D.; Deya, P.M. On the directionality of anion–π interactions. Phys. Chem. Chem. Phys. 2011, 13, 5696–5702. [Google Scholar]
- Robertazzi, A.; Krull, F.; Knapp, E.-W.; Gamez, P. Recent advances in anion-πinteractions. CrystEngComm 2011, 13, 3293–3300. [Google Scholar] [CrossRef]
- Pointer, D.J.; Wilford, J.B. Crystal and molecular structure of 3,3'-thio-bis-(2-methyl-1-phenylimidazo-[1,5-a]pyridinium) bistetrafluoroborate; a potent new sulphur-containing curariform agent. J. Chem. Soc. Chem. Commun. 1978, 816–817. [Google Scholar]
- Frank, B.; Beckert, R.; Rau, S.; Görls, H. 2-Azaanthraquinones: Building blocks for new ring-fused imidazoles and their transformation into benzo[f]isoindole-4,9-diones. Z. Naturforsch. 2005, B60, 771–779. [Google Scholar]
- Kunz, D.; Deißler, C.; Gierz, V.; Rominger, F.; Oeser, T. Reduction of dipyrido ureas via 6-alkyloxydipyrido[1,2-c;2',1'-e]imidazolium salts. Z. Naturforsch. 2010, B65, 861–872. [Google Scholar]
- Brown, D.H.; Skelton, B.W. Nickel complexes of a bis(benzimidazolin-2-ylidene)pyridine pincer ligand with four- and five-coordinate geometries. Dalton Trans. 2011, 40, 8849–8858. [Google Scholar] [CrossRef]
- Laus, G.; Wurst, K.; Kahlenberg, V.; Kopacka, H.; Kreutz, C.; Schottenberger, H. N-Heterocyclic carbene (NHC) derivatives of 1,3-di(benzyloxy)imidazolium salts. Z. Naturforsch. 2010, B65, 776–782. [Google Scholar]
- Scheele, U.J.; Dechert, S.; Meyer, F. Bridged dinucleating N-heterocyclic carbene ligands and their double helical mercury(II) complexes. Inorg. Chim. Acta 2006, 359, 4891–4900. [Google Scholar] [CrossRef]
- Weigand, J.J.; Feldmann, K.-O.; Henne, F.D. arbene-Stabilized phosphorus(III)-centered cations [LPX2]+ and [L2PX]2+ (L = NHC; X = Cl, CN, N3). J. Am. Chem. Soc. 2010, 132, 16321–16323. [Google Scholar]
- Maas, G.; Stang, P.J. Structures of dication ethers. Crystal and molecular structures of bis(1,3-dimethyl-2-imidazoliniumyl) ether ditriflate and bis[1,2-bis(dimethylamino)-3-cyclopropenyl-iumyl] ether ditriflate. J. Org. Chem. 1983, 48, 3038–3043. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic potentials mapped on Hirshfeld surfaces provide direct insight into intermolecular interactions in crystals. Cryst. Eng. Comm. 2008, 10, 377–388. [Google Scholar]
- Dean, P.M.; Pringle, J.M.; Forsyth, C.M.; Scott, J.L.; MacFarlane, D.R. Interactions in bisamide ionic liquids—insights from a Hirshfeld surface analysis of their crystalline states. New J. Chem. 2008, 32, 2121–2126. [Google Scholar] [CrossRef]
- Albrecht, M.; Müller, M.; Mergel, O.; Rissanen, K.; Valkonen, A. CH-Directed anion–π interactions in the crystals of pentafluorobenzyl-substituted ammonium and pyridinium salts. Chem. Eur. J. 2010, 16, 5062–5069. [Google Scholar] [CrossRef]
- Laus, G.; Hummel, M.; Többens, D.M.; Gelbrich, T.; Kahlenberg, V.; Wurst, K.; Griesser, U.J.; Schottenberger, H. The 1:1 and 1:2 salts of 1,4-diazabicyclo[2.2.2]octane and bis(trifluoro-methylsulfonyl)amine: Thermal behaviour and polymorphism. CrystEngComm 2011, 13, 5439–5446. [Google Scholar] [CrossRef]
- Lin, B.-L.; Kang, P.; Stack, T.D.P. Unexpected Ccarbene-X (X: I, Br, Cl) reductive elimination from N-heterocyclic carbene copper halide complexes under oxidative conditions. Organometallics 2010, 29, 3683–3685. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Sproll, S.M. Nitrogen-Rich polymers based on 5-bromo-1-vinyl-1H-tetrazole. Eur. J. Org. Chem. 2010, 1169–1175. [Google Scholar]
- Linninger, C.S.; Herdtweck, E.; Hoffmann, S.D.; Herrmann, W.A.; Kühn, F.E. A new palladium(II) complex of a functionalized N-heterocyclic carbene: Synthesis, characterization and application in Suzuki-Miyaura cross-coupling reactions. J. Mol. Struct. 2008, 890, 192–197. [Google Scholar] [CrossRef]
- Domasevitch, K.V.; Boldog, I. Polar hydrogen-bonded organic chains in 4,4'-bipyrazolium bromide and perchlorate monohydrates. Acta Cryst. 2005, C61, o373–o376. [Google Scholar]
- Peters, K.; Peters, E.M.; Reinhardt, R.; Quast, H. Crystal structure of (1R*,7S*,9S*,15R*)-2,6, 10,14-tetraazatricyclo[13.1.0.07,9]hexadeca-2,4,10,12-tetraene bis(hydrobromide). Z. Krist. New Cryst. Struct. 1998, 213, 609–610. [Google Scholar]
- Gelders, Y.G.; De Ranter, C.J.; Van Rooijen-Reiss, C. 7,8-Didehydro-4,5-epoxy-17-(2-propenyl)morphinan-3,6-diol hydrobromide [nalorphine hydrobromide]. Cryst. Struct. Commun. 1979, 8, 995–1003. [Google Scholar]
- Seley, K.L.; Januszczyk, P.; Hagos, A.; Zhang, L.; Dransfield, D.T. Synthesis and antitumor activity of thieno-separated tricyclic purines. J. Med. Chem. 2000, 43, 4877–4883. [Google Scholar] [CrossRef]
- Burla, M.C.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G. More power for direct methods: SIR2002. Z. Kristallogr. 2002, 217, 629–635. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).