Optimizing the Morphology and Optical Properties of MoS2 Using Different Substrate Placement: Numerical Simulation and Experimental Verification
Abstract
1. Introduction
2. Materials and Methods
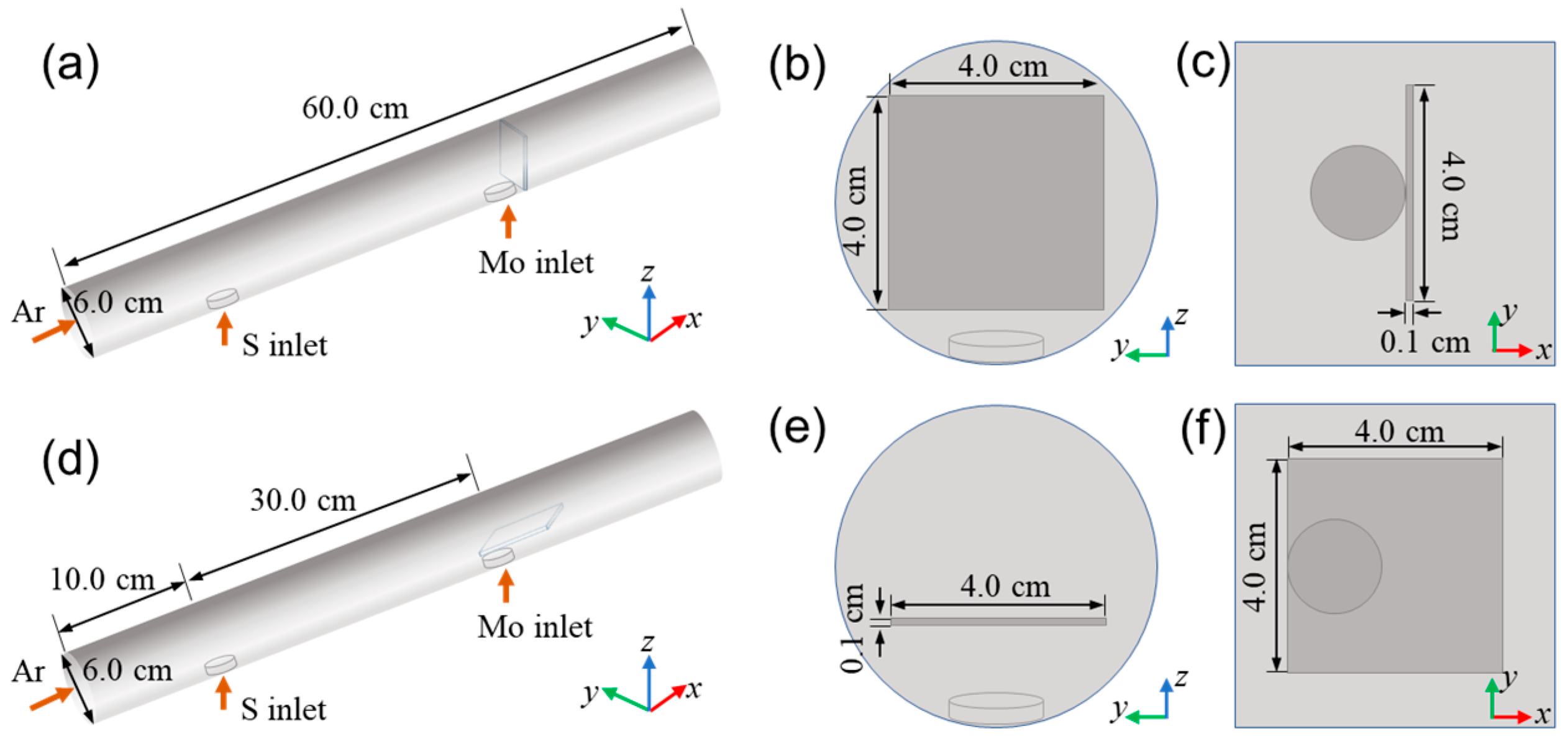
2.1. The Numerical Models
2.2. Synthesis of MoS2
2.3. Optical Measurement of PL and Raman Spectra
3. Results
3.1. The Simulation Results
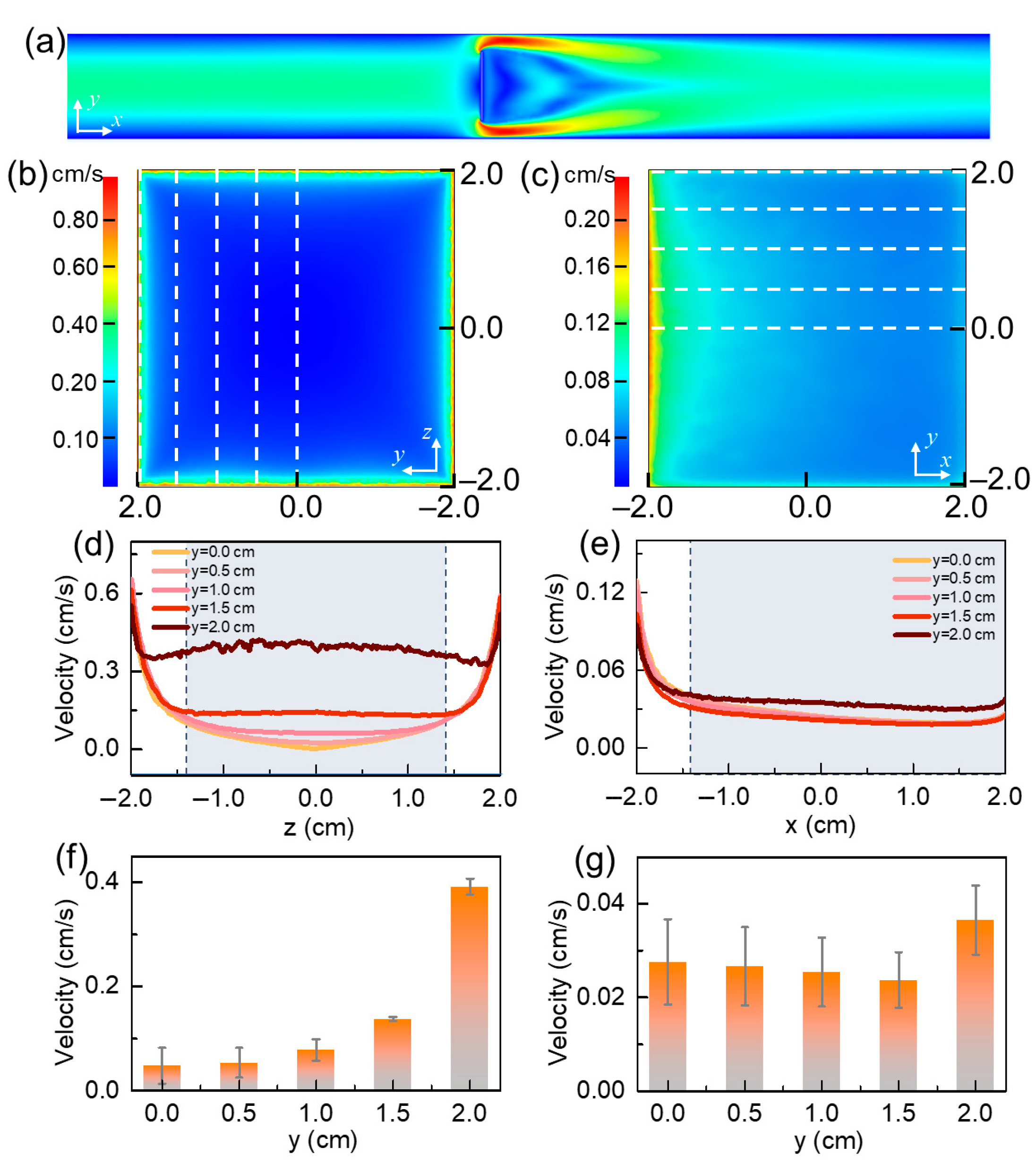
3.1.1. Velocity Distribution
3.1.2. Precursor Molar Concentration
3.2. The Morphology of MoS2 Deposited on SiO2/Si Substrate
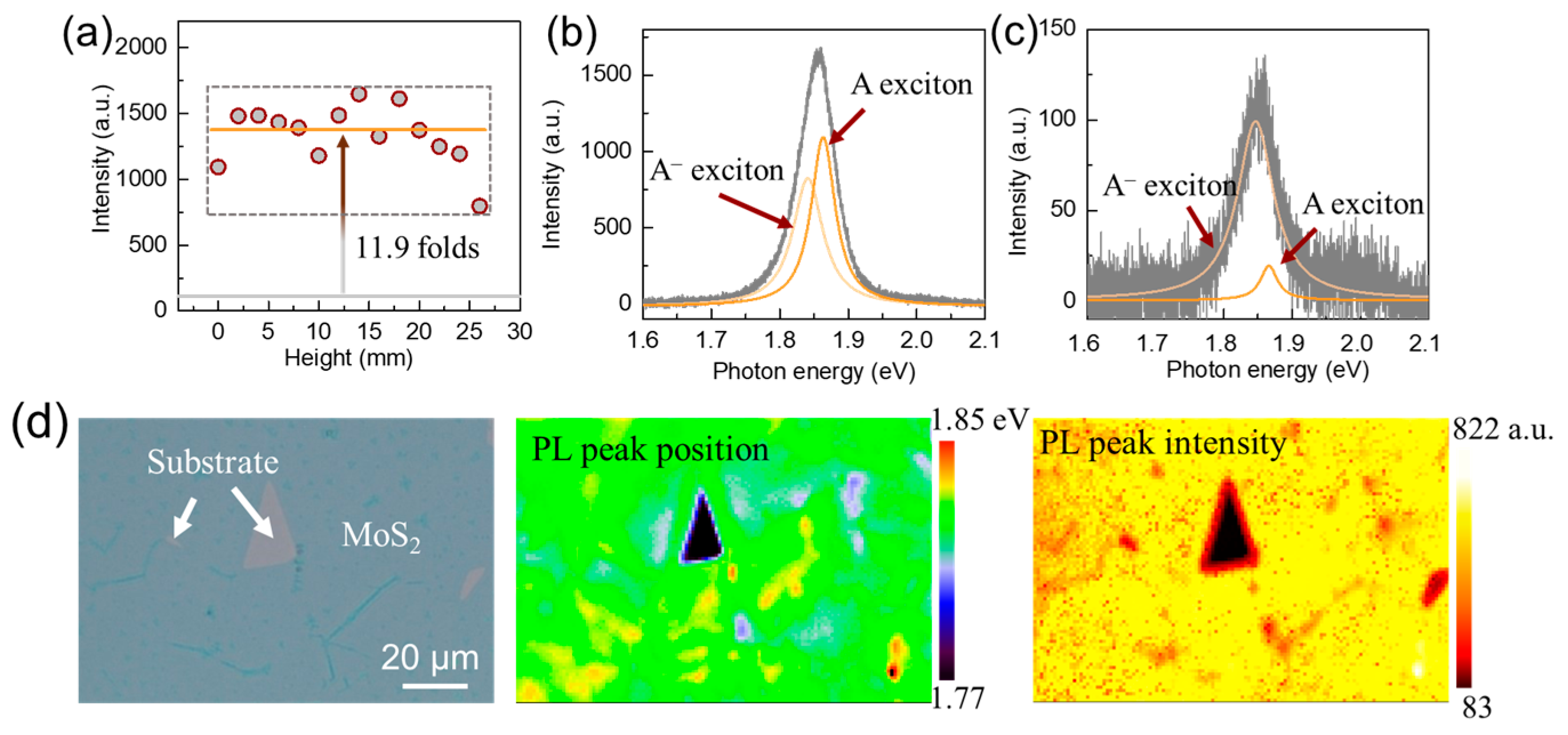
3.3. PL Emissions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, B.; Xiao, Q.L.; Wang, S.H.; Zhang, H. 2D Layered Materials: Synthesis, Nonlinear Optical Properties, and Device Applications. Laser Photonics Rev. 2019, 13, 1800327. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M. Recent Progress in Emerging 2D Transition-Metal Dichalcogenide-Based Gas Sensors: A Review. IEEE Sens. J. 2024, 24, 25221–25226. [Google Scholar] [CrossRef]
- Liu, X.F.; Guo, Q.B.; Qiu, J.R. Emerging Low-Dimensional Materials for Nonlinear Optics and Ultrafast Photonics. Adv. Mater. 2017, 29, 1605886. [Google Scholar] [CrossRef]
- Liao, F.; Wang, Y.; Peng, T.; Peng, J.; Gu, Z.Q.; Yu, H.K.; Chen, T.; Yu, J.X.; Gu, F.X. Highly Efficient Nonlinear Optical Conversion in Waveguiding GaSe Nanoribbons with Pump Pulses Down to a Femto-Joule Level. Adv. Opt. Mater. 2018, 6, 1701012. [Google Scholar] [CrossRef]
- Yu, J.X.; Xing, S.; Dai, G.Y.; Linghu, S.Y.; Gu, F.X. Direct-Bandgap Bilayer WSe2/Microsphere Monolithic Cavity for Low-Threshold Lasing. Adv. Mater. 2021, 34, 2106502. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Yu, J.X.; Gu, Z.Q.; Yang, Z.Y.; Hasan, T.; Linghu, S.Y.; Peng, J.; Fang, W.; Zhuang, S.L.; Gu, M.; et al. Enhancing Monolayer Photoluminescence on Optical Micro/nanofibers for Low-threshold Lasing. Sci. Adv. 2019, 5, eaax7398. [Google Scholar] [CrossRef]
- Yu, J.X.; Yang, L.Q.; Gu, F.X. Metal Substrate Modulation for Realizing a More Compact Whispering-gallery Microcavity. Appl. Phys. Express 2021, 14, 064001. [Google Scholar] [CrossRef]
- Gong, Y.J.; Ye, G.L.; Lei, S.D.; Shi, G.; He, Y.M.; Lin, J.H.; Zhang, X.; Vajtai, R.; Pantelides, S.T.; Zhou, W.; et al. Synthesis of Millimeter-Scale Transition Metal Dichalcogenides Single Crystals. Adv. Funct. Mater. 2016, 26, 2009–2015. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, S.P.; Li, L.; Wei, Z.; Wang, Q.Q.; Sun, H.C.; Tian, J.P.; Guo, Y.T.; Liu, J.Y.; Yu, H.; et al. Chemical Vapor Deposition of 4 Inch Wafer-Scale Monolayer MoSe2. Small Sci. 2022, 2, 2200062. [Google Scholar] [CrossRef]
- Sergeyev, D.; Duisenova, A.; Shunkeyev, K. Electronic and Optical Properties of One-Dimensional Van Der Waals Nanodevices Based on MoS2(n,n) and MoSe2(n,n) Nanotubes. Crystals 2024, 14, 1055. [Google Scholar] [CrossRef]
- Johari, M.H.; Sirat, M.S.; Mohamed, M.A.; Mustaffa, A.F.; Mohmad, A.R. Computational Fluid Dynamics In -sights into Chemical Vapor Deposition of Homogeneous MoS2 Film with Solid Precursors. Cryst. Res. Technol. 2023, 58, 2300139. [Google Scholar] [CrossRef]
- Zhou, D.; Shu, H.B.; Hu, C.L.; Jiang, L.; Liang, P.; Chen, X.S. Unveiling the Growth Mechanism of MoS2 with Chemical Vapor Deposition: From Two-Dimensional Planar Nucleation to Self-Seeding Nucleation. Cryst. Growth Des. 2018, 18, 1012–1019. [Google Scholar] [CrossRef]
- Kim, T.S.; Dhakal, K.P.; Park, E.; Noh, G.; Chai, H.J.; Kim, Y.; Oh, S.; Kang, M.; Park, J.; Kim, J.; et al. Gas-Phase Alkali Metal-Assisted MOCVD Growth of 2D Transition Metal Dichalcogenides for Large-Scale Precise Nucleation Control. Small 2022, 18, 2106368. [Google Scholar] [CrossRef]
- Zhu, L.J.; Yang, P.F.; Huan, Y.H.; Pan, S.Y.; Zhang, Z.Q.; Cui, F.F.; Shi, Y.P.; Jiang, S.L.; Xie, C.Y.; Hong, M.; et al. Scalable Salt-templated Directed Synthesis of High-Quality MoS2 Nanosheets Powders towards Energetic and Environmental Applications. Nano Res. 2020, 13, 3098–3104. [Google Scholar] [CrossRef]
- Zhou, J.D.; Lin, J.H.; Huang, X.W.; Zhou, Y.; Chen, Y.; Xia, J.; Wang, H.; Xie, Y.; Yu, H.M.; Lei, J.C.; et al. A Library of Atomically Thin Metal Chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef]
- Wang, S.S.; Rong, Y.M.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape Evolution of Monolayer MoS2 Crystals Grown by Chemical Vapor Deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Zhou, D.; Lang, J.; Yoo, N.; Unocic, R.R.; Wu, Q.H.; Li, B. Fluid-Guided CVD Growth for Large-Scale Monolayer Two-Dimensional Materials. ACS Appl. Mater. Interfaces 2020, 12, 26342–26349. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.P.; Zheng, K.; Feng, C.N.; Yao, R.; Tao, L.Q.; Yu, J.B.; Chen, X.P. Promoting Crystal Distribution Uniformity Based on the CVD Method with the Aid of Finite Element Methods. Cryst. Growth Des. 2020, 20, 777–782. [Google Scholar] [CrossRef]
- Shivayogimath, A.; Thomsen, J.D.; Mackenzie, D.M.A.; Geisler, M.; Stan, R.M.; Holt, A.J.; Bianchi, M.; Crovetto, A.; Whelan, P.R.; Carvalho, A.; et al. A Universal Approach for the Synthesis of Two-dimensional Binary Compounds. Nat. Commun. 2019, 10, 2957. [Google Scholar] [CrossRef]
- Buchkov, K.; Rafailov, P.; Minev, N.; Videva, V.; Strijkova, V.; Lukanov, T.; Dimitrov, D.; Marinova, V. Meta tungstate Chemical Vapor Deposition of WSe2: Substrate Effects, Shapes, and Morphologies. Crystals 2024, 14, 184. [Google Scholar] [CrossRef]
- Cho, Y.J.; Sim, Y.; Lee, J.H.; Hoang, N.T.; Seong, M.J. Size and Shape control of CVD-grown monolayer MoS2. Curr. Appl. Phys. 2023, 45, 99–104. [Google Scholar] [CrossRef]
- Loginov, A.B.; Kuvatov, M.M.; Ismagilov, R.R.; Sapkov, I.V.; Fedotov, P.V.; Kleshch, V.I.; Obraztsova, E.D.; Obraztsov, A.N. Evolution of Transition Metal Dichalcogenide Film Properties during Chemical Vapor Deposition: From Monolayer Islands to Nanowalls. Nanotechnology 2024, 35, 415601. [Google Scholar] [CrossRef]
- Liao, F.; Huang, H.D.; Xie, Q.Q.; Zeng, Y.H.; Liang, L.; Gu, F.X.; Zuo, Z.W. Broadband Tunable Resonance Modes from Multi-composition Monolayer MoS2(1−x)Se2x with SiO2 Microsphere Cavity. Appl. Phys. Lett. 2024, 124, 243103. [Google Scholar] [CrossRef]
- Politano, G.G.; Versace, C. Recent Advances in the Raman Investigation of Structural and Optical Properties of Graphene and Other Two-Dimensional Materials. Crystals 2023, 13, 1357. [Google Scholar] [CrossRef]
- Carozo, V.; Wang, Y.X.; Fujisawa, K.; Carvalho, B.R.; Mccreary, A.; Feng, S.M.; Lin, Z.; Zhou, C.J.; Perea-Lopez, N.; Elias, A.L.; et al. Optical Identification of Sulfur Vacancies: Bound Excitons at the Edges of Monolayer Tungsten Disulfide. Sci. Adv. 2017, 3, e1602813. [Google Scholar] [CrossRef] [PubMed]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable Photoluminescence of Monolayer MoS2 via Chemical Doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef]
- Ross, J.S.; Wu, S.F.; Yu, H.Y.; Ghimire, N.J.; Jones, A.M.; Aivazian, G.; Yan, J.Q.; Mandrus, D.G.; Xiao, D.; Yao, W.; et al. Electrical Control of Neutral and Charged Excitons in a Monolayer Semiconductor. Nat. Commun. 2013, 4, 1474. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Chen, Y.F.; Huang, Y.L.; Gogoi, P.K.; Li, M.Y.; Li, L.J.; Trevisanutto, P.E.; Wang, Q.X.; Pennycook, S.J.; Wee, A.T.S.; et al. Point Defects and Localized Excitons in 2D WSe2. ACS Nano 2019, 13, 6050–6059. [Google Scholar] [CrossRef]
- Zhou, M.F.; Wang, W.H.; Lu, J.P.; Ni, Z.H. How Defects Influence the Photoluminescence of TMDCs. Nano Res. 2021, 14, 29–39. [Google Scholar] [CrossRef]
- Komsa, H.P.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A.V. From Point to Extended Defects in Two-dimensional MoS2: Evolution of Atomic Structure under Electron Irradiation. Phys. Rev. B 2013, 88, 035301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, F.; Zeng, Y.; Xie, Q.; Yang, Y.; Linghu, S.; Liang, L.; Zuo, Z. Optimizing the Morphology and Optical Properties of MoS2 Using Different Substrate Placement: Numerical Simulation and Experimental Verification. Crystals 2025, 15, 59. https://doi.org/10.3390/cryst15010059
Liao F, Zeng Y, Xie Q, Yang Y, Linghu S, Liang L, Zuo Z. Optimizing the Morphology and Optical Properties of MoS2 Using Different Substrate Placement: Numerical Simulation and Experimental Verification. Crystals. 2025; 15(1):59. https://doi.org/10.3390/cryst15010059
Chicago/Turabian StyleLiao, Feng, Yuhan Zeng, Qingqing Xie, Yupeng Yang, Shuangyi Linghu, Li Liang, and Zewen Zuo. 2025. "Optimizing the Morphology and Optical Properties of MoS2 Using Different Substrate Placement: Numerical Simulation and Experimental Verification" Crystals 15, no. 1: 59. https://doi.org/10.3390/cryst15010059
APA StyleLiao, F., Zeng, Y., Xie, Q., Yang, Y., Linghu, S., Liang, L., & Zuo, Z. (2025). Optimizing the Morphology and Optical Properties of MoS2 Using Different Substrate Placement: Numerical Simulation and Experimental Verification. Crystals, 15(1), 59. https://doi.org/10.3390/cryst15010059





