Single Crystal Growth and Structural Study of the New MCu2Zn20 (M = Nb, Hf) Compounds
Abstract
:1. Introduction
2. Methods
2.1. Synthesis
2.2. Energy Dispersion Spectroscopy
2.3. X-Ray Diffraction
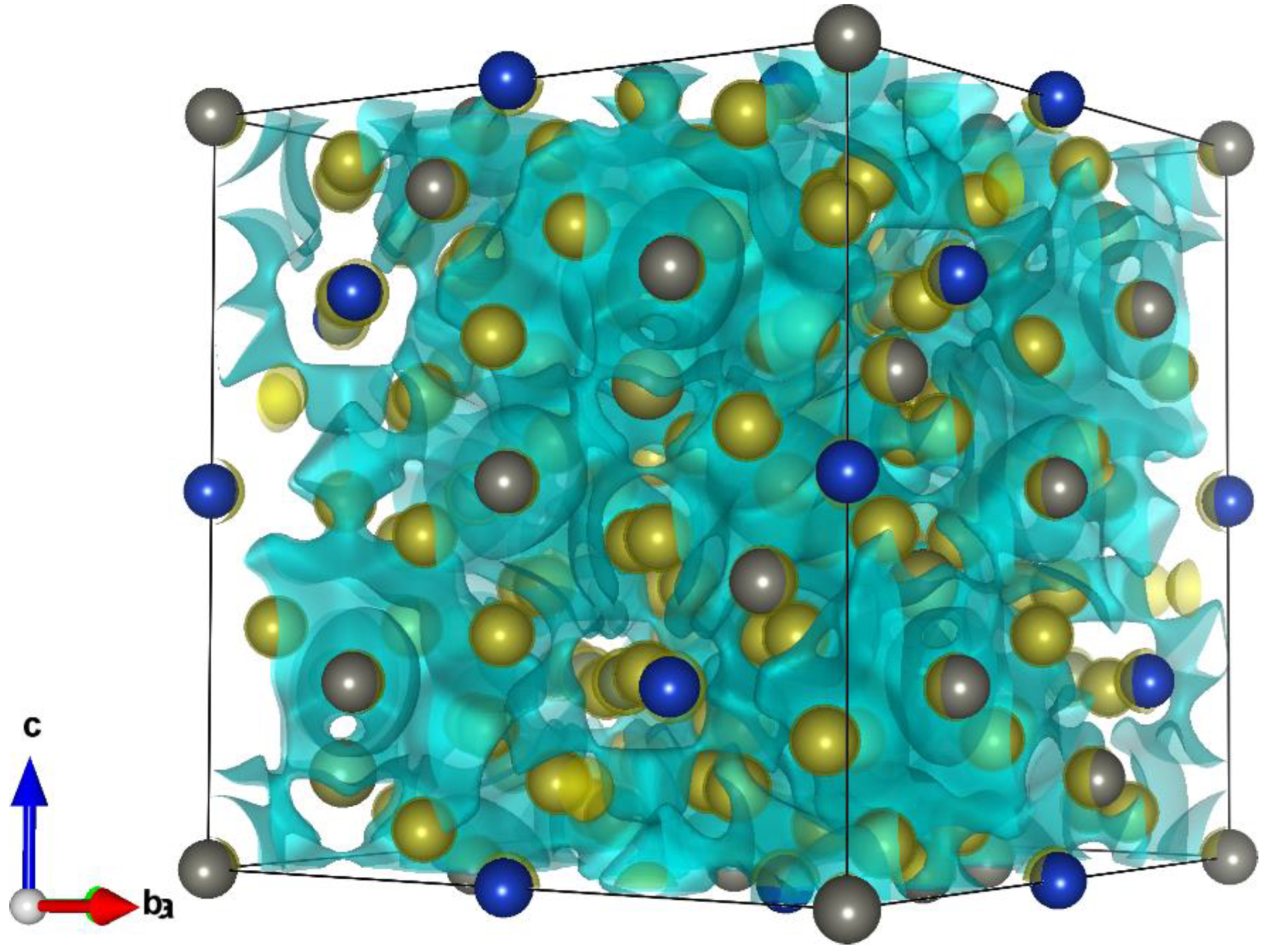
2.4. Density Functional Theory
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kripyakevich, P.I.; Zarechnyuk, O.S. RCr2Al20 Compounds in Systems of Rare Earth Metals and Calcium, and their Crystal Structures. Dopov. Akad. Nauk Ukr. RSR Ser. A 1968, 30, 364–367. [Google Scholar]
- Nasch, T.; Jeitschko, W.; Rodewald, U.C. Ternary Rare Earth Transition Metal Zinc Compounds RT2Zn20 with T = Fe, Ru, Co, Rh, and Ni. Z. Naturforsch. B 1997, 52, 1023–1030. [Google Scholar] [CrossRef]
- Gross, N.; Nasch, T.; Jeitschko, W. Ternary Intermetallics with High Zinc Content: TT′2Zn20 (T = Zr, Hf, Nb; T′ = Mn, Fe, Ru, Co, Rh, Ni) with CeCr2Al20-type Structure. J. Solid State Chem. 2001, 161, 288–293. [Google Scholar] [CrossRef]
- Niemann, S.; Jeitschko, W. Ternary Aluminides AT2Al20 (A = Rare Earth Elements and Uranium; T = Ti, Nb, Ta, Mo, and W) with CeCr2Al20-type Structure. J. Solid State Chem. 1995, 114, 337–341. [Google Scholar] [CrossRef]
- Canfield, P.C.; Jia, S.; Mun, E.D.; Bud’ko, S.L.; Samolyuk, G.D.; Torikachvili, M.S. Myriad of Correlated Electron Effects Found in the RT2Zn20 Family. Phys. B Condens. Matter 2008, 403, 844–846. [Google Scholar] [CrossRef]
- Thiede, V.M.T.; Jeitschko, W.; Niemann, S.; Ebel, T. EuTa2Al20, Ca6W4Al43 and Other Compounds with CeCr2Al20 and Ho6Mo4Al43 Type Structures and Some Magnetic Properties of These Compounds. J. Alloys Comp. 1998, 267, 23–31. [Google Scholar] [CrossRef]
- Pöttgen, R.; Janka, O. CeCr2Al20-type Intermetallics—Structure-property Relationships. Rev. Inorg. Chem. 2023, 43, 357–383. [Google Scholar] [CrossRef]
- Koldemir, A.; Klenner, S.; Pöttgen, R. Crystal Structure of Europium Dichromium Icosaaluminum, EuCr2Al20. Z. Für Kristallographie-New Cryst. Struct. 2023, 238, 507–509. [Google Scholar] [CrossRef]
- Yasmin, N.; Noor, M.F.B.; Besara, T. Structural Trends and Itinerant Magnetism of the New Cage-structured Compound HfMn2Zn20. J. Phys. Condens. Matter 2024, 36, 275801. [Google Scholar] [CrossRef]
- Zagorac, D.; Müller, H.; Ruehl, S.; Zagorac, J.; Rehme, S. Recent Developments in the Inorganic Crystal Structure Database: Theoretical Crystal Structure Data and Related Features. J. Appl. Crystallogr. 2019, 52, 918–925. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shimizu, T.; Yamaura, J.; Hiroi, Z. Crystal Chemistry and Magnetic Properties of Manganese Zinc Alloy “YMn2Zn20” Comprising a Mn Pyrochlore Lattice. J. Solid State Chem. 2012, 191, 246–256. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shimizu, T.; Yamaura, J.-i.; Kiuchi, Y.; Hiroi, Z. Itinerant-electron Magnet of the Pyrochlore Lattice: Indium-doped YMn2Zn20. J. Phys. Soc. Jpn. 2010, 79, 093712. [Google Scholar] [CrossRef]
- Benbow, E.M.; Latturner, S.E. Mixed-metal Flux Synthesis of Quaternary RMn2TrxZn20−x Compounds with Tr = Al, In. J. Solid State Chem. 2006, 179, 3989–3996. [Google Scholar] [CrossRef]
- Yogi, M.; Kubota, F.; Uehara, H.; Higa, N.; Sugiyama, Y.; Wakiya, K.; Umehara, I. NMR Studies of Structural Stabilization by Site-Selective Element Substitution in 1-2-20 System. JPS Conf. Proc. 2020, 29, 015004. [Google Scholar] [CrossRef]
- Svanidze, E.; Kindy II, M.; Georgen, C.; Fulfer, B.W.; Lapidus, S.H.; Chan, J.Y.; Morosan, E. Magnetic and Crystallographic Properties of ZrM2−dZn20+d (M = Cr−Cu). J. Magn. Magn. Mater. 2016, 416, 401–407. [Google Scholar] [CrossRef]
- Schumacher, L.; Kösters, J.; Hensmann, L.; Pöttgen, R. The Solid Solutions RETi2Al20−xGax (0 ≤ x ≤ 10) with RE = Eu, Gd, Tb, Dy and Yb−First Gallides with CeCr2Al20-type Structure. Z. Für Kristallographie-Cryst. Mater. 2024, 239, 367–376. [Google Scholar] [CrossRef]
- Swatek, P.; Kaczorowski, D. Magnetic and Electrical Properties of UCr2Al20 Single Crystals. J. Solid State Chem. 2012, 191, 191–194. [Google Scholar] [CrossRef]
- Schumacher, L.; Schreiner, F.; Koldemir, A.; Janka, O.; Hansen, M.R.; Pöttgen, R. Cadmium-rich Intermetallic Phases ARh2Cd20—Structure, Magnetic Behavior, 151Eu Mössbauer and 113Cd Solid-state NMR Spectroscopy. Dalton Trans. 2025. Advance Article. [Google Scholar] [CrossRef]
- Jia, S.; Bud’ko, S.L.; Samolyuk, G.D.; Canfield, P.C. Nearly Ferromagnetic Fermi-liquid Behaviour in YFe2Zn20 and High-temperature Ferromagnetism of GdFe2Zn20. Nat. Phys. 2007, 3, 334–338. [Google Scholar] [CrossRef]
- Jia, S.; Ni, N.; Bud’ko, S.L.; Canfield, P.C. Magnetic Properties of GdxY1−xFe2Zn20: Dilute, Large-S Moments in a Nearly Ferromagnetic Fermi Liquid. Phys. Rev. B 2007, 76, 184410. [Google Scholar] [CrossRef]
- Jia, S.; Ni, N.; Bud’ko, S.L.; Canfield, P.C. Magnetic Properties of RFe2Zn20 and RCo2Zn20 (R = Y, Nd, Sm, Gd-Lu). Phys. Rev. B 2009, 80, 104403. [Google Scholar] [CrossRef]
- Jia, S.; Ni, N.; Samolyuk, G.D.; Safa-Sefat, A.; Dennis, K.; Ko, H.; Miller, G.J.; Bud’ko, S.L.; Canfield, P.C. Variation of the Magnetic Ordering in GdT2Zn20 (T = Fe, Ru, Os, Co, Rh and Ir) and Its Correlation with the Electronic Structure of Isostructural YT2Zn20. Phys. Rev. B 2008, 77, 104408. [Google Scholar] [CrossRef]
- Wang, C.H.; Lawrence, J.M.; Bauer, E.D.; Kothapalli, K.; Gardner, J.S.; Ronning, F.; Gofryk, K.; Thompson, J.D.; Nakotte, H.; Trouw, F. Unusual Signatures of the Ferromagnetic Transition in the Heavy Fermion Compound UMn2Al20. Phys. Rev. B 2010, 82, 094406. [Google Scholar] [CrossRef]
- Kase, N.; Shimura, Y.; Kittaka, S.; Sakakibara, T.; Nakatsuji, S.; Nakano, T.; Takeda, N.; Akimitsu, J. Antiferromagnetic Transition of the Caged Compound TmTi2Al20. J. Phys. Conf. Ser. 2015, 592, 012052. [Google Scholar] [CrossRef]
- Hirose, Y.; Doto, H.; Honda, F.; Li, D.; Aoki, D.; Haga, Y.; Settai, R. New Heavy-fermion Antiferromagnet UPd2Cd20. J. Phys. Condens. Matter 2016, 28, 425601. [Google Scholar] [CrossRef] [PubMed]
- Torikachvili, M.S.; Jia, S.; Mun, E.D.; Hannahs, S.T.; Black, R.C.; Neils, W.K.; Martien, D.; Bud’ko, S.L.; Canfield, P.C. Six Closely Related YbT2Zn20 (T = Fe, Co, Ru, Rh, Os, Ir) Heavy Fermion Compounds with Large Local Moment Degeneracy. Proc. Natl. Acad. Sci. USA 2007, 104, 9960–9963. [Google Scholar] [CrossRef] [PubMed]
- Verbovytsky, Y.; Łątka, K.; Tomala, K. The Crystal Structure and Magnetic Properties of the GdV2Al20 and GdCr2Al20 Ternary Compounds. J. Alloys Comp. 2007, 442, 334–336. [Google Scholar] [CrossRef]
- Tian, W.; Christianson, A.D.; Zarestky, J.L.; Jia, S.; Bud’ko, S.L.; Canfield, P.C.; Piccoli, P.M.B.; Schultz, A.J. Magnetic Order in TbCo2Zn20 and TbFe2Zn20. Phys. Rev. B 2010, 81, 144409. [Google Scholar] [CrossRef]
- Yamamoto, R.; Onimaru, T.; Yamane, Y.; Shimura, Y.; Umeo, K.; Takabatake, T. Magnetic Properties of a Diluted Nd System Y1−xNdxCo2Zn20 for x = 0.20 with a Γ6 Doublet Ground State. JPS Conf. Proc. 2020, 29, 015006. [Google Scholar] [CrossRef]
- Hirose, Y.; Toda, M.; Yoshiuchi, S.; Yasui, S.; Sugiyama, K.; Honda, F.; Hagiwara, M.; Kindo, K.; Settai, R.; Ōnuki, Y. Metamagnetic Transition in Heavy Fermion Compounds YbT2Zn20 (T: Co, Rh, Ir). J. Phys. Conf. Ser. 2011, 273, 012003. [Google Scholar] [CrossRef]
- Ramesh Kumar, K.; Nair, H.S.; Christian, R.; Thamizhavel, A.; Strydom, A.M. Magnetic, Specific Heat and Electrical Transport Properties of Frank-Kasper Cage Compounds RTM2Al20 [R = Eu, Gd and La; TM = V, Ti]. J. Phys. Condens. Matter 2016, 28, 436002. [Google Scholar] [CrossRef]
- Yaniv, G.; Vidal, D.; Fuks, D.; Meshi, L. Bonding and Stability of Ternary Structures in the CeT2Al20 (T = Ta, W, Re) and YRe2Al20 Alloys. Metals 2020, 10, 422. [Google Scholar] [CrossRef]
- Yamamoto, R.; Le, M.D.; Adroja, D.T.; Shimura, Y.; Takabatake, T.; Onimaru, T. Inelastic Neutron Scattering Study of Crystalline Electric Field Excitations in the Caged Compounds NdT2Zn20 (T = Co, Rh, and Ir). Phys. Rev. B 2023, 107, 075114. [Google Scholar] [CrossRef]
- Yamane, Y.; Onimaru, T.; Shimura, Y.; Umeo, K.; Takabatake, T. Non-Kramers Γ3 Doublet Ground State in a Diluted Pr System Y1−xPrxCo2Zn20. JPS Conf. Proc. 2020, 29, 015001. [Google Scholar] [CrossRef]
- Kubo, T.; Tou, H.; Kotegawa, H.; Harima, H.; Higashinaka, R.; Nakamaka, A.; Aoki, Y.; Sato, H. Development of Short-range Correlations of 4f Electrons in the Non-Kramers Γ3 System PrNb2Al20 at Low Temperatures Investigated by NMR Measurements. JPS Conf. Proc. 2020, 29, 015003. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Hidaka, H.; Amitsuka, H.; Zherlitsyn, S.; Wosnitza, J.; Yamane, Y.; Onimaru, T. Logarithmic Elastic Response in the Dilute Non-Kramers System Y1−xPrxIr2Zn20. JPS Conf. Proc. 2020, 29, 015002. [Google Scholar] [CrossRef]
- Afzal, M.A.; Ōnuki, Y.; Aoki, D.; Harima, H.; Higashinaka, R.; Aoki, Y.; Matsuda, T.D. Large Cyclotron Effective Masses in Antiferromagnet SmTi2Al20 Compared with Conventional Masses in Ferromagnet SmNi2Cd20 and Antiferromagnet SmPd2Cd20. J. Phys. Soc. Jpn. 2024, 93, 054710. [Google Scholar] [CrossRef]
- Omasa, K.; Matsuoka, E.; Aoki, D.; Sugawara, H. de Haas-van Alphen Effect in SmTi2Al20. JPS Conf. Proc. 2020, 29, 015007. [Google Scholar] [CrossRef]
- Kubo, T.; Matsuoka, E.; Kotegawa, H.; Tou, H.; Nakamura, A.; Aoki, D.; Harima, H.; Sugawara, H. Fermi Surface of the Heavy-fermion Superconductor PrTi2Al20. J. Phys. Soc. Jpn. 2020, 89, 084704. [Google Scholar] [CrossRef]
- Magishi, K.; Hisada, A.; Saito, T.; Koyama, K.; Namiki, T.; Nishimura, K. NMR Study of Caged Cubic Compound NdTr2Al20 (Tr = Ti and V). JPS Conf. Proc. 2020, 29, 015005. [Google Scholar] [CrossRef]
- Slack, G.A. New Materials and Performance Limits for Thermoelectric Cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Sales, B.C. Novel Thermoelectric Materials. Curr. Opin. Solid State Mater. Sci. 1997, 2, 284–289. [Google Scholar] [CrossRef]
- Beekman, M.; Morelli, D.T.; Nolas, G.S. Better Thermoelectrics through Glass-like Crystals. Nat. Mater. 2015, 14, 1182–1185. [Google Scholar] [CrossRef]
- Wei, K.; Neu, J.N.; Lai, Y.; Chen, K.-W.; Hobbis, D.; Nolas, G.S.; Graf, D.E.; Siegrist, T.; Baumbach, R.E. Enhanced Thermoelectric Performance of Heavy-fermion Compounds YbTM2Zn20 (TM = Co, Rh, Ir) at Low Temperatures. Sci. Adv. 2019, 5, eaaw6183. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.D.; Jia, S.; Bud’ko, S.L.; Canfield, P.C. Thermoelectric Power of the YbT2Zn20 (T = Fe, Ru, Os, Ir, Rh, and Co) Heavy Fermions. Phys. Rev. B 2012, 86, 115110. [Google Scholar] [CrossRef]
- Galeano-Cabral, J.R.; Karr, E.; Schundelmier, B.; Oladehin, O.; Choi, E.S.; Siegrist, T.; Ordonez, J.; Shastri, S.; Petkov, V.; Baumbach, R.E.; et al. Enhanced Thermoelectric Properties of Heavy-fermion Compounds YbxCeySmzIr2Zn20 (x + y + z = 1). Phys. Rev. Mater. 2023, 7, 025406. [Google Scholar] [CrossRef]
- Nakamura, N.; Higashinaka, R.; Aoki, Y.; Sato, H.; Matsuda, T.D. Superconducting Properties of Cage Compounds YbTr2Al20 with Tr = Mo and Ta. Solid State Commmun. 2023, 363, 115098. [Google Scholar] [CrossRef]
- Winiarski, M.J.; Wiendlocha, B.; Sternik, M.; Wiśniewski, P.; O’Brien, J.R.; Kaczorowski, D.; Klimczuk, T. Rattling-enhanced Superconductivity in MV2Al20 (M = Sc, Lu, Y) Intermetallic Cage Compounds. Phys. Rev. B 2016, 93, 134507. [Google Scholar] [CrossRef]
- Hiroi, Z.; Onosaka, A.; Okamoto, Y.; Yamaura, J.; Harima, H. Rattling and Superconducting Properties of the Cage Compound GaxV2Al20. J. Phys. Soc. Jpn. 2012, 81, 124707. [Google Scholar] [CrossRef]
- Onosaka, A.; Okamoto, Y.; Yamaura, J.-i.; Hiroi, Z. Superconductivity in the Einstein Solid AxV2Al20 (A = Al and Ga). J. Phys. Soc. Jpn. 2012, 81, 023703. [Google Scholar] [CrossRef]
- Onimaru, T.; Matsumoto, K.T.; Inoue, Y.F.; Umeo, K.; Saiga, Y.; Matsushita, Y.; Tamura, R.; Nishimoto, K.; Ishii, I.; Suzuki, T.; et al. Superconductivity and Structural Phase Transitions in Caged Compounds RT2Zn20 (R = La, Pr, T = Ru, Ir). J. Phys. Soc. Jpn. 2010, 79, 033704. [Google Scholar] [CrossRef]
- Panday, S.R.; Dzero, M. Superconductivity in Ce-based Cage Compounds. J. Phys. Condens. Matter 2023, 35, 335601. [Google Scholar] [CrossRef]
- Tachibana, M. Beginner’s Guide to Flux Crystal Growth; Springer: Tokyo, Japan, 2017. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction CrysAlisPro, version 1.171.42.53a; Rigaku Corporation: Oxford, UK, 2022.
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for Guided Crystal Structure Analysis. J. Appl. Crystallogr. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab Initio Molecular-dynamics Simulation of the Liquid-metal-amorphous-semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- CrystalMaker, version 10.8.1; CrystalMaker Software Ltd.: Oxford, UK, 2023.
- Slater, J.C. Atomic Radii in Crystals. J. Chem. Phys. 1964, 41, 3199–3204. [Google Scholar] [CrossRef]
- Baker, H.; Henry, S.D.; Okamoto, H. ASM Handbook. Volume 3: Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
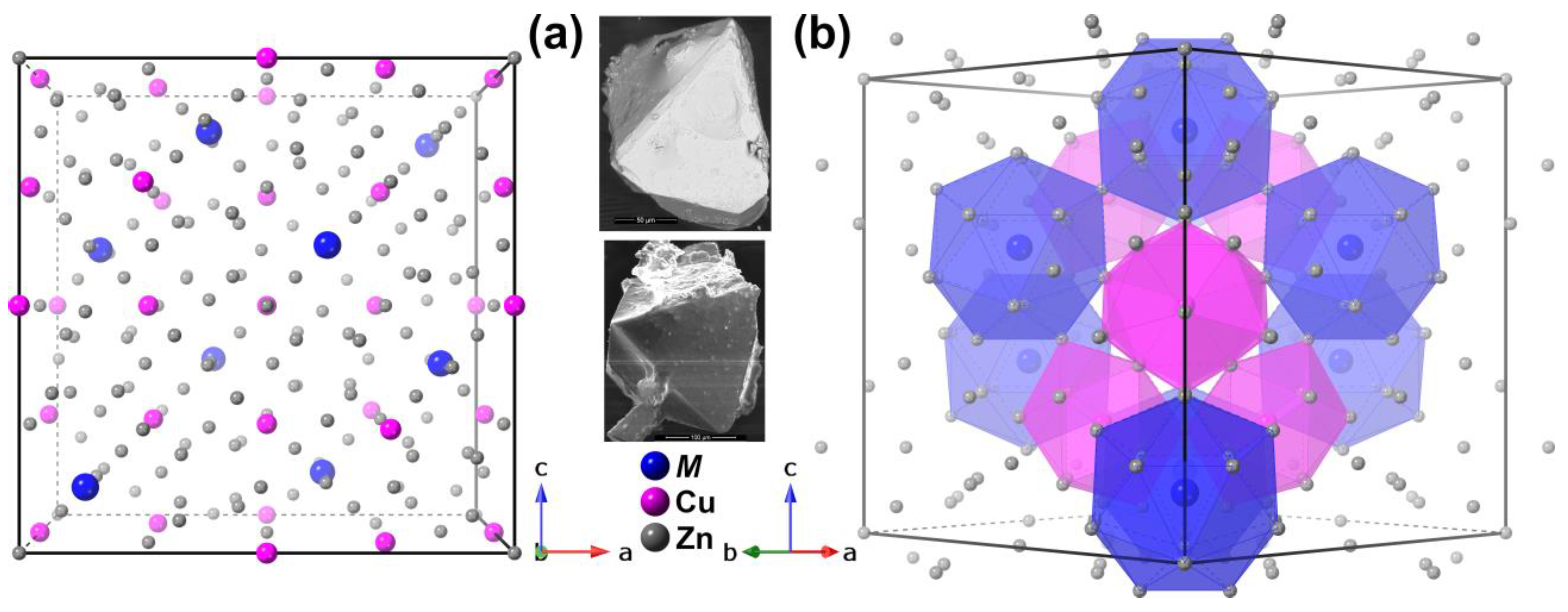
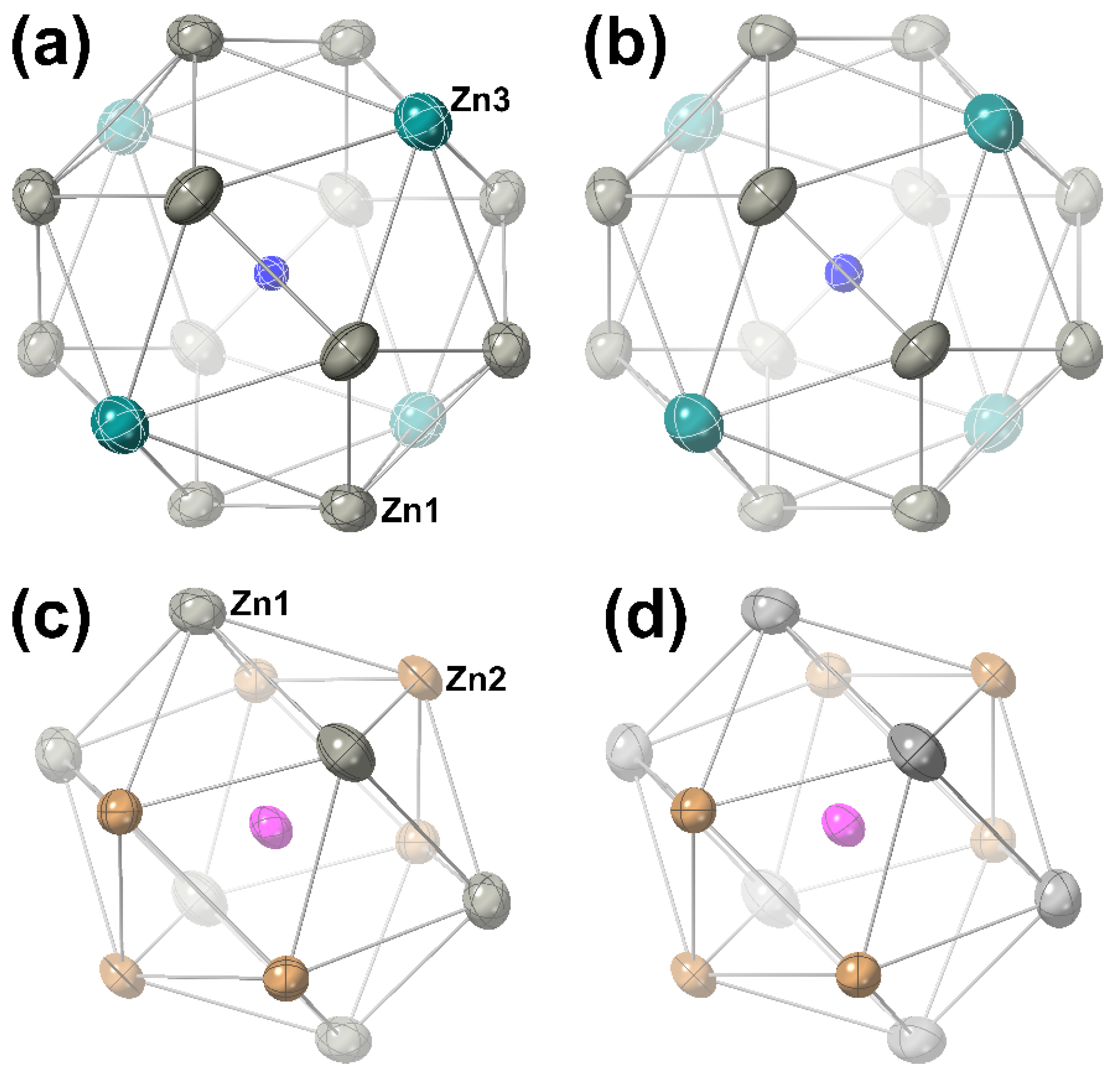
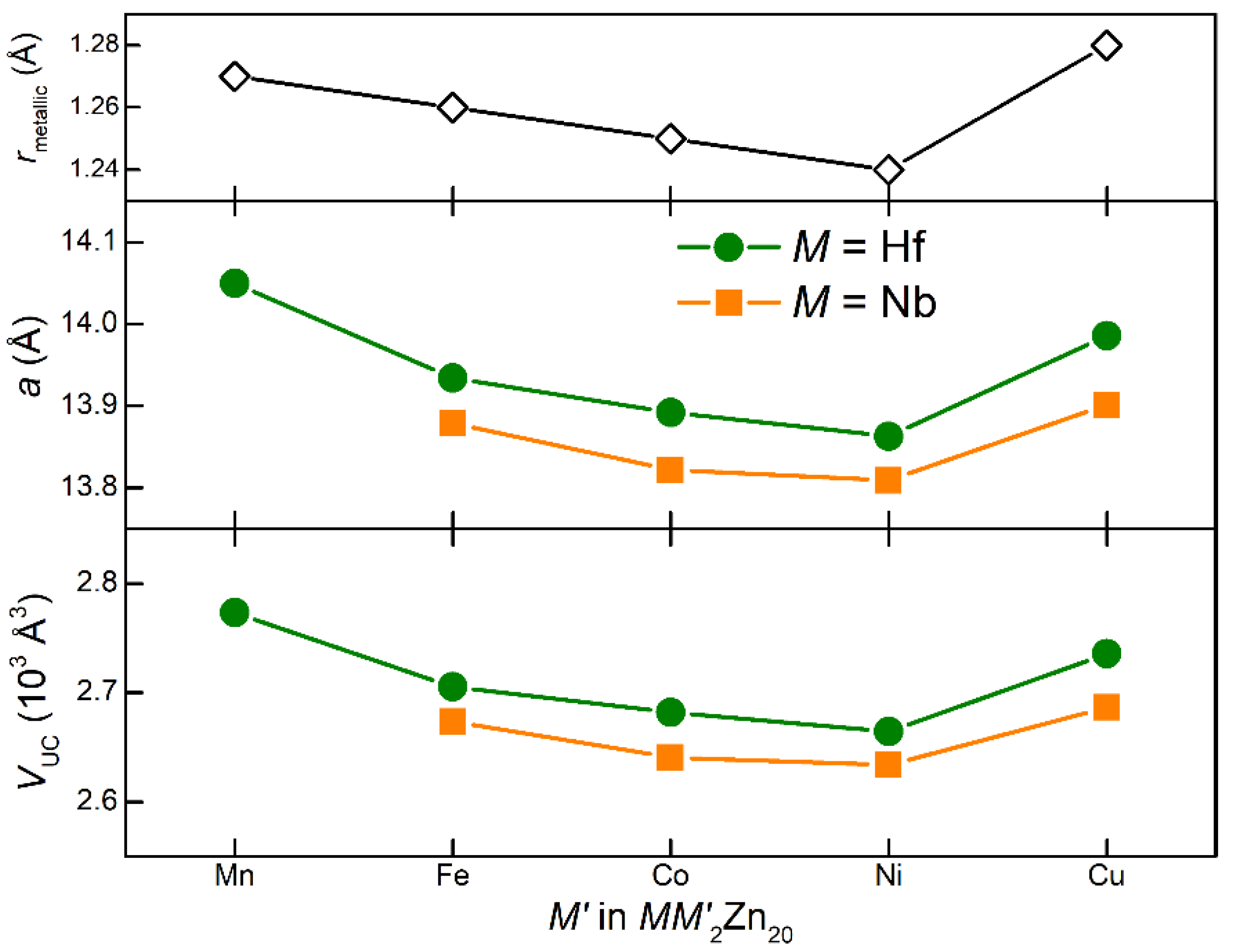
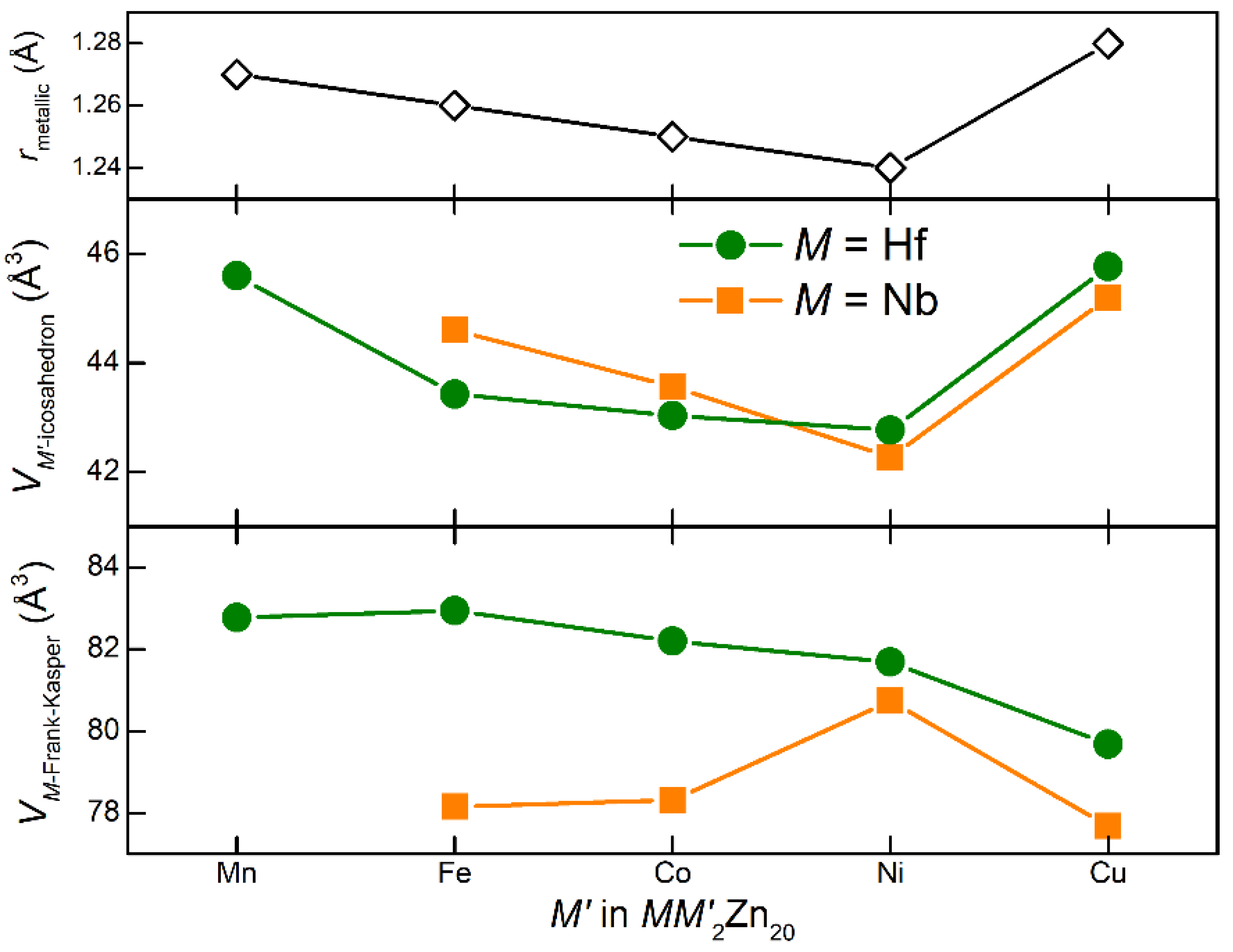

| NbCu2Zn20 | Hf0.96Cu1.67Zn20.33 | |
|---|---|---|
| Molecular weight (g/mol) | 1527.60 | 1607.14 [1613.18] |
| Space group | (#227) | (#227) |
| a (Å) | 13.9013(2) | 13.9856(2) |
| V (Å3) | 2686.35(8) | 2735.52(7) |
| Z | 8 | 8 |
| ρcalc (g/cm3) | 7.554 | 7.804 [7.834] |
| Absorption coefficient µ (mm−1) | 38.801 | 44.634 [44.850] |
| Absorption corrections Tmin, Tmax | 0.222, 0.390 | 0.04, 0.12 [0.107, 0.280] |
| Crystal size (mm3) | 0.038 × 0.062 × 0.068 | 0.048 × 0.076 × 0.094 |
| Data collection range (°) | 2.54 < θ < 44.81 | 2.52 < θ < 44.76 |
| h range | −20 ≤ h ≤ 21 | −23 ≤ h ≤ 26 |
| k range | −27 ≤ k ≤ 26 | −27 ≤ k ≤ 27 |
| l range | −27 ≤ l ≤ 26 | −27 ≤ l ≤ 27 |
| Reflections collected | 3049 | 6235 |
| Independent reflections | 574 | 577 |
| Parameters refined | 17 | 17 |
| Restraints | 0 | 6 [0] |
| Δρmin, Δρmax (e/Å−3) | −0.81, 0.71 | −3.51, 0.94 [−4.75, 1.32] |
| Rint | 0.019 | 0.032 |
| R1(F) for all data a | 0.0160 | 0.0160 [0.0198] |
| wR2(FO2) b | 0.0390 | 0.0276 [0.0473] |
| Goodness-of-fit on F2 | 0.9991 | 0.9989 [0.9872] |
| CSD # | 2201921 | 2201922 |
| M | Atom | Site | SOF | x | y | z | Ueq (Å2) |
|---|---|---|---|---|---|---|---|
| Nb | Nb | 8a | 1 | 1/8 | 1/8 | 1/8 | 0.0055(1) |
| Cu | 16d | 1 | 1/2 | 1/2 | 1/2 | 0.0084(1) | |
| Zn1 | 96g | 1 | 0.06126(1) | =x | 0.32041(2) | 0.0130(1) | |
| Zn2 | 48f | 1 | 0.48739(2) | 1/8 | 1/8 | 0.0090(1) | |
| Zn3 | 16c | 1 | 0 | 0 | 0 | 0.0144(1) | |
| Hf | Hf | 8a | 0.963(2) | 1/8 | 1/8 | 1/8 | 0.0061(1) |
| Cu11 | 16d | 0.837(2) | 1/2 | 1/2 | 1/2 | 0.0084(1) | |
| Zn12 | 0.163(2) | ||||||
| Zn1 | 96g | 1 | 0.06101(2) | =x | 0.32106(2) | 0.0139(1) | |
| Zn2 | 48f | 1 | 0.48752(3) | 1/8 | 1/8 | 0.0096(1) | |
| Zn3 | 16c | 1 | 0 | 0 | 0 | 0.0162(1) |
| M = Nb | M = Hf | % Change | |
|---|---|---|---|
| Lattice parameter (Å) | 13.9013(2) | 13.9856(2) | 0.61 |
| Unit cell volume (Å3) | 2686.35(8) | 2735.52(7) | 1.83 |
| Frank–Kasper polyhedron of M | |||
| M—Zn1 (Å) | 2.992(1) | 3.020(1) | 0.94 |
| M—Zn3 (Å) | 3.010(1) | 3.028(1) | 0.60 |
| Volume (Å3) | 77.676(3) | 79.678(3) | 2.58 |
| Icosahedron of Cu | |||
| Cu—Zn1 (Å) | 2.772(1) | 2.778(1) | 0.22 |
| Cu—Zn2 (Å) | 2.464(1) | 2.478(1) | 0.57 |
| Volume (Å3) | 45.197(3) | 45.764(3) | 1.25 |
| Other measures | |||
| M—M (Å) | 6.019(1) | 6.056(1) | 0.62 |
| Cu—Cu (Å) | 4.915(1) | 4.945(1) | 0.61 |
| M—Cu (Å) | 5.763(1) | 5.798(1) | 0.61 |
| M—Zn2 (Å) (not part of cage) | 5.038(1) | 5.070(1) | 0.64 |
| Cu—Zn3 (Å) (not part of cage) | 4.915(1) | 4.945(1) | 0.61 |
| M’ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Mn | Fe | Co | Ni | Cu | Ru | Rh | Os | Ir | ||
| M | Sc | ● | ● | ||||||||
| Y | ● b | ● | ● | ● | ● | ● | ● | ||||
| Zr | ● a | ● a | ● | ● | ● | ● | ● | ● | |||
| Nb | ● | ● | ● | o | |||||||
| Hf | ● c | ● | ● | ● | o | ● | ● | ||||
| Ce | ● b | ● | ● | ● | |||||||
| Pr | ● b | ● | ● | ||||||||
| Nd | ● b | ● | ● | ● | |||||||
| Sm | ● b | ● | ● | ● | |||||||
| Gd | ● b | ● | ● | ● | ● | ● | ● | ||||
| Tb | ● | ● | ● | ● | |||||||
| Dy | ● b | ● | ● | ● | ● | ● | |||||
| Ho | ● | ● | ● | ● | ● | ||||||
| Er | ● b | ● | ● | ● | ● | ● | |||||
| Tm | ● | ● | ● | ● | ● | ||||||
| Yb | ● b | ● | ● | ● | ● | ● | ● | ||||
| Lu | ● | ● | ● | ● | ● | ||||||
| U | ● | ● | ● | ● | ● | ● | |||||
| ZrCu2Zn20 | % Larger than HfCu2Zn20 | % Larger than NbCu2Zn20 | |
| Lattice parameter (Å) | 14.0116 | 0.19 | 0.79 |
| Unit cell volume (Å3) | 2750.83 | 0.56 | 2.40 |
| M Frank–Kasper polyhedron (Å3) | 79.979 | 0.38 | 2.96 |
| Cu Icosahedron (Å3) | 46.085 | 0.70 | 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longworth, S.M.; Mou, M.M.; Yasmin, N.; Noor, M.F.B.; Sakidja, R.; Besara, T. Single Crystal Growth and Structural Study of the New MCu2Zn20 (M = Nb, Hf) Compounds. Crystals 2025, 15, 391. https://doi.org/10.3390/cryst15050391
Longworth SM, Mou MM, Yasmin N, Noor MFB, Sakidja R, Besara T. Single Crystal Growth and Structural Study of the New MCu2Zn20 (M = Nb, Hf) Compounds. Crystals. 2025; 15(5):391. https://doi.org/10.3390/cryst15050391
Chicago/Turabian StyleLongworth, Sarah M., Marium M. Mou, Nusrat Yasmin, Md Fahel Bin Noor, Ridwan Sakidja, and Tiglet Besara. 2025. "Single Crystal Growth and Structural Study of the New MCu2Zn20 (M = Nb, Hf) Compounds" Crystals 15, no. 5: 391. https://doi.org/10.3390/cryst15050391
APA StyleLongworth, S. M., Mou, M. M., Yasmin, N., Noor, M. F. B., Sakidja, R., & Besara, T. (2025). Single Crystal Growth and Structural Study of the New MCu2Zn20 (M = Nb, Hf) Compounds. Crystals, 15(5), 391. https://doi.org/10.3390/cryst15050391







