Mono- and Polynuclear Hg(II) Complexes with Mixed Ligands: Nicotinamide and Oxalate, Nitrate, or Sulphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Complexes
2.2.1. [Hg2(ox)2(NA)4]n·3nH2O (1): A solution with a volume of 10 mL was obtained by dissolving 2 mmol (0.244 g) of nicotinamide in distilled water. Then, we added it to a water solution (20 mL) of 1 mmol (0.525 g) mercurous nitrate. We stirred the mixture for 10 min at room temperature, and after that, we mixed it with a 20 mL volume of an ammonium oxalate (1 mmol, 0.124 g) solution. The solution obtained was refluxed for 1 h and left to slowly evaporate at room temperature. After two weeks, colorless crystals were separated. Yield: 2.85% (31.90 mg) (based on Hg2(NO3)2)
Hg2C28H30N8O15
2.2.2. [Hg(NO3)2(NA)2(H2O)2]·2NA (2): We added 3 mL solution of diluted nitric acid (5%) to a water solution (10 mL) of mercurous acetate (1 mmol, 0.519 g), and we gently stirred the mixture for two minutes. After this, we added a volume of 15 mL water–ethanol (1:2) nicotinamide solution (2 mmol, 0.244 g) and we refluxed the final mixture for 1h. After cooling and filtering, we left the filtrate to slowly evaporate. After several days, the appearance of colorless crystals was observed. Yield: 28.03% (238 mg) (based on Hg2(CH3COO)2)
HgC24H28N10O12
2.2.3. [Hg2(SO4)2(H2O)2(NA)4]·6H2O (3): A water solution (10 mL) of mercurous acetate (1 mmol, 0.519 g) was mixed with 3 mL of diluted sulfuric acid solution (3%). Then, 15 mL of nicotinamide (2 mmol, 0.244 g) was added to the water–ethanol (1:2) solution, and the mixture obtained was refluxed for 1h. After cooling and filtering, we left the solution to slowly evaporate. In a few days, colorless crystals were separated. The single-crystal X-ray diffraction analysis revealed that these crystals correspond to the compound C6H7N2O+·HSO4−, indicating that they are formed from protonated nicotinamide and an acid sulfate anion. Because the synthesis of the complex failed and the mercurous acetate had no role in our attempt, we tried to use the obtained compound to achieve our goal. Accordingly, a 10 mL solution (1 mmol, 0.22 g) of this compound was mixed with a 5 mL solution of mercurous acetate (0.5 mmol, 0.26 g), and the final solution was refluxed for 1h. After several days, colorless crystals appeared, and they proved to be the desired coordination compound, having the formula [Hg2(SO4)2(H2O)2(NA)4]·6H2O. Yield: 14.25% (43 mg) (based on Hg2(CH3COO)2)
HgC12H20N4O10S
2.3. Single Crystal X-Ray Diffraction and Refinement
2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.5. NMR Spectroscopy
2.6. Photoluminescence
2.7. Thermogravimetric Analysis (TG)
2.8. Elemental Analysis
3. Results
3.1. Structural Characterization
3.2. FT-IR Spectra
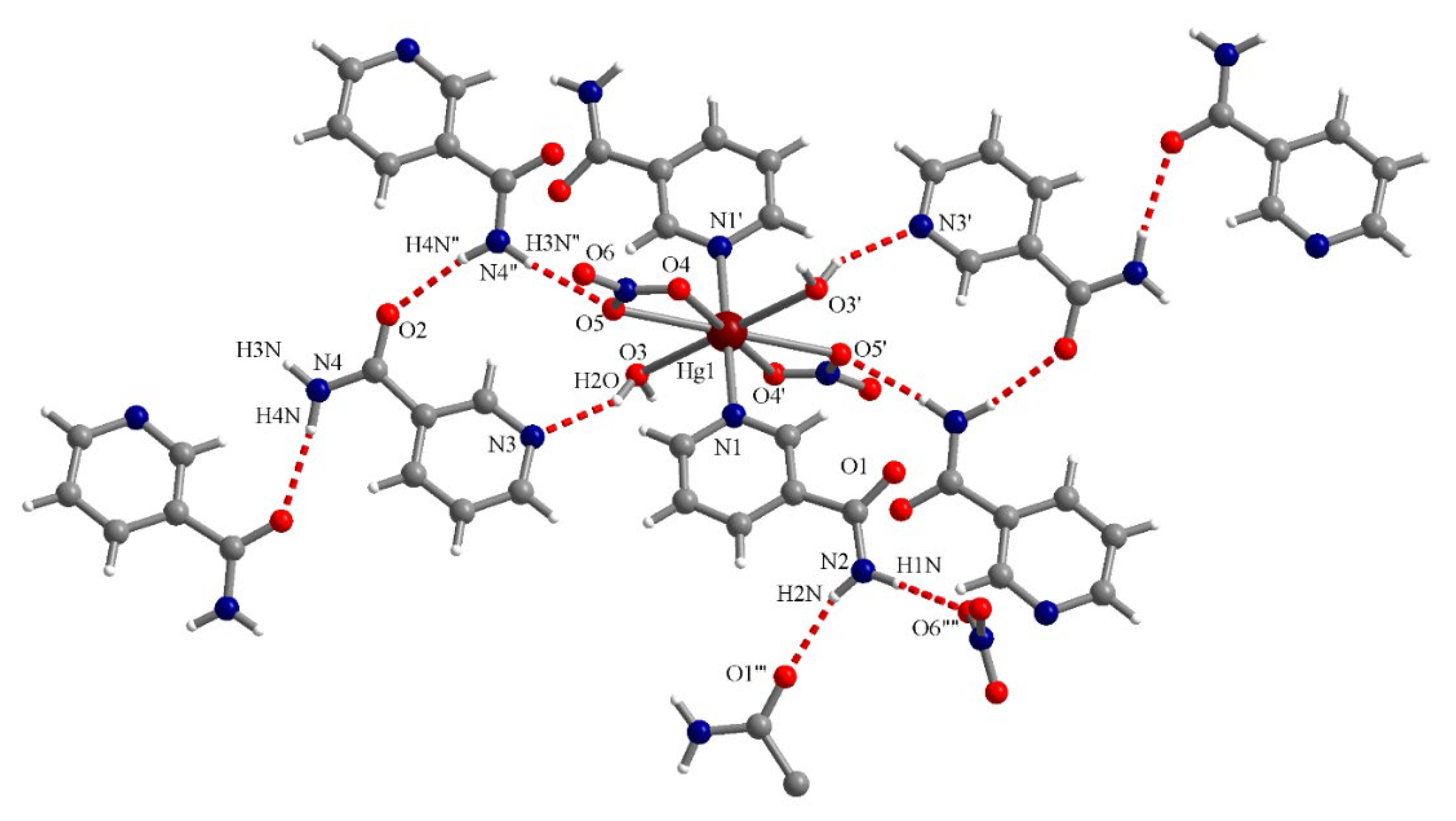
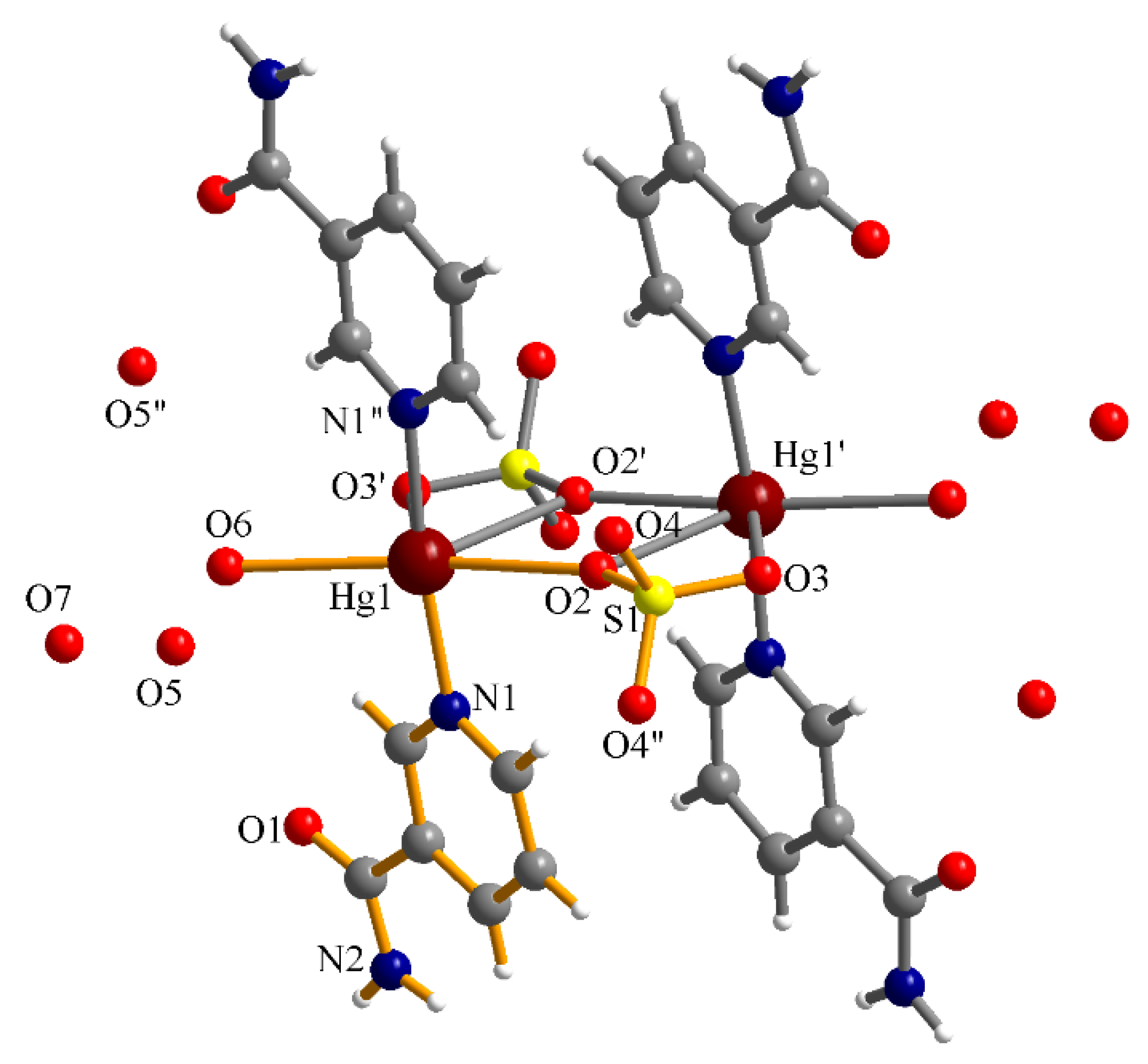
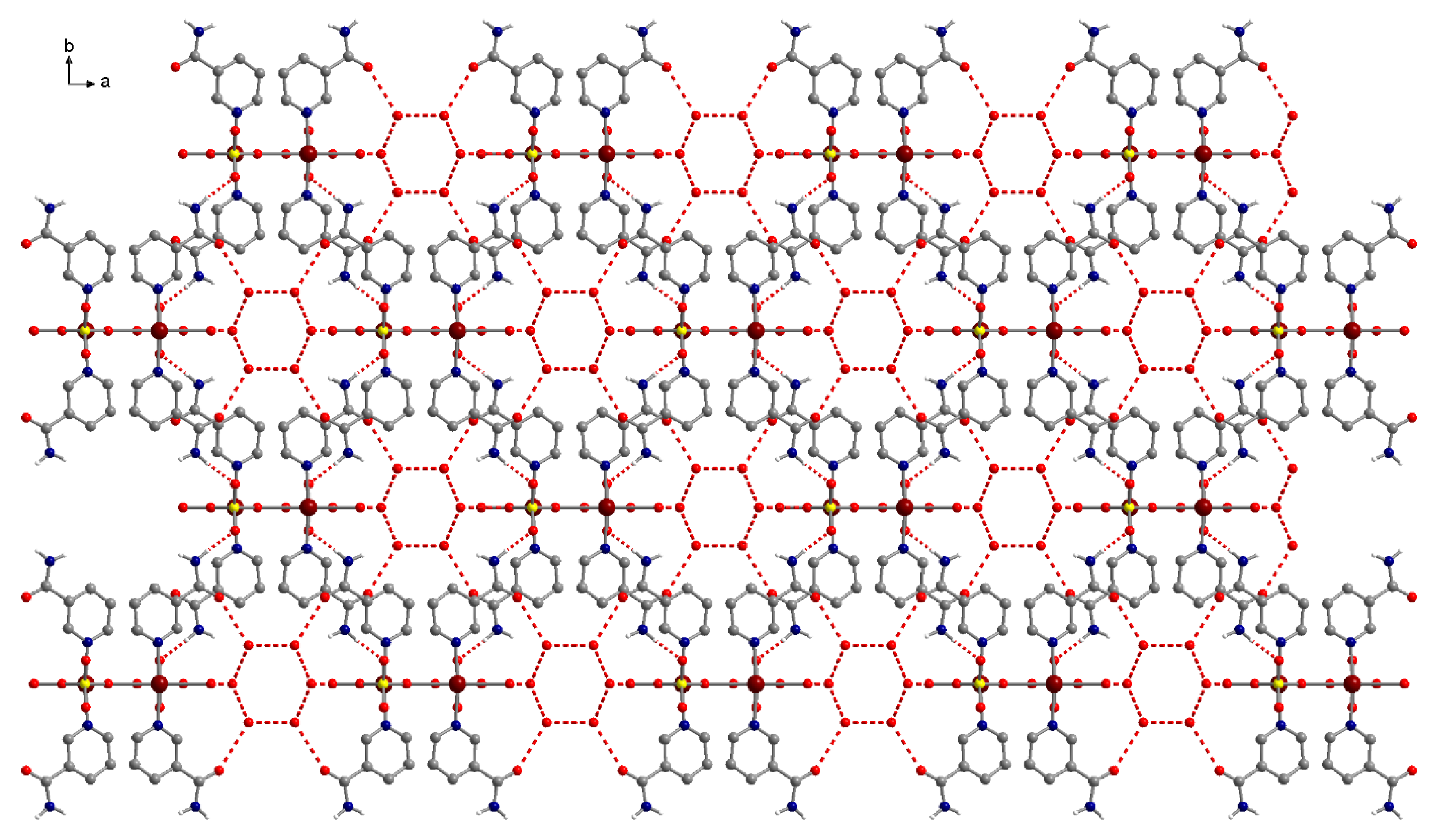
3.3. Crystal Structure Descriptions
3.3.1. Complex (1)
3.3.2. Complex (2)
3.3.3. Complex (3)
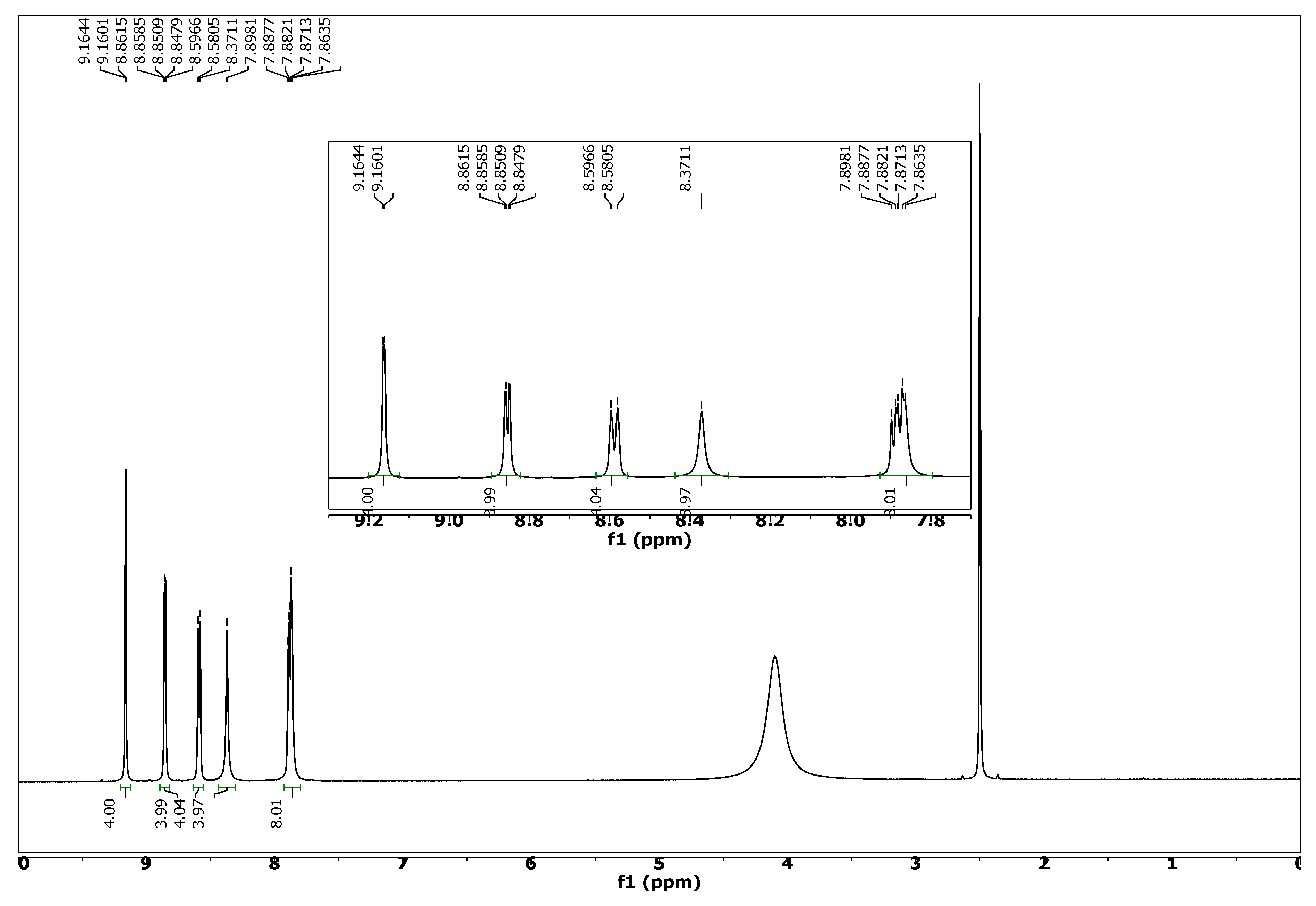
3.4. Solution Phase Magnetic Resonance Spectroscopy (NMR)
3.5. Thermogravimetric Analysis
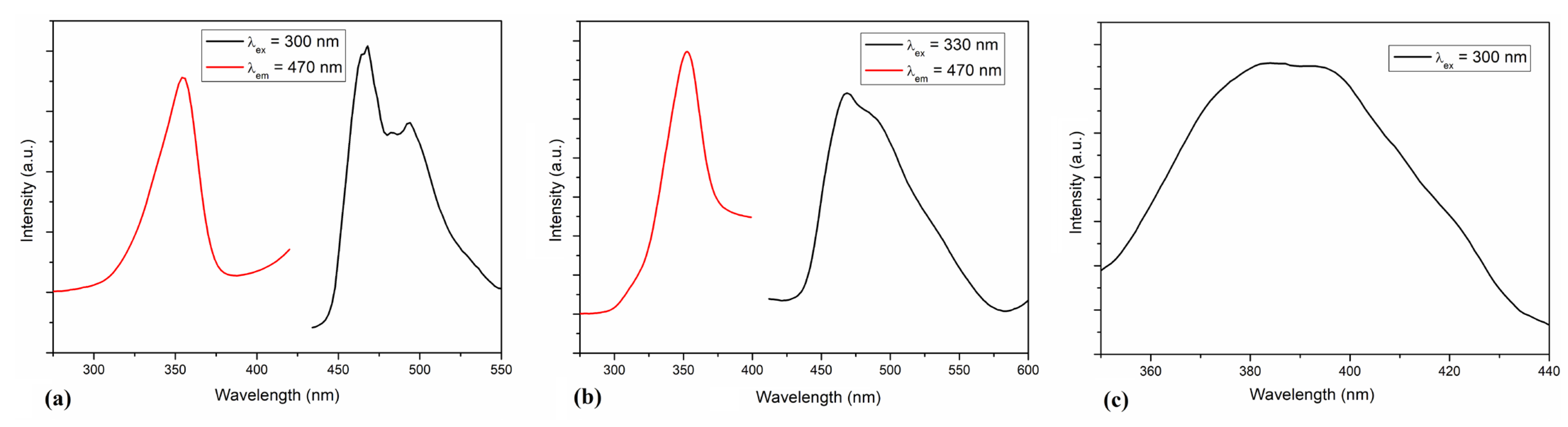
3.6. Luminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bozkurt, N.; Dilek, N.; Delibaș, N.C.; Necefoğlu, H.; Hökelek, T. Di-µ-nicotinamide-k2N1:O; k2O: N1-bis[aquabis(3-chlorobenzoato-k2O,O’) cadmium]. Acta Cryst. 2013, E69, m389–m390. [Google Scholar]
- Zhao, X.-L.; Mak, T.C.W. Silver cages with encapsulated acetylenediide as building blocks for hydrothermal synthesis of supramolecular complexes with n-cyanopyridine and pyridine-n-carboxamide (n = 3,4). Dalton Trans. 2004, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Kozlevčar, B.; Leban, I.; Turel, I.; Šegedin, P.; Petric, M.; Pohleven, F.; White, A.J.P.; Williams, D.J.; Sieler, J. Complexes of copper(II) acetate with nicotinamide: Preparation, characterization and fungicidal activity: Crystal structures of [Cu2(O2CCH3)4(nia)] and [Cu2(O2CCH3)4(nia)2]. Polyhedron 1999, 18, 755–762. [Google Scholar] [CrossRef]
- Monfared, H.H.; Kalantari, Z.; Kamyabi, M.-A.; Janiak, C. Synthesis, structural characterization and electrochemical studies of a nicotinamide-bridged dinuclear copper complex derived from a tridentate hydrazone Schiff base ligand. Z. Anorg. Allg. Chem. 2007, 633, 1945–1948. [Google Scholar] [CrossRef]
- Özbek, F.E.Ö.; Sertçelik, M.; Yüksek, M.; Elmalı, A.; Şahin, E. The superiority of the classical synthesis compared to the hydrothermal synthesis upon the structural, optical absorption and fluorescent properties of new Cd(II) 3-fluorobenzoate complexes with pyridine-3-carboxamide/pyridine-3-carboxylate. Inorg. Chim. Acta 2020, 509, 119694. [Google Scholar] [CrossRef]
- Malekhoseini, A.; Montazerozohori, M.; Naghiha, R.; Kokdan, E.P.; Joohari, S. Antimicrobial/antioxidant and cytotoxicity activities of some new mercury(II) complexes. Chem. Rev. Lett. 2023, 6, 166–182. [Google Scholar]
- Aliabadi, A.; Motieiyan, E.; Hosseinabadi, F.; Ghadermazi, M.; Abdolmaleki, S. One-pot synthesis, crystallographic characterization, evaluation as in vitro antibacterial and cytotoxic agents of two mercury(II) complexes containing pyridine dicarboxylic acid derivatives. J. Mol. Struct. 2021, 1226, 129405. [Google Scholar] [CrossRef]
- Maqsood, F.; Al-Rawi, S.S.; Ibrahim, A.H.; Jamil, F.; Zafar, A.; Iqbal, M.A.; Shoukat, U.S.; Asad, M.; Zia, S.U.; Ahmad, F.; et al. Recent trends in medicinal applications of mercury based organometallic and coordination compounds. Rev. Inorg. Chem. 2025, 45, 375–396. [Google Scholar] [CrossRef]
- Alshater, H.; Al-Sulami, A.I.; Aly, S.A.; Abdalla, E.M.; Sakr, M.A.; Hassan, S.S. Antitumor and antibacterial activity of Ni(II), Cu(II), Ag(I), and Hg(II) complexes with ligand derived from thiosemicarbazones: Characterization and theoretical studies. Molecules 2023, 28, 2590. [Google Scholar] [CrossRef]
- Akhtari, E.; Mohammadi, K.; Hayati, P.; Keshavarzi, S.; Tavallaei, O.; Derakhshankhah, H.; Karamveysi, H.; Mahammadpour, M.; Retailleau, P. In Vitro Anticancer and Antibacterial Activity of a New Hg(II) Pyridinedicarboxylate Coordination Supramolecular Compound. Appl. Organomet. Chem. 2025, 39, e70173. [Google Scholar] [CrossRef]
- Shahzad, A.; Khan, E.; Said, M.; Khan, G.S.; Sied, M.G.; Noor, A.; Zahoor, M.; Ullah, R.; Bari, A. Complexes of 1,3-diisobutyl thiourea with copper(I), zinc(II) and mercury(II): Their antioxidant and antibacterial evaluation. Crystals 2021, 11, 989. [Google Scholar] [CrossRef]
- Kim, J.A.; Park, H.; Kim, J.C.; Lough, A.J.; Pyun, S.Y.; Roh, J.; Lee, B.M. 1D copper(II) and Zn(II) coordination polymers containing an unusual twisted oxalate bridge. Inorg. Chim. Acta 2008, 361, 2087–2093. [Google Scholar] [CrossRef]
- Yesilel, O.Z.; Erer, H.; Odabasoglu, M.; Buyukgungor, O. A novel copper(II)-hydrogen oxalate coordination polymer showing a new coordination mode. J. Inorg. Organomet. Polym. Mater. 2010, 20, 78–82. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Lorenzo-Luis, P.A.; Ruiz-Perez, C. A new coordination mode in oxalate-bridged polymers: Molecular and crystal structure of [Cu3(C2O4)2(C2H8N2)4(ClO4)2]n·2H2O. CrystEngComm 2001, 3, 60–63. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Zhao, K.; Xu, J.Q.; Yu, J.H.; Li, G.H.; Zhang, X.; Bie, H.Y.; Wang, T.G. Novel oxalate coordination mode and roles: Synthesis, structure and fluorescence property of [Cd2(µ5-ox)(µ3-OH)2] with 3-D structure. Inorg. Chem. Commun. 2004, 7, 1154–1156. [Google Scholar] [CrossRef]
- Louka, F.R.Y.; Mautner, F.A.; Vicente, R.; Massoud, S.S. µ2-oxalato-bridged tricopper(II) complex derived from 1,4,8,12-tetraazacyclopentadecane: Synthesis, structure and magnetic characterization. Inorg. Chem. Commun. 2008, 4, 438–441. [Google Scholar] [CrossRef]
- Christensen, A.N.; Norby, P.; Hanson, J.C. A crystal structure determination of HgC2O4 from synchrotron X-ray and neutron powder diffraction data. Z. Krystallograph. 1994, 209, 874–877. [Google Scholar] [CrossRef]
- Colmenero, F.; Timon, V. Mechanical anomalies in mercury oxalate and the deformation of the mercury cube coordination environment under pressure. Appl. Phys. A 2021, 127, 395. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, X.; Qin, S.; Lei, C.; Liang, F. Structure and fluorescent properties of mercury(II) pyridine-2,3-dicarboxylate coordination polymers tuned by ancillary ligands and alkaline-earth metal ions. CrystEngComm 2011, 13, 2029–2038. [Google Scholar] [CrossRef]
- Khutia, A.; Sanz-Miguel, P.J.; Lippert, B. Molecular arhitectures derived from metal ions and the flexible 3,3’-bipyridine ligand: Unexpected dimer with Hg(II). Bioinorg. Chem. Appl. 2010, 2010, 169054. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Morsali, A.; Zeller, M. Mercury(II) acetate/thiocyanate coordination polymers with n-donor ligands, spectroscopic, thermal and structural studies. Inorg. Chim. Acta 2009, 362, 217–225. [Google Scholar] [CrossRef]
- Ramazani, A.; Mahmoudi, G.; Dolatyari, L.; Morsali, A.; Hu, M.L. New mixed-anion mercury(II) complex, spectroscopic, thermal and structural studies of [Hg(bipy)2(CH3COO)2](SO4)0.5NaCl. J. Coord. Chem. 2007, 60, 2115–2120. [Google Scholar] [CrossRef]
- Soldin, Z.; Kukovec, B.M.; Matkovic-Calogovic, D.; Popovic, Z. The solvent effect on composition and dimensionality of mercury (II) complexes with picolinic acid. Molecules 2021, 26, 5002–5015. [Google Scholar] [CrossRef]
- Popovic, Z.; Pavlovic, G.; Soldin, Z. Iodo (picolinato-k2N, O)(picolinic acid, k2N, O) mercury(II). Acta Cryst. 2006, C62, m272–m274. [Google Scholar]
- Hayati, P.; Rezvani, A.R.; Morsali, A.; Retailleau, P.; Garcia-Granda, S. Influences of temperature, power ultrasound and reaction time on the morphological properties of two new mercury(II) coordination supramolecular compounds. Ultrason. Sonochem. 2017, 34, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Morsali, A.; Masoomi, M.Y. Structure and properties of mercury(II) coordination polymers. Coord. Chem. Rev. 2009, 253, 1882–1905. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Yazicilar, T.K.; Andac, O.; Kutuk, H.; Bekdemir, Y.; Harrison, W.T.A. Bis(p-nitrobenzoxasulfamato) complexes of cadmium(II) and mercury(II)–synthesis, spectra and X-ray crystal structures. Z. Anorg. Allg. Chem. 2002, 628, 1908–1912. [Google Scholar] [CrossRef]
- Morozov, I.V.; Sherezhkin, V.N.; Troyanov, S.I. Modes of coordination of the NO3- anions in inorganic nitrates. Russ. Bull. Chem. 2008, 57, 439–450. [Google Scholar] [CrossRef]
- Polonius, J.M.F.-A.; Roitzsch, M. [Hg(9-methyl-1-deazapurine)2](NO3)2.H2O: A complex with a distorted hexagonal bipyramidal metal ion coordination sphere. Inorg. Chim. Acta 2005, 358, 1225–1230. [Google Scholar]
- Dziewulska-Kulaczkowska, A.; Mazur, L.; Ferenc, W. Thermal, spectroscopic and structural studies of zinc(II) complex with nicotinamide. J. Therm. Anal. Calorim. 2009, 96, 255–260. [Google Scholar] [CrossRef]
- Lian, Z.; Zhao, N.; Yang, F.; Liu, P. Crystal structure of trans-trans-trans-diaquabis(nicotinamide)-dinitratocadmium(II)-nicotinamide(1:2), Cd(H2O)2(C6H6N2O)2(NO3)2.2C6H6N2O. Z. Crystallogr. NCS 2011, 226, 289–290. [Google Scholar]
- Papatriantafyllopoulou, C.; Manesi, E.; Escuer, A.; Perlepes, S.P. The sulphate ligand as a promising “player” in 3d-metal cluster chemistry. Inorg. Chim. Acta 2009, 362, 634–650. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N.; Dushina, S.V. Synthesis and thermal analysis of a Ni(II) complex of nicotinamide. Russ. J. Gen. Chem. 2017, 87, 613–618. [Google Scholar] [CrossRef]
- Cakir, S.; Bicer, E.; Aoki, K.; Coskun, E. Structural features of a new [Fe(nicotinamide)2(H2O)4] center dot [Fe(H2O)6] center dot (SO4)2 center dot 2H(2)O complex. Cryst. Res. Technol. 2006, 41, 314–320. [Google Scholar] [CrossRef]
- Dakovic, M.; Popovic, Z.; Giester, G.; Rajic-Linaric, M. Synthesis, spectroscopic and structural investigation of Zn(NCS)2(nicotinamide)2 and Hg(SCN)2(nicotinamide)]n. Polyhedron 2008, 27, 465–472. [Google Scholar] [CrossRef]
- Soldin, Z.; Kukovec, B.-M.; Perokovic, V.P.; Dakovic, M.; Popovic, Z. Cadmium(II) coordination compounds and mercury(II) inorganic-organic hybrid with a quaternary nicotinamide salt, 3-carbamoyl-1-carboxymethylpyridinium bromide. Inorg. Chim. Acta 2025, 582, 122662. [Google Scholar] [CrossRef]
- Amani, V.; Shokouhmanesh, M.; Khavasi, H.R. Binuclear mercury(II) complexes containing N-(naphthalene-1-yl) isonicotinamide ligand: X-ray studies and spectroscopic characterization. Inorg. Chem. Res. 2025, 9, 8–13. [Google Scholar]
- Kotai, L.; Lippart, J.; Gacs, I. Deuterium isotope separation in the chemical reaction of aluminum amalgam and water. Eur. Chem. Bull. 2012, 1, 37–38. [Google Scholar]
- Ramalingam, S.; Periandy, S.; Govindarajan, M.; Mohan, S. FT-IR and FT-Raman vibrational spectra and molecular structure investigation of nicotinamide: A combined experimental and theoretical study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 1552–1558. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B–Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 79–82. [Google Scholar]
- Andros, L.; Juric, M.; Planinic, P.; Zilic, D.; Ravkin, B.; Molcanov, K. New mononuclear oxalate complexes of copper (II) with 2D and 3D architecture: Synthesis, crystal structure and spectroscopic characterization. Polyhedron 2010, 29, 1291–1298. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v 2.1; Institut de Química Teòrica i Computacional-Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Escriva, E.; Folgado, J.V.; Espallargas, G.M.; Soto, L.; Sancho, A.; Perello, L.; Ortiz, R. New dinuclear copper complexes incorporating bis(imidazolyl) based ligands and bidentate-monodentate oxalate bridges. Crystal structure and magnetic properties of [Cu2(BIM)2(C2O4)2].4H2O and [Cu2(BIK)2(C2O4)2] (BIM = bis(2-imidazolyl)methane), BIK = bis(2-imidazolyl)ketone). Exploring magneto-structural correlations. Polyhedron 2016, 112, 137–144. [Google Scholar]
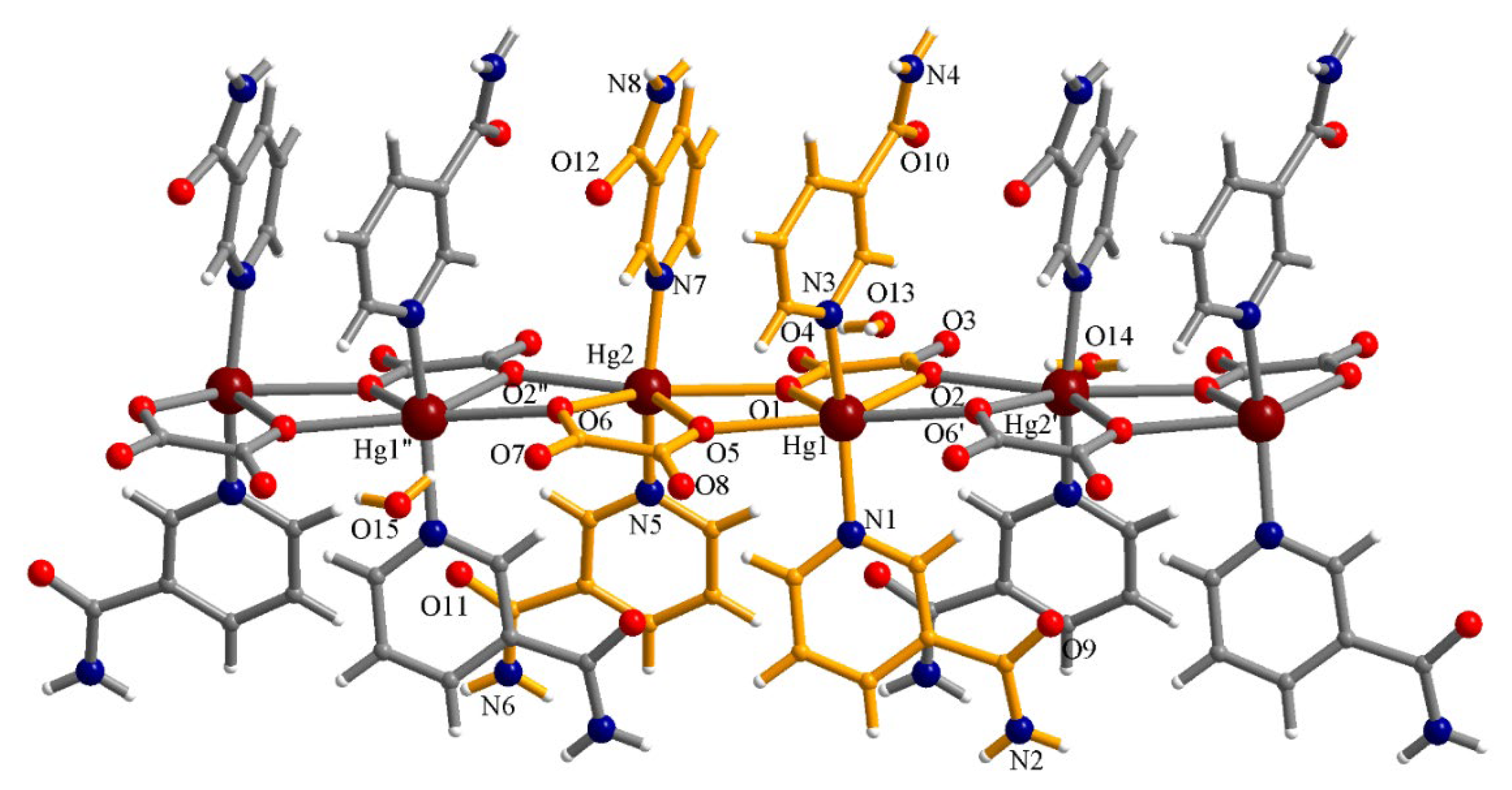
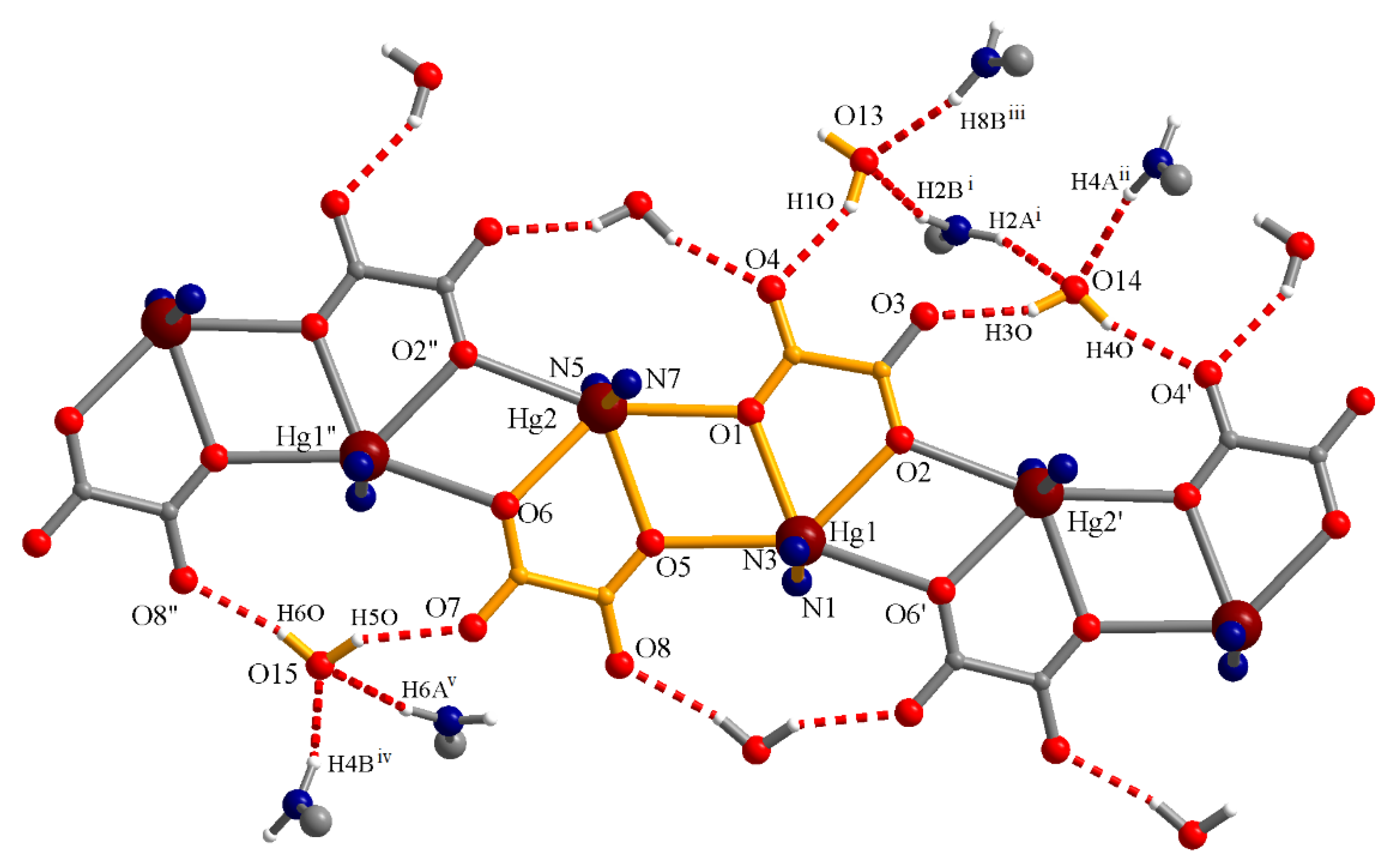
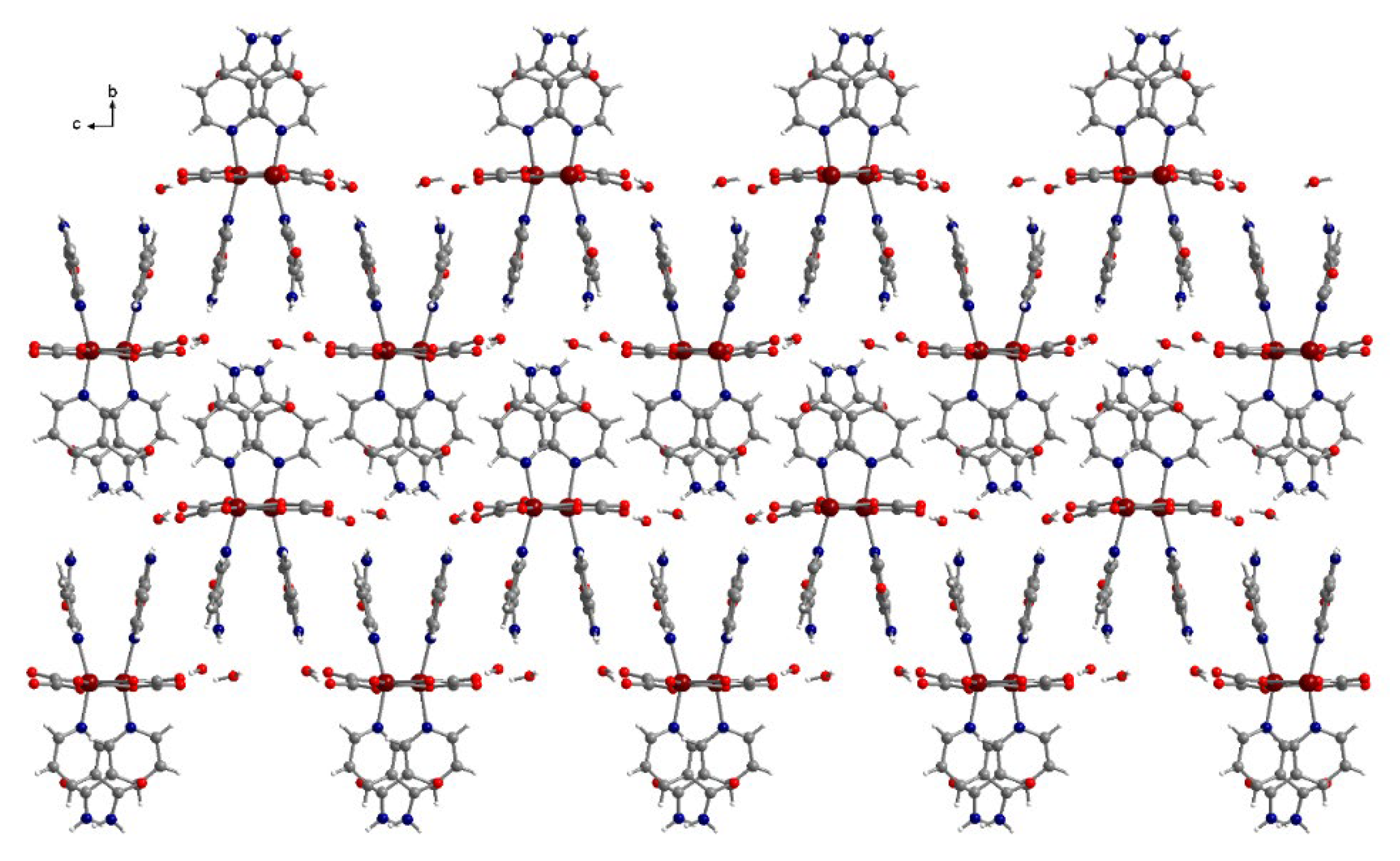
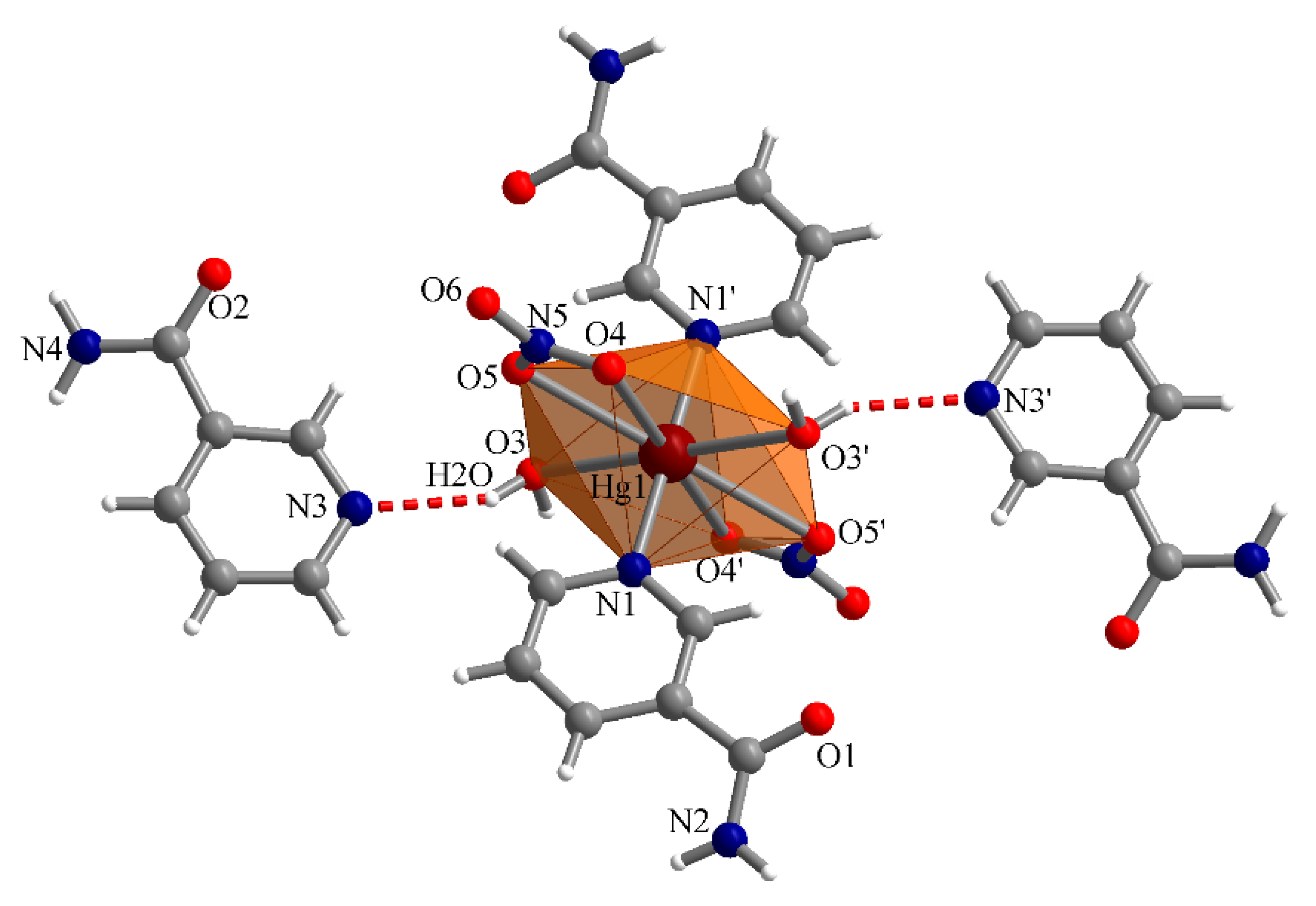
| Compound | (1) | (2) | (3) |
|---|---|---|---|
| Chemical formula | C28H30N8O15Hg2 | C24H28N10O12Hg | C12H16N4O10SHg |
| M (g mol−1) | 1119.78 | 849.15 | 612.97 |
| Temperature, (K) | 293(2) | 293(2) | 293(2) |
| Wavelength, (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n | C2/m |
| a (Å) | 7.8085(3) | 7.4611(4) | 16.5391(8) |
| b (Å) | 31.3979(11) | 22.4487(10) | 18.0007(7) |
| c (Å) | 14.2847(5) | 9.4543(4) | 6.9969(4) |
| α (°) | 90 | 90 | 90 |
| β (°) | 101.671(4) | 108.570(5) | 113.293(6) |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 3429.8(2) | 1501.07(13) | 1913.30(18) |
| Z | 4 | 2 | 4 |
| Dc (g cm−3) | 2.169 | 1.879 | 2.128 |
| Size of the crystal (mm) | 0.3 × 0.1 × 0.08 | 0.2 × 0.04 × 0.02 | 0.2 × 0.02 × 0.01 |
| μ (mm−1) | 9.025 | 5.207 | 8.213 |
| F(000) | 2136 | 836 | 1184 |
| Goodness-of-fit on F2 | 1.044 | 1.044 | 1.210 |
| Final R1, wR2 [I > 2σ(I)] | 0.0283, 0.0583 | 0.0266, 0.0617 | 0.0389, 0.1054 |
| R1, wR2 (all data) | 0.0400, 0.0619 | 0.0381, 0.0670 | 0.0405, 0.1061 |
| Atoms | Bond Distance | Atoms | Angle |
|---|---|---|---|
| Hg1–O1 | 2.437(3) | O1–Hg1–O2 | 65.79(11) |
| Hg1–O2 | 2.492(4) | O1–Hg1–O5 | 69.66(11) |
| Hg1–O5 | 2.629(3) | O2–Hg1–O5 | 135.18(11) |
| Hg1–O6 | 2.633(3) | O5–Hg1–O6 | 158.91(12) |
| Hg1–N1 | 2.163(4) | N3–Hg1–N1 | 155.55(16) |
| Hg1–N3 | 2.158(4) | N3–Hg1–O6 | 86.42(13) |
| Hg2–O1 | 2.604(3) | N1–Hg1–O6 | 89.49(16) |
| Hg2–O2 | 2.626(3) | O5–Hg2–O6 | 64.90(11) |
| Hg2–O5 | 2.509(3) | O2–Hg2–O6 | 65.59(11) |
| Hg2–O6 | 2.469(4) | O1–Hg2–O2 | 160.44(12) |
| Hg2–N5 | 2.175(4) | N5–Hg2–O2 | 89.26(13) |
| Hg2–N7 | 2.158(4) | N5–Hg2–N7 | 157.51(17) |
| D–H∙∙∙A | d(H∙∙∙A)(Å) | d(D∙∙∙A)(Å) | <<(DHA)(°) |
|---|---|---|---|
| O13–H10∙∙∙O4 | 2.022 | 2.796 | 150.87 |
| O14–H30∙∙∙O3 | 2.015 | 2.825 | 158.62 |
| O14–H40∙∙∙O4′ | 1.987 | 2.809 | 162.01 |
| O15–H50∙∙∙O7 | 2.040 | 2.793 | 147.29 |
| O15–H60∙∙∙O8″ | 1.999 | 2.833 | 167.01 |
| N8iii–H8Biii∙∙∙O13 | 2.137 | 2.969 | 162.62 |
| N2i–H2Bi∙∙∙O13 | 2.165 | 2.995 | 162.08 |
| N2i–H2Ai∙∙∙O14 | 2.205 | 2.963 | 146.86 |
| N4–H4A∙∙∙O14v | 2.154 | 2.999 | 167.5 |
| N4iv–H4Biv∙∙∙O15 | 2.194 | 3.016 | 159.6 |
| N6–H6A∙∙∙O15iii | 2.299 | 3.122 | 160.47 |
| D–H∙∙∙A | d(H∙∙∙A)(Å) | d(D∙∙∙A)(Å) | <(DHA)(°) |
|---|---|---|---|
| O3–H2O∙∙∙N3 | 2.125 | 2.901 | 151.4 |
| N4″–H4N″∙∙∙O2 | 2.115 | 2.963 | 168.6 |
| N4″–H3N″∙∙∙O5 | 2.181 | 3.037 | 173.5 |
| N2–H1N∙∙∙O6″″ | 2.120 | 2.956 | 164.2 |
| N2–H2N∙∙∙O1′″ | 2.071 | 2.906 | 163.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pricop, L.; Hanganu, A.; Ganciarov, M.; Mădălan, A.M. Mono- and Polynuclear Hg(II) Complexes with Mixed Ligands: Nicotinamide and Oxalate, Nitrate, or Sulphate. Crystals 2025, 15, 835. https://doi.org/10.3390/cryst15100835
Pricop L, Hanganu A, Ganciarov M, Mădălan AM. Mono- and Polynuclear Hg(II) Complexes with Mixed Ligands: Nicotinamide and Oxalate, Nitrate, or Sulphate. Crystals. 2025; 15(10):835. https://doi.org/10.3390/cryst15100835
Chicago/Turabian StylePricop, Laurențiu, Anamaria Hanganu, Mihaela Ganciarov, and Augustin M. Mădălan. 2025. "Mono- and Polynuclear Hg(II) Complexes with Mixed Ligands: Nicotinamide and Oxalate, Nitrate, or Sulphate" Crystals 15, no. 10: 835. https://doi.org/10.3390/cryst15100835
APA StylePricop, L., Hanganu, A., Ganciarov, M., & Mădălan, A. M. (2025). Mono- and Polynuclear Hg(II) Complexes with Mixed Ligands: Nicotinamide and Oxalate, Nitrate, or Sulphate. Crystals, 15(10), 835. https://doi.org/10.3390/cryst15100835








