Molecular Dynamics Study of the Ni Content-Dependent Mechanical Properties of NMC Cathode Materials
Abstract
1. Introduction
2. Method
3. Results and Discussion
3.1. Model Validation
3.2. Elastic Modulus
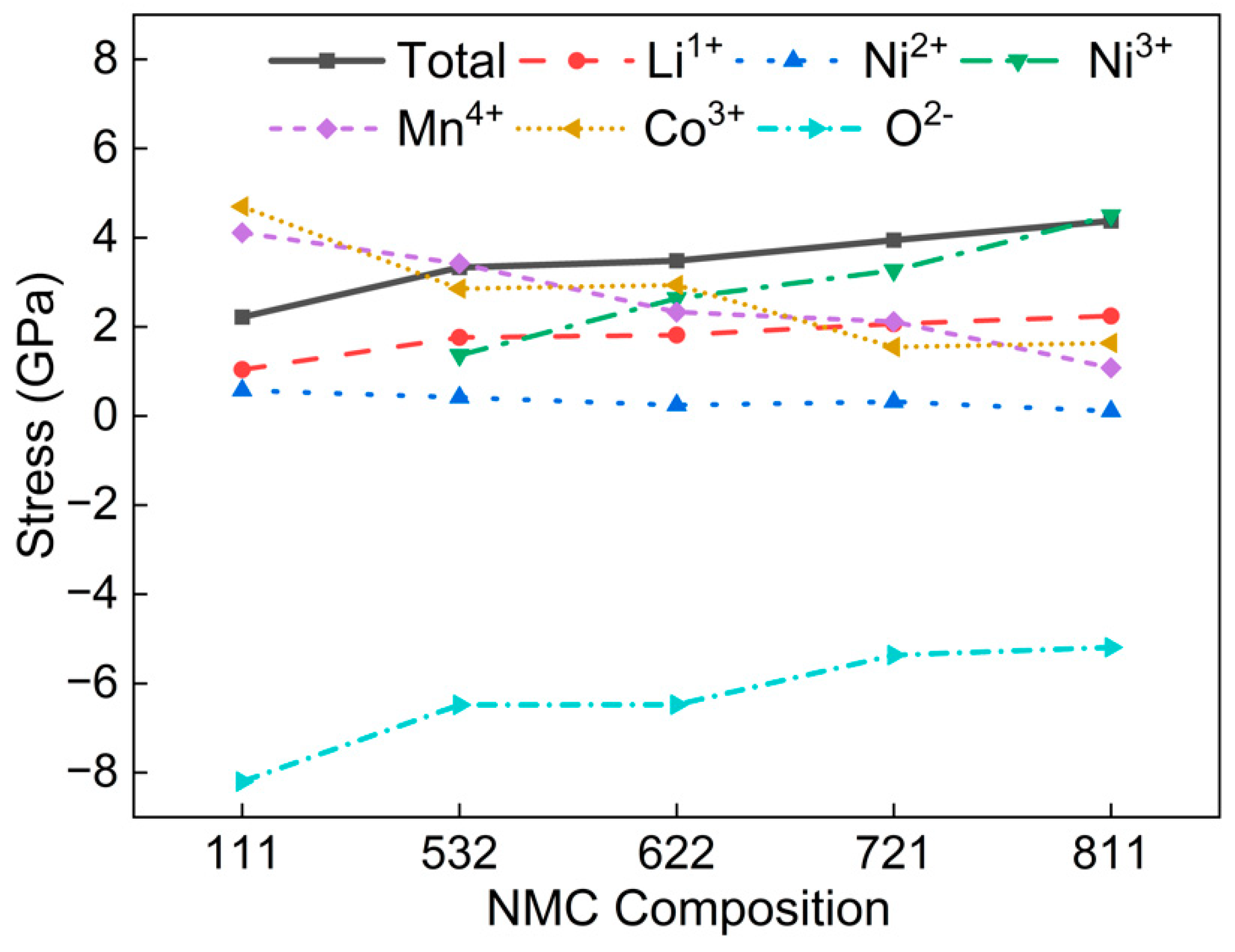
3.3. Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- He, P.; Yu, H.; Li, D.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R Rep. 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Yu, Y. A review on covalent organic frameworks for rechargeable zinc-ion batteries. Chin. Chem. Lett. 2024, 35, 108865. [Google Scholar] [CrossRef]
- Yao, J.; Wang, X.; Hu, P.; Fan, J.; Yang, X.; Jiang, W.; Jiang, S.; Dong, P.; Zhang, Y.; Duan, J.; et al. Local Electron Spin-State Modulation at Mn Site for Advanced Sodium-Ion Batteries with Fast-Kinetic NaNi0.33Fe0.33Mn0.33O2 Cathode. Adv. Funct. Mater. 2024, 2419967. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Fan, J.; Yao, J.; Luo, L.; Zhou, Z.; Dong, P.; Xiao, W. In situ constructed MgO parclose-concerted fabrication of Silicon/carbon hybrids via a high-efficiency and expedited electrochemical process in molten salt. Chem. Eng. J. 2024, 484, 149428. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x< −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef]
- Mo, J.Y.; Jeon, W. The Impact of Electric Vehicle Demand and Battery Recycling on Price Dynamics of Lithium-Ion Battery Cathode Materials: A Vector Error Correction Model (VECM) Analysis. Sustainability 2018, 10, 2870. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling─Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; de Picciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179–187. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Zhao, E.; He, L.; Wang, B.; Li, X.; Zhang, J.; Wu, Y.; Chen, J.; Zhang, S.; Liang, T.; Chen, Y.; et al. Structural and mechanistic revelations on high capacity cation-disordered Li-rich oxides for rechargeable Li-ion batteries. Energy Storage Mater. 2019, 16, 354–363. [Google Scholar] [CrossRef]
- Stoyanova, R. Lithium/nickel mixing in the transition metal layers of lithium nickelate: High-pressure synthesis of layered Li[LixNi1−x]O2 oxides as cathode materials for lithium-ion batteries. Solid State Ion. 2003, 161, 197–204. [Google Scholar] [CrossRef]
- Capitaine, F. A new variety of LiMnO2 with a layered structure. Solid State Ion. 1996, 89, 197–202. [Google Scholar] [CrossRef]
- Min, K.; Kim, K.; Jung, C.; Seo, S.-W.; Song, Y.Y.; Lee, H.S.; Shin, J.; Cho, E. A comparative study of structural changes in lithium nickel cobalt manganese oxide as a function of Ni content during delithiation process. J. Power Sources 2016, 315, 111–119. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater 2009, 8, 320–324. [Google Scholar] [CrossRef]
- Guo, J.; Jiao, L.F.; Yuan, H.T.; Li, H.X.; Zhang, M.; Wang, Y.M. Effect of synthesis condition on the structural and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 prepared by the metal acetates decomposition method. Electrochim. Acta 2006, 51, 3731–3735. [Google Scholar] [CrossRef]
- Hwang, B.J.; Santhanam, R.; Chen, C.H. Effect of synthesis conditions on electrochemical properties of LiNi1−yCoyO2 cathode for lithium rechargeable batteries. J. Power Sources 2003, 114, 244–252. [Google Scholar] [CrossRef]
- Ren, H.; Huang, Y.; Wang, Y.; Li, Z.; Cai, P.; Peng, Z.; Zhou, Y. Effects of different carbonate precipitators on LiNi1/3Co1/3Mn1/3O2 morphology and electrochemical performance. Mater. Chem. Phys. 2009, 117, 41–45. [Google Scholar] [CrossRef]
- Ahn, W.; Lim, S.N.; Jung, K.-N.; Yeon, S.-H.; Kim, K.-B.; Song, H.S.; Shin, K.-H. Combustion-synthesized LiNi0.6Mn0.2Co0.2O2 as cathode material for lithium ion batteries. J. Alloys Compd. 2014, 609, 143–149. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Optimization of Ni2+/Ni3+ ratio in layered Li(Ni,Mn,Co)O2 cathodes for better electrochemistry. J. Power Sources 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Shen, Y.; Bo, S.-H. Fracture behavior in battery materials. J. Phys. Energy 2020, 2, 022002. [Google Scholar] [CrossRef]
- Zhu, M.; Park, J.; Sastry, A.M. Fracture Analysis of the Cathode in Li-Ion Batteries: A Simulation Study. J. Electrochem. Soc. 2012, 159, A492. [Google Scholar] [CrossRef]
- Iqbal, N.; Choi, J.; Lee, C.; Khan, A.; Tanveer, M.; Lee, S. A Review on Modeling of Chemo-mechanical Behavior of Particle–Binder Systems in Lithium-Ion Batteries. Multiscale Sci. Eng. 2022, 4, 79–93. [Google Scholar] [CrossRef]
- Iqbal, N.; Lee, S. Anisotropic model to describe chemo-mechanical response of Ni-rich cathode materials. Int. J. Mech. Sci. 2024, 269, 109034. [Google Scholar] [CrossRef]
- Iqbal, N.; Choi, J.; Shah, S.F.; Lee, C.; Lee, S. Mathematical modeling and simulations of stress mitigation by coating polycrystalline particles in lithium-ion batteries. Appl. Math. Mech.-Engl. Ed. 2024, 45, 947–962. [Google Scholar] [CrossRef]
- Asheri, A.; Rezaei, S.; Glavas, V.; Xu, B.-X. Microstructure impact on chemo-mechanical fracture of polycrystalline lithium-ion battery cathode materials. Eng. Fract. Mech. 2024, 309, 110370. [Google Scholar] [CrossRef]
- Taghikhani, K.; Weddle, P.J.; Berger, J.R.; Kee, R.J. A chemo-mechanical grain boundary model and its application to understand the damage of Li-ion. J. Electrochem. Soc. 2021, 168, 080511. [Google Scholar] [CrossRef]
- Taghikhani, K.; Weddle, P.J.; Hoffman, R.M.; Berger, J.R.; Kee, R.J. Electro-chemo-mechanical finite-element model of single-crystal and polycrystalline NMC cathode particles embedded in an argyrodite solid electrolyte. Electrochim. Acta 2023, 460, 142585. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, K.; Liu, Y.; Stein, P.; Xu, B.-X. A chemo-mechanical grain boundary model and its application to understand the damage of Li-ion battery materials. Scr. Mater. 2020, 183, 45–49. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Wu, L.; Lee, W.H.; Zhang, J. First Principles Study on the Electrochemical, Thermal and Mechanical Properties of LiCoO2 for Thin Film Rechargeable Battery. Mater. Today Proc. 2014, 1, 82–93. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J. Ab initio study of anisotropic mechanical properties of LiCoO 2 during lithium intercalation and deintercalation process. J. Appl. Phys. 2015, 118, 225101. [Google Scholar] [CrossRef]
- Howey, D.A.; Roberts, S.A.; Viswanathan, V.; Mistry, A.; Beuse, M.; Khoo, E.; DeCaluwe, S.C.; Sulzer, V. Free Radicals: Making a Case for Battery Modeling. Electrochem. Soc. Interface 2020, 29, 30–34. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Sastry, A.M.; Lu, W. Molecular Dynamics Simulations of SOC-Dependent Elasticity of LixMn2O4 Spinels in Li-Ion Batteries. J. Electrochem. Soc. 2013, 160, 5. [Google Scholar] [CrossRef]
- Tyagi, R.; Srinivasan, S. Molecular dynamics modeling of lithium ion intercalation induced change in the mechanical properties of LixMn2O4. J. Chem. Phys. 2020, 153, 164712. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, K.-R.; Lee, B.-J. Interatomic Potential of Li–Mn–O and Molecular Dynamics Simulations on Li Diffusion in Spinel Li1– xMn2O4. J. Phys. Chem. C 2017, 121, 13008–13017. [Google Scholar] [CrossRef]
- Lee, E.; Lee, K.-R.; Lee, B.-J. An interatomic potential for the Li-Co-O ternary system. Comput. Mater. Sci. 2018, 142, 47–58. [Google Scholar] [CrossRef]
- Asadi, A.; Aghamiri, S.F.; Talaie, M.R. Molecular dynamics simulation of a LixMn2O4 spinel cathode material in Li-ion batteries. RSC Adv. 2016, 6, 115354–115363. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.S. Atomistic Simulation Study of Mixed-Metal Oxide (LiNi1/3Co1/3Mn1/3O2) Cathode Material for Lithium Ion Battery. J. Phys. Chem. C 2012, 116, 6484–6489. [Google Scholar] [CrossRef]
- He, X.; Ding, X.; Xu, R. Anisotropic mechanical properties of LiNixMnyCo1-x-yO2 cathodes for Li-ion batteries: A first-principles theoretical study. Acta Mater. 2024, 267, 119751. [Google Scholar] [CrossRef]
- Liu, J.; Lin, W.; Wang, Z.; Wang, Y.; Chen, T.; Zheng, J. Elastic Mechanics Study of Layered Li (NixMnyCoz)O2. PRX Energy 2024, 3, 013012. [Google Scholar] [CrossRef]
- Cheng, E.J.; Hong, K.; Taylor, N.J.; Choe, H.; Wolfenstine, J.; Sakamoto, J. Mechanical and physical properties of LiNi0.33Mn0.33Co0.33O2 (NMC). J. Eur. Ceram. Soc. 2017, 37, 3213–3217. [Google Scholar] [CrossRef]
- Xu, R.; Sun, H.; de Vasconcelos, L.S.; Zhao, K. Mechanical and Structural Degradation of LiNixMnyCozO2 Cathode in Li-Ion Batteries: An Experimental Study. J. Electrochem. Soc. 2017, 164, A3333. [Google Scholar] [CrossRef]
- Sharma, N.; Meng, D.; Wu, X.; De Vasconcelos, L.S.; Li, L.; Zhao, K. Nanoindentation measurements of anisotropic mechanical properties of single crystalline NMC cathodes for Li-ion batteries. Extrem. Mech. Lett. 2023, 58, 101920. [Google Scholar] [CrossRef]
- Pedone, A.; Malavasi, G.; Menziani, M.C.; Cormack, A.N.; Segre, U. A new self-consistent empirical interatomic potential model for oxides, silicates, and silica-based glasses. J Phys Chem B 2006, 110, 11780–11795. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Du, R.; Tang, M.; Wang, B.; Zhang, J. Effect of Ni2+ on Lithium-Ion Diffusion in Layered LiNi1−x−yMnxCoyO2 Materials. Crystals 2021, 11, 465. [Google Scholar] [CrossRef]
- Akimoto, J.; Gotoh, Y.; Oosawa, Y. Synthesis and Structure Refinement of LiCoO2Single Crystals. J. Solid State Chem. 1998, 141, 298–302. [Google Scholar] [CrossRef]
- Wang, C.; Ma, X.; Zhou, L.; Cheng, J.; Sun, J.; Zhou, Y. Study on the rapid synthesis of LiNi1−xCoxO2 cathode material for lithium secondary battery. Electrochim. Acta 2007, 52, 3022–3027. [Google Scholar] [CrossRef]
- Cauranta, D.; Baffie, N.; Garcia, B.; Pereira-Ramosb, J.P. Synthesis by a soft chemistry route and characterization of LiNixCo1−xO2 (0 ≤ x ≤ 1) cathode materials. Solid State Ionics. 1996, 91, 45–54. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Morgan, L.M.; Islam, M.M.; Yang, H.; O’Regan, K.; Patel, A.N.; Ghosh, A.; Kendrick, E.; Marinescu, M.; Offer, G.J.; Morgan, B.J.; et al. From Atoms to Cells: Multiscale Modeling of LiNixMnyCozO2 Cathodes for Li-Ion Batteries. ACS Energy Lett. 2022, 7, 108–122. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.-K.; Liu, J.; Amine, K. Li(Ni1/3Co1/3Mn1/3)O2 as a suitable cathode for high power applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Bie, X.; Du, F.; Wang, Y.; Zhu, K.; Ehrenberg, H.; Nikolowski, K.; Wang, C.; Chen, G.; Wei, Y. Relationships between the crystal/interfacial properties and electrochemical performance of LiNi0.33Co0.33Mn0.33O2 in the voltage window of 2.5–4.6V. Electrochim. Acta 2013, 97, 357–363. [Google Scholar] [CrossRef]
- Ishidzu, K.; Oka, Y.; Nakamura, T. Lattice volume change during charge/discharge reaction and cycle performance of Li[NixCoyMnz]O2. Solid State Ion. 2016, 288, 176–179. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Electronic Structure and Comparative Properties of LiNixMnyCozO2 Cathode Materials. J. Phys. Chem. C 2017, 121, 6002–6010. [Google Scholar] [CrossRef]
- Sun, H.-H.; Manthiram, A. Impact of Microcrack Generation and Surface Degradation on a Nickel-Rich Layered Li[Ni0.9Co0.05Mn0.05]O2 Cathode for Lithium-Ion Batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Dahbi, M.; Saadoune, I.; Amarilla, J.M. LixNi0.7Co0.3O2 electrode material: Structural, physical and electrochemical investigations. Electrochim. Acta 2008, 53, 5266–5271. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Schmidt, A.; Xu, J.; Geßwein, H.; Mönig, R.; Hartmann, P.; Sommer, H.; Brezesinski, T.; Janek, J. Anisotropic Lattice Strain and Mechanical Degradation of High- and Low-Nickel NCM Cathode Materials for Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 3286–3294. [Google Scholar] [CrossRef]
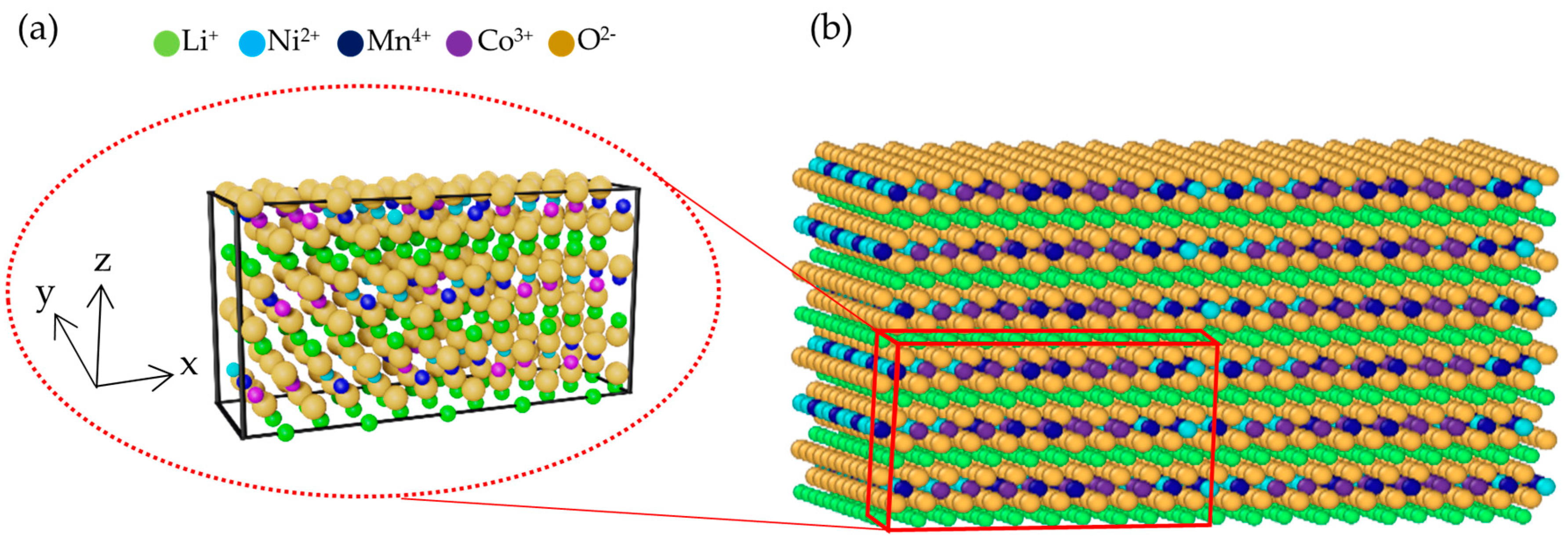
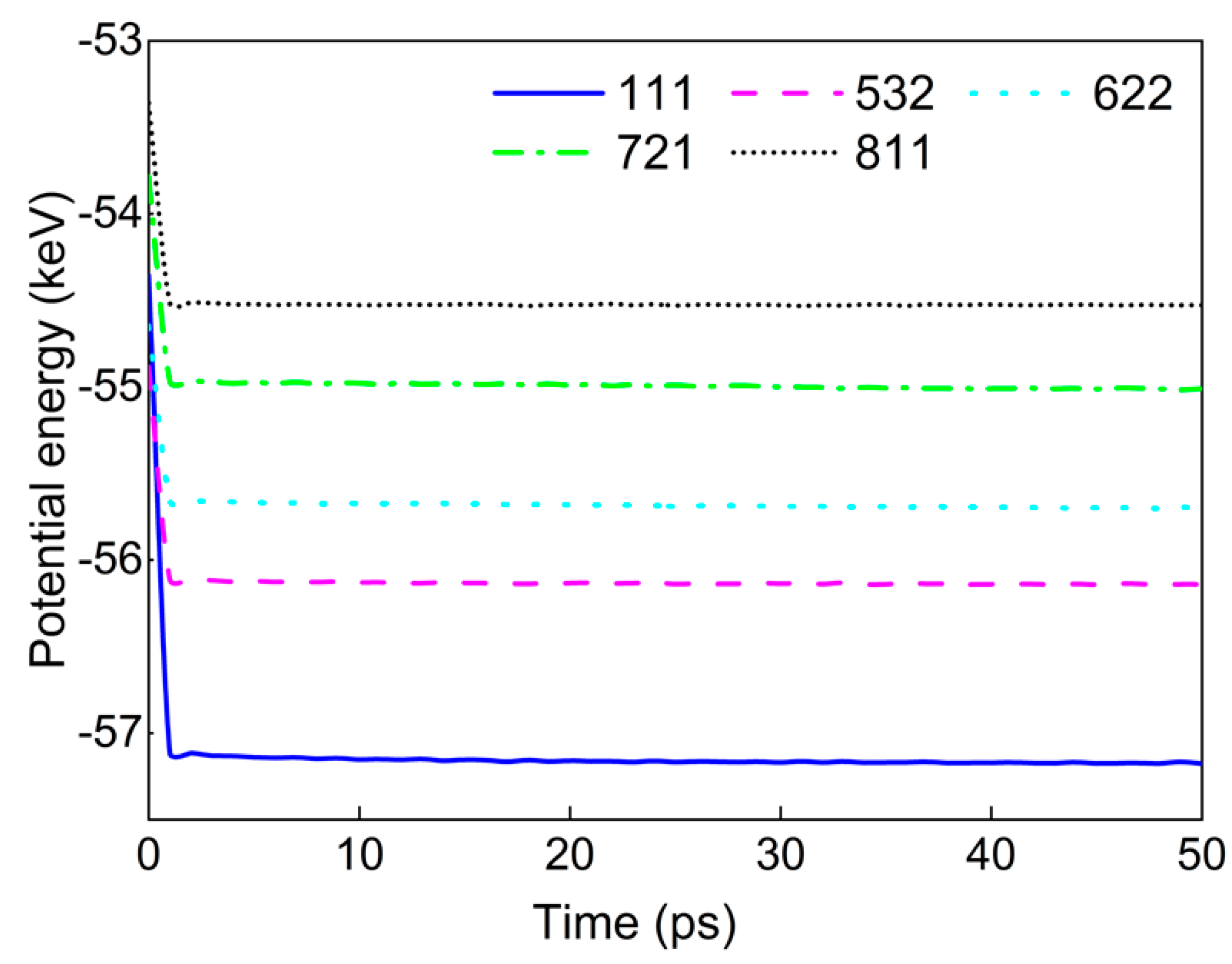
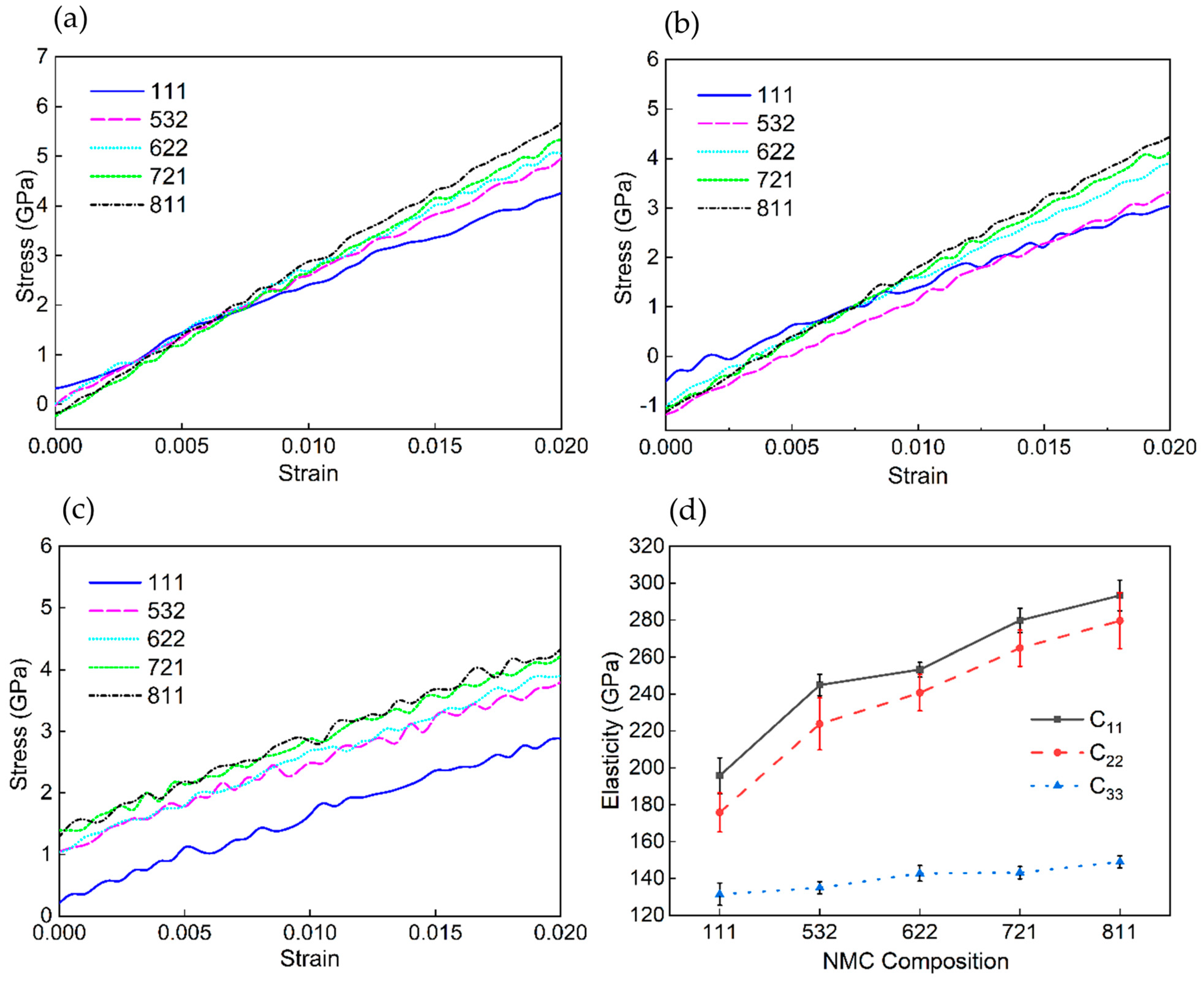
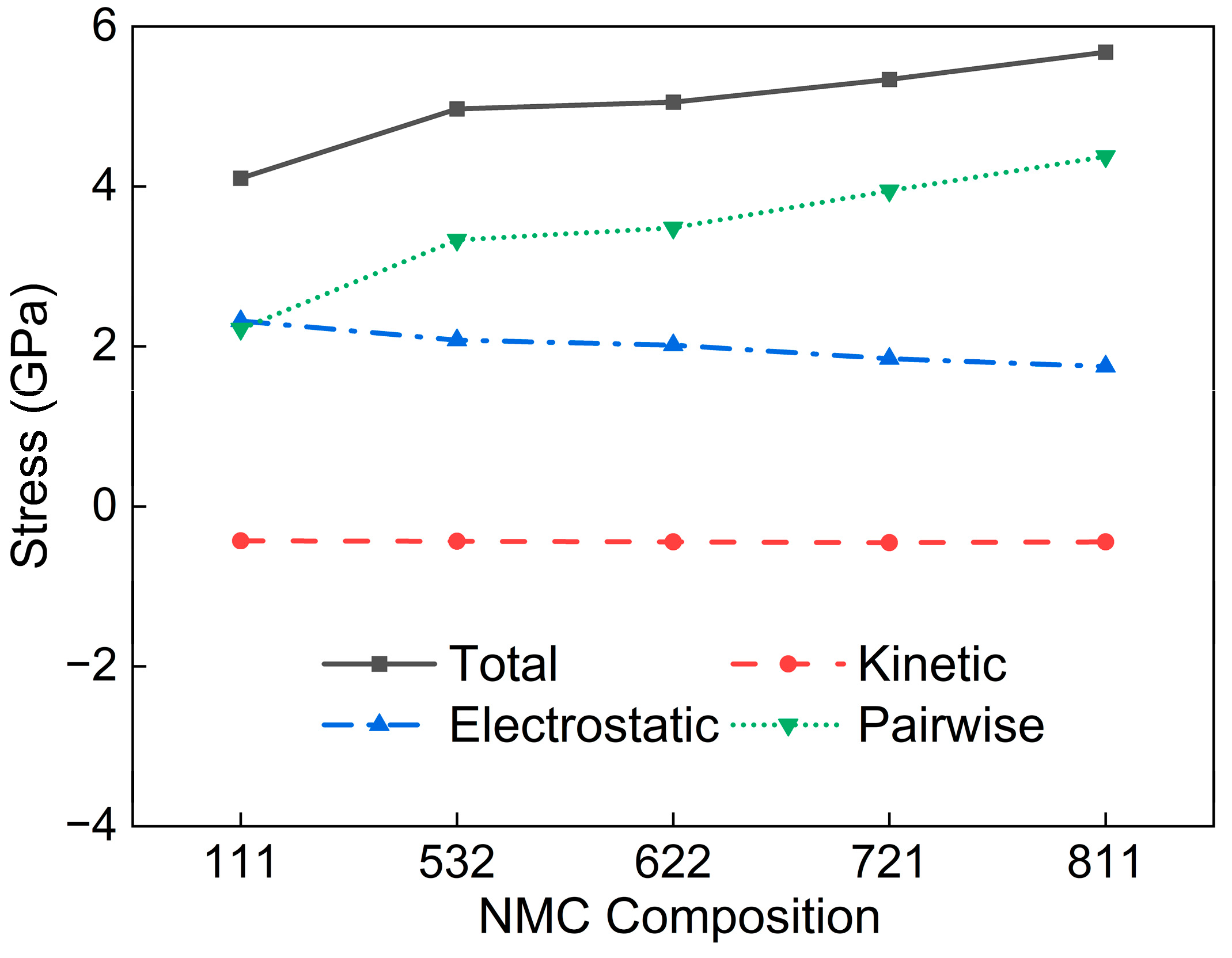
| Interaction Pair | D0 (eV) | r0 (Å) | α (Å−1) |
|---|---|---|---|
| Li+ − O2− | 0.001114 | 2.681360 | 3.429506 |
| Ni2+ − O2− | 0.029356 | 2.500754 | 2.679137 |
| Ni3+ − O2− | 0.029356 | 2.500754 | 2.679137 |
| Mn4+ − O2− | 0.029658 | 2.440000 | 3.012000 |
| Co3+ − O2− | 0.010958 | 2.400628 | 3.461272 |
| O2− − O2− | 0.042395 | 3.358701 | 1.659316 |
| NMC | Ni2+ | Ni3+ | Mn4+ | Co3+ |
|---|---|---|---|---|
| 111 | 0.33 | 0 | 0.33 | 0.33 |
| 532 | 0.3 | 0.2 | 0.3 | 0.2 |
| 622 | 0.2 | 0.4 | 0.2 | 0.2 |
| 721 | 0.2 | 0.5 | 0.2 | 0.1 |
| 811 | 0.1 | 0.7 | 0.1 | 0.1 |
| NMC | Charge Weight | C11 (GPa) | |
|---|---|---|---|
| Simulation Result | Reference Result [Ref.] | ||
| 111 | 50% | 142.0 | 199 [50] |
| 60% | 195.7 | ||
| 80% | 326.5 | ||
| 100% | 490.4 | ||
| Ions | Partial Charges (qi) |
|---|---|
| Li+ | +0.6 |
| Ni2+ | +1.2 |
| Ni3+ | +1.8 |
| Mn4+ | +2.4 |
| Co3+ | +1.8 |
| O2− | −1.2 |
| NMC | Simulation Result (Å) | Reference Result (Å) | |||
|---|---|---|---|---|---|
| x-Axis | z-Axis | x-Axis | [Ref.] | z-Axis | |
| 111 | 2.955 | 14.703 | 2.862 | [61] | 14.238 |
| 2.865 | [62] | 14.249 | |||
| 2.865 | [63] | 14.25 | |||
| 2.868 | [47] | 14.213 | |||
| 2.8125 | [64] | 14.42 | |||
| 532 | 2.951 | 14.685 | 2.925 | [64] | 14.42 |
| 622 | 2.936 | 14.610 | 2.8683 | [65] | 14.2241 |
| 2.91 | [64] | 14.39 | |||
| 721 | 2.942 | 14.638 | 2.8565 | [66] | 14.1576 |
| 811 | 2.932 | 14.590 | 2.83 | [67] | 14.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, I.U.; Lee, S. Molecular Dynamics Study of the Ni Content-Dependent Mechanical Properties of NMC Cathode Materials. Crystals 2025, 15, 272. https://doi.org/10.3390/cryst15030272
Haq IU, Lee S. Molecular Dynamics Study of the Ni Content-Dependent Mechanical Properties of NMC Cathode Materials. Crystals. 2025; 15(3):272. https://doi.org/10.3390/cryst15030272
Chicago/Turabian StyleHaq, Ijaz Ul, and Seungjun Lee. 2025. "Molecular Dynamics Study of the Ni Content-Dependent Mechanical Properties of NMC Cathode Materials" Crystals 15, no. 3: 272. https://doi.org/10.3390/cryst15030272
APA StyleHaq, I. U., & Lee, S. (2025). Molecular Dynamics Study of the Ni Content-Dependent Mechanical Properties of NMC Cathode Materials. Crystals, 15(3), 272. https://doi.org/10.3390/cryst15030272







