Particle Size and Dispersity Control in High-Quality Mid-Wave Infrared HgSe Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
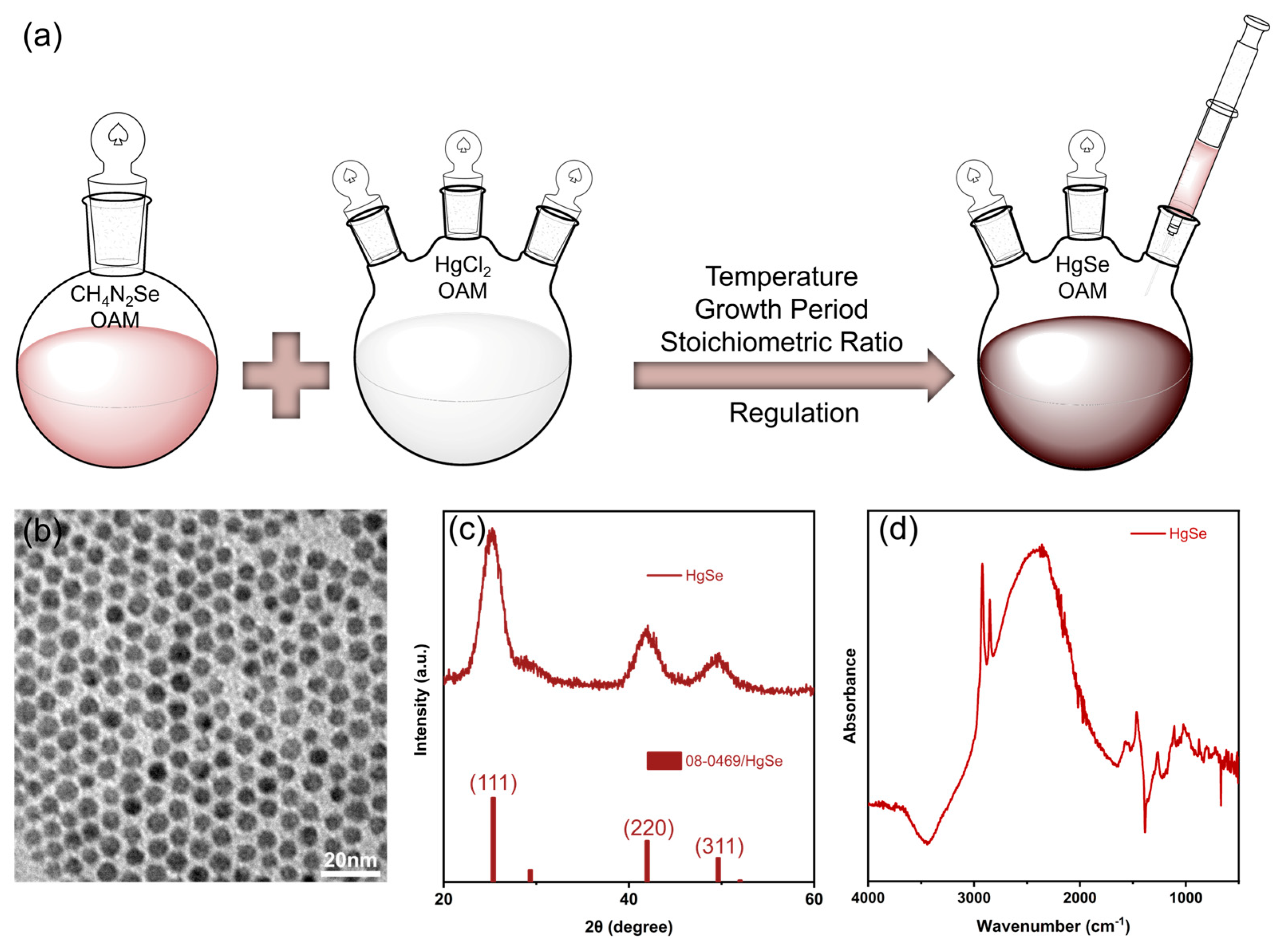
2.2. Synthesis of Precursors
2.3. Synthesis of HgSe QDs
2.4. Ligand Exchange
2.5. Film Preparation
2.6. Characterizaiton
3. Results and Discussions
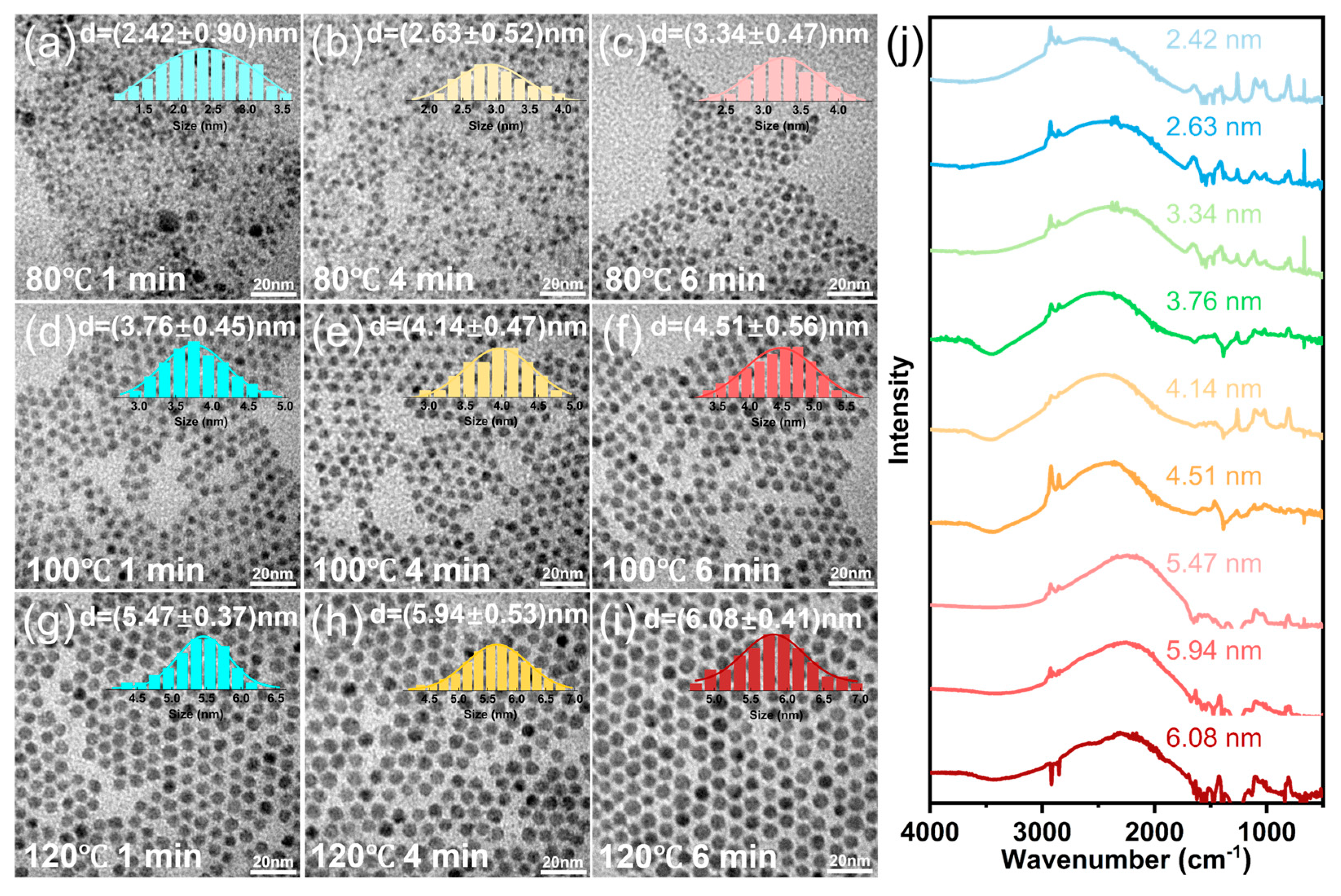
3.1. The Regulation of Reaction Temperature and the Growth Period
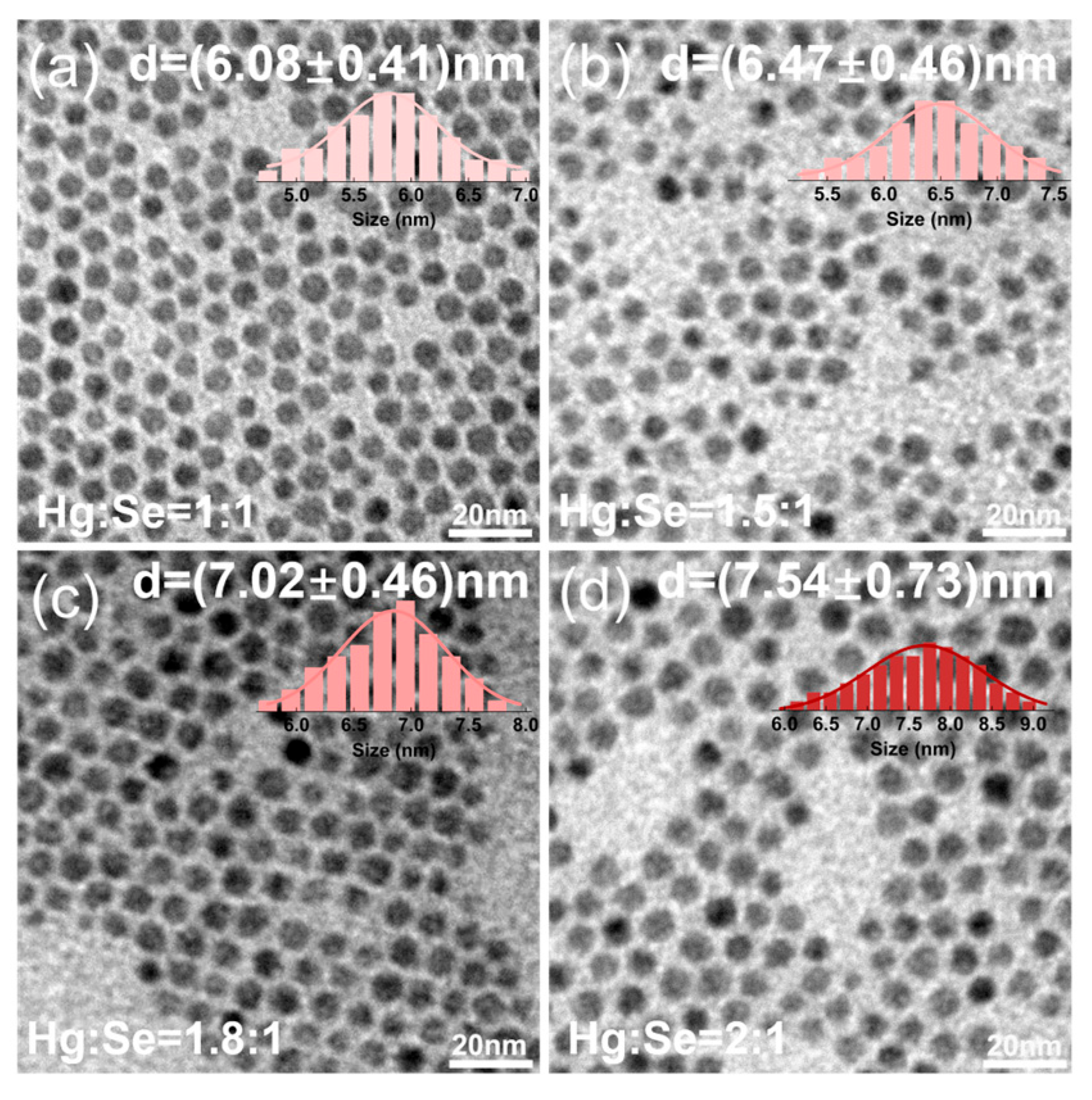
3.2. The Regulation of the Hg:Se Stoichiometric Ratio
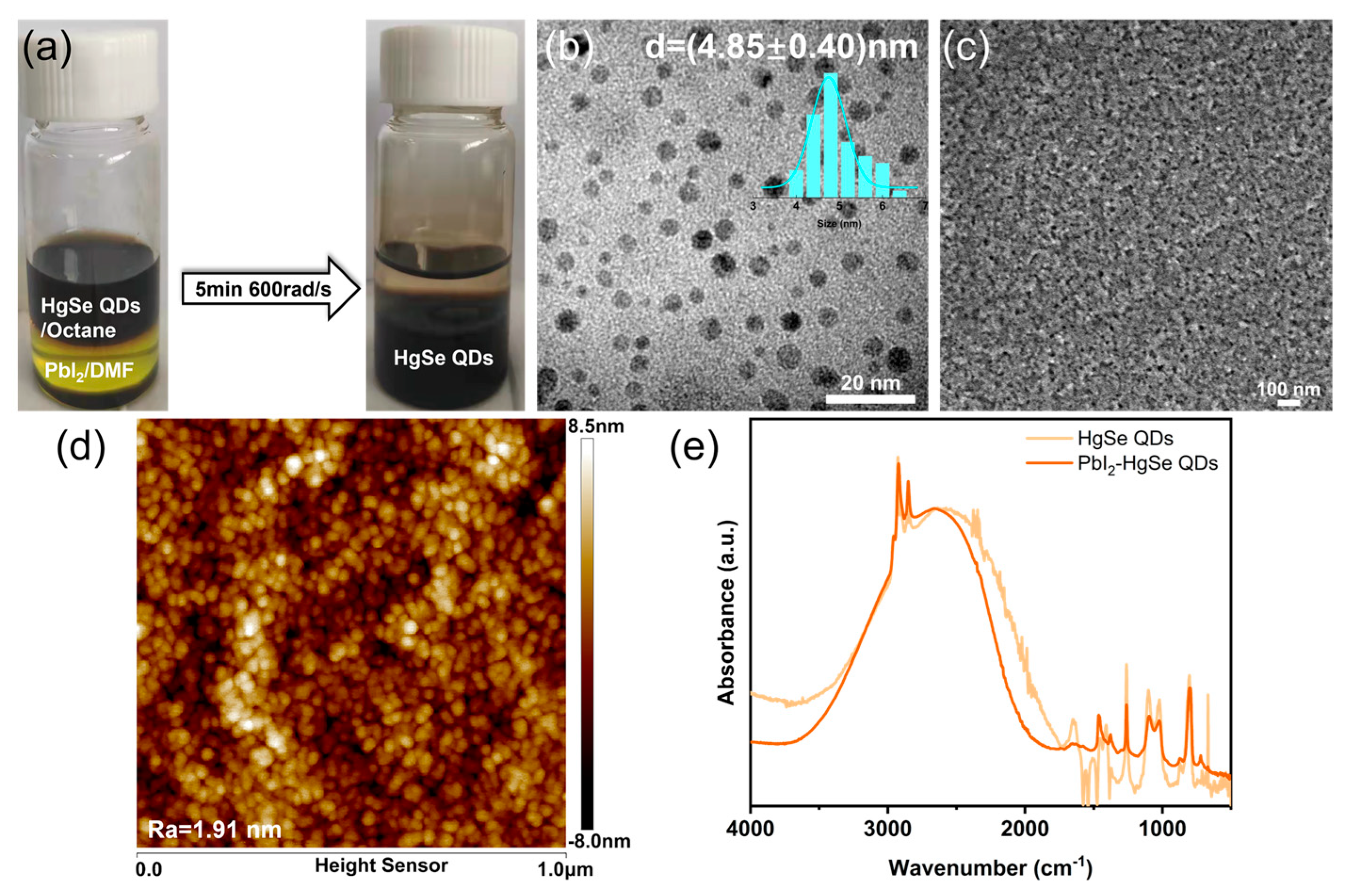
3.3. Phase Transfer and Film Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QDs | Quantum dots |
References
- Lhuillier, E.; Guyot-Sionnest, P. Recent Progresses in Mid Infrared Nanocrystal Optoelectronics. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photonics Lasers Med. 2012, 1, 255–266. [Google Scholar] [CrossRef]
- Rogalski, A. Recent progress in infrared detector technologies. Infrared Phys. Techn. 2011, 54, 136–154. [Google Scholar] [CrossRef]
- Keuleyan, S.; Lhuillier, E.; Brajuskovic, V.; Guyot-Sionnest, P. Mid-infrared HgTe colloidal quantum dot photodetectors. Nat. Photonics 2011, 5, 489–493. [Google Scholar] [CrossRef]
- Guyot-Sionnest, P.; Roberts, J.A. Background limited mid-infrared photodetection with photovoltaic HgTe colloidal quantum dots. Appl. Phys. Lett. 2015, 107, 395–423. [Google Scholar] [CrossRef]
- Deng, Z.; Guyot-Sionnest, P. Colloidal Quantum Dots Intraband Photodetectors. ACS Nano 2014, 8, 11707–11714. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, A. HgCdTe infrared detector material: History, status and outlook. Rep. Prog. Phys. 2005, 68, 2267–2336. [Google Scholar] [CrossRef]
- Deng, Z.; Guyot-Sionnest, P. Intraband Luminescence from HgSe/CdS Core/Shell Quantum Dots. ACS Nano 2016, 10, 2121–2127. [Google Scholar] [CrossRef]
- Zhao, X.; Mu, G.; Tang, X.; Chen, M. Mid-IR Intraband Photodetectors with Colloidal Quantum Dots. Coatings 2022, 12, 467. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Guo, T.; Cao, S. A Long Wave-Infrared Miniatured Quantum Dot Spectrometer. Anal. Chem. 2024, 96, 14090–14098. [Google Scholar] [CrossRef]
- Yu, C.; Shan, Y.; Zhu, J.; Sun, D.; Zheng, X.; Zhang, N.; Hou, J.; Fang, Y.; Dai, N.; Liu, Y. Heterojunctions of Mercury Selenide Quantum Dots and Halide Perovskites with High Lattice Matching and Their Photodetection Properties. Materials 2024, 17, 1864. [Google Scholar] [CrossRef]
- Sevim Ünlütürk, S.; Taşcıoğlu, D.; Özçelik, S. Colloidal quantum dots as solution-based nanomaterials for infrared technologies. Nanotechnology 2024, 36, 082001. [Google Scholar] [CrossRef]
- Livache, C.; Martinez, B.; Goubet, N.; Gréboval, C.; Qu, J.; Chu, A.; Royer, S.; Ithurria, S.; Silly, M.G.; Dubertret, B.; et al. A colloidal quantum dot infrared photodetector and its use for intraband detection. Nat. Commun. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, I.; Özdemir, O.; Christodoulou, S.; Gupta, S.; Dalmases, M.; Torre, I.; Konstantatos, G. Mid- and Long-Wave Infrared Optoelectronics via Intraband Transitions in PbS Colloidal Quantum Dots. Nano Lett. 2020, 20, 1003–1008. [Google Scholar] [CrossRef]
- Li, S.S.; Xia, J.B. Intraband optical absorption in semiconductor coupled quantum dots. Phys. Rev. B 1997, 55, 15434–15437. [Google Scholar] [CrossRef]
- Sato, S.A.; Lucchini, M.; Volkov, M.; Schlaepfer, F.; Gallmann, L.; Keller, U.; Rubio, A. Role of intraband transitions in photocarrier generation. Phys. Rev. B 2018, 98, 035202. [Google Scholar] [CrossRef]
- Qu, J.; Goubet, N.; Livache, C.; Martinez, B.; Amelot, D.; Gréboval, C.; Chu, A.; Ramade, J.; Cruguel, H.; Ithurria, S.; et al. Intraband Mid-Infrared Transitions in Ag2Se Nanocrystals: Potential and Limitations for Hg-Free Low-Cost Photodetection. J. Phys. Chem. C 2018, 122, 18161–18167. [Google Scholar] [CrossRef]
- Park, M.; Choi, D.; Choi, Y.; Shin, H.-B.; Jeong, K.S. Mid-Infrared Intraband Transition of Metal Excess Colloidal Ag2Se Nanocrystals. ACS Photonics 2018, 5, 1907–1911. [Google Scholar] [CrossRef]
- Palosz, W.; Trivedi, S.; DeCuir, E.; Wijewarnasuriya, P.S.; Thon, S.M.; Cheng, Y.; Lu, C.; Jensen, J.L. Synthesis and Characterization of Large PbSe Colloidal Quantum Dots. Part. Part. Syst. Char. 2021, 38, 2000285. [Google Scholar] [CrossRef]
- Ponomarenko, V.P.; Popov, V.S.; Shuklov, I.A.; Ivanov, V.V.; Razumov, V.F. Photosensors based on colloidal quantum dots. Russ. Chem. Rev+ 2024, 93, RCR5113. [Google Scholar] [CrossRef]
- Pierini, S.; Capitani, F.; Scimeca, M.; Kozlov, S.; Pierucci, D.; Alchaar, R.; Abadie, C.; Khalili, A.; Cavallo, M.; Dang, T.H.; et al. Vanishing Confinement Regime in Terahertz HgTe Nanocrystals Studied under Extreme Conditions of Temperature and Pressure. J. Phys. Chem. Lett. 2022, 13, 6919–6926. [Google Scholar] [CrossRef]
- Lee, W.; Smith, A.M. Interdiffusion-enhanced cation exchange for HgSe and HgCdSe nanocrystals with infrared bandgaps. Nat. Synth. 2024, 3, 1243–1254. [Google Scholar] [CrossRef]
- Chen, M.; Shen, G.; Guyot-Sionnest, P. State-Resolved Mobility of 1 cm2/(Vs) with HgSe Quantum Dot Films. J. Phys. Chem. Lett. 2020, 11, 2303–2307. [Google Scholar] [CrossRef]
- Sokolova, D.; Dyomkin, D.V.; Katsaba, A.V.; Bocharova, S.I.; Razumov, V.F. Effect of different ligand types on sensitivity of infrared photodetectors based on colloidal HgSe quantum dots. Infrared Phys. Techn. 2022, 123, 104188. [Google Scholar] [CrossRef]
- Chen, M.; Shen, G.; Guyot-Sionnest, P. Size Distribution Effects on Mobility and Intraband Gap of HgSe Quantum Dots. J. Phys. Chem. C 2020, 124, 16216–16221. [Google Scholar] [CrossRef]
- Liu, L.; Long, Z.; Shi, K.; Zhong, H. A General Crystallization Picture of Quantum Dots: The Underlying Physical Chemistry. CCS Chem. 2025, 7, 926–949. [Google Scholar] [CrossRef]
- Wang, S.; Guo, T.; Cao, S. The Influence of Synthetic Parameters on HgSe QDs. ACS Omega 2023, 8, 44804–44811. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Voznyy, O.; Sabatini, R.; Garcia de Arquer, F.P.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic-inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nat. Mater. 2017, 16, 258–263. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Jia, Y.; Liu, X.; Liu, T.; Fu, T.; Li, J.; Weng, B.; Zhang, X.; Liu, Y. Manipulation of Phase-Transfer Ligand-Exchange Dynamics of PbS Quantum Dots for Efficient Infrared Photovoltaics. J. Phys. Chem. C 2019, 123, 30137–30144. [Google Scholar] [CrossRef]
- Lin, Q.; Yun, H.J.; Liu, W.; Song, H.-J.; Makarov, N.S.; Isaienko, O.; Nakotte, T.; Chen, G.; Luo, H.; Klimov, V.I.; et al. Phase-Transfer Ligand Exchange of Lead Chalcogenide Quantum Dots for Direct Deposition of Thick, Highly Conductive Films. J. Am. Chem. Soc. 2017, 139, 6644–6653. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, S.; Wang, L.; Zhang, K.; Song, J.; Wang, L.; Li, L.-S. Particle Size and Dispersity Control in High-Quality Mid-Wave Infrared HgSe Quantum Dots. Crystals 2025, 15, 872. https://doi.org/10.3390/cryst15100872
Zang S, Wang L, Zhang K, Song J, Wang L, Li L-S. Particle Size and Dispersity Control in High-Quality Mid-Wave Infrared HgSe Quantum Dots. Crystals. 2025; 15(10):872. https://doi.org/10.3390/cryst15100872
Chicago/Turabian StyleZang, Shuaipu, Lingshi Wang, Kun Zhang, Jiaojiao Song, Lei Wang, and Lin-Song Li. 2025. "Particle Size and Dispersity Control in High-Quality Mid-Wave Infrared HgSe Quantum Dots" Crystals 15, no. 10: 872. https://doi.org/10.3390/cryst15100872
APA StyleZang, S., Wang, L., Zhang, K., Song, J., Wang, L., & Li, L.-S. (2025). Particle Size and Dispersity Control in High-Quality Mid-Wave Infrared HgSe Quantum Dots. Crystals, 15(10), 872. https://doi.org/10.3390/cryst15100872




