Effect of Nano-Silica on Mechanical Properties and Cytotoxicity of Calcium-Silicate-Based Root Canal Filling Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Raw Materials
2.2. Experimental Method
2.3. Paste Preparation and Performance Testing
3. Measurement and Analysis of Hydration Products
3.1. Cytotoxicity Measurement
3.2. Phase and Micromorphology Analysis
4. Results
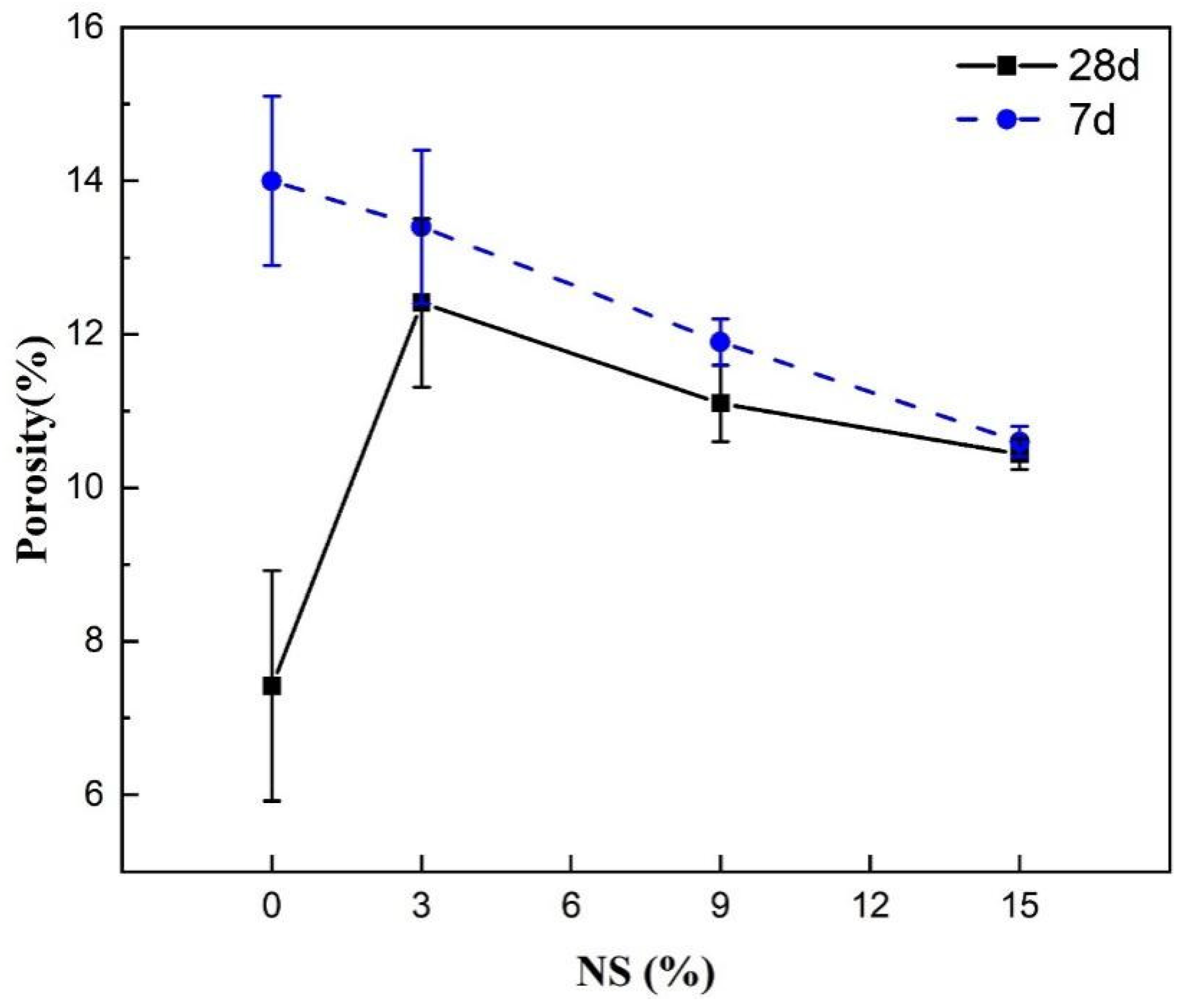
4.1. Physical Properties of the Material and Their Effects on the Compressive Strength
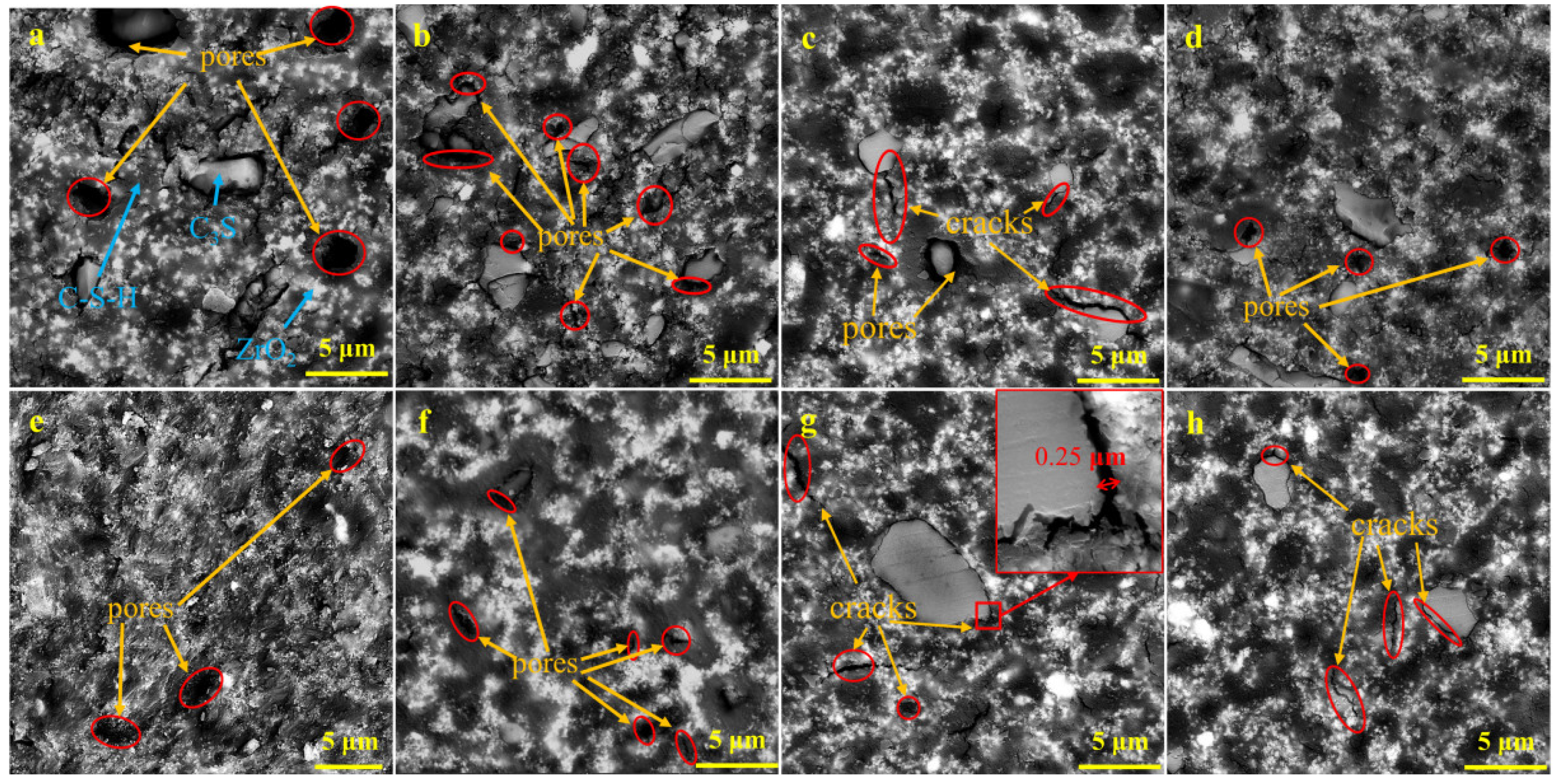
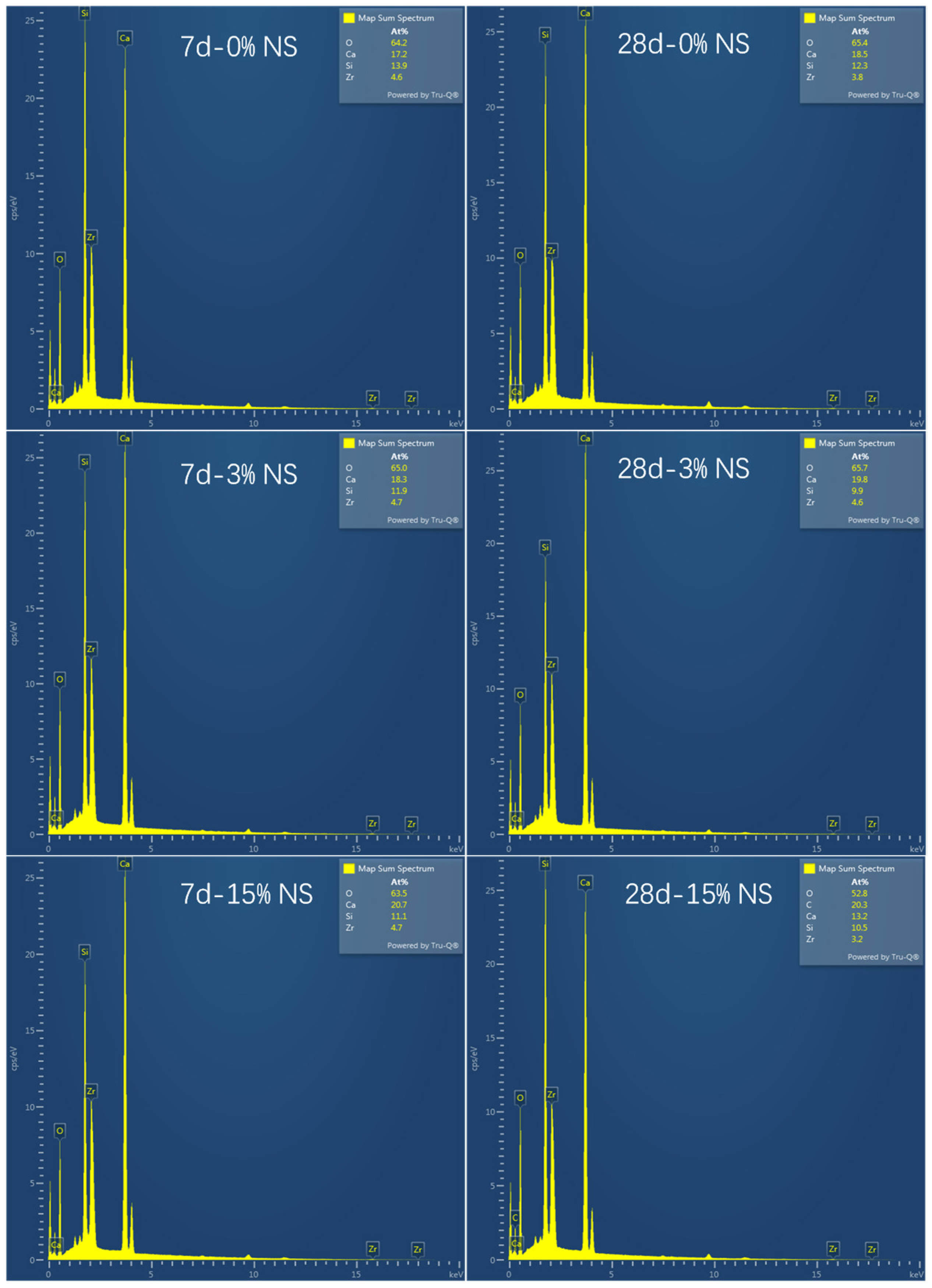
4.2. Effect of Microstructure and Composition on Compressive Strength
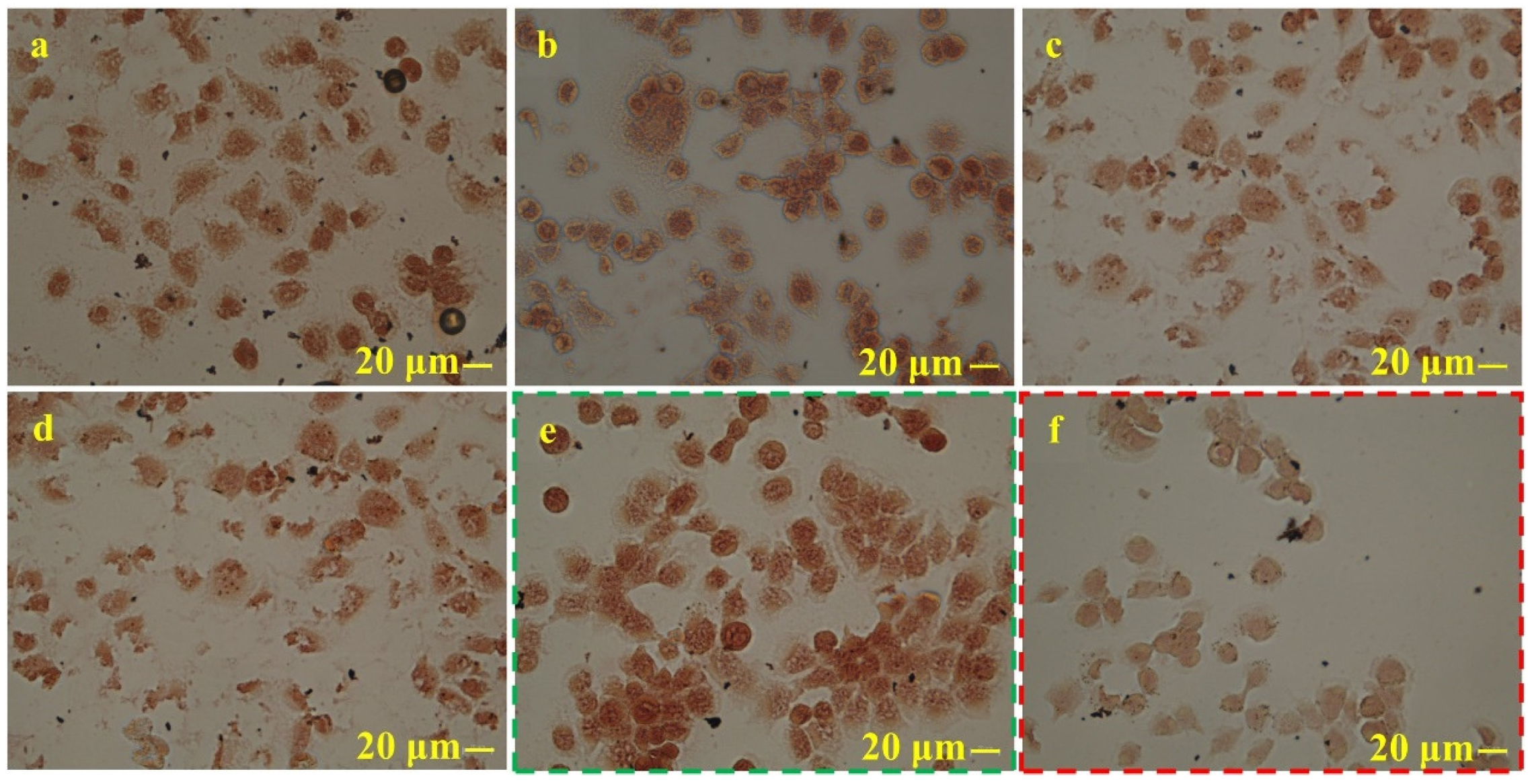
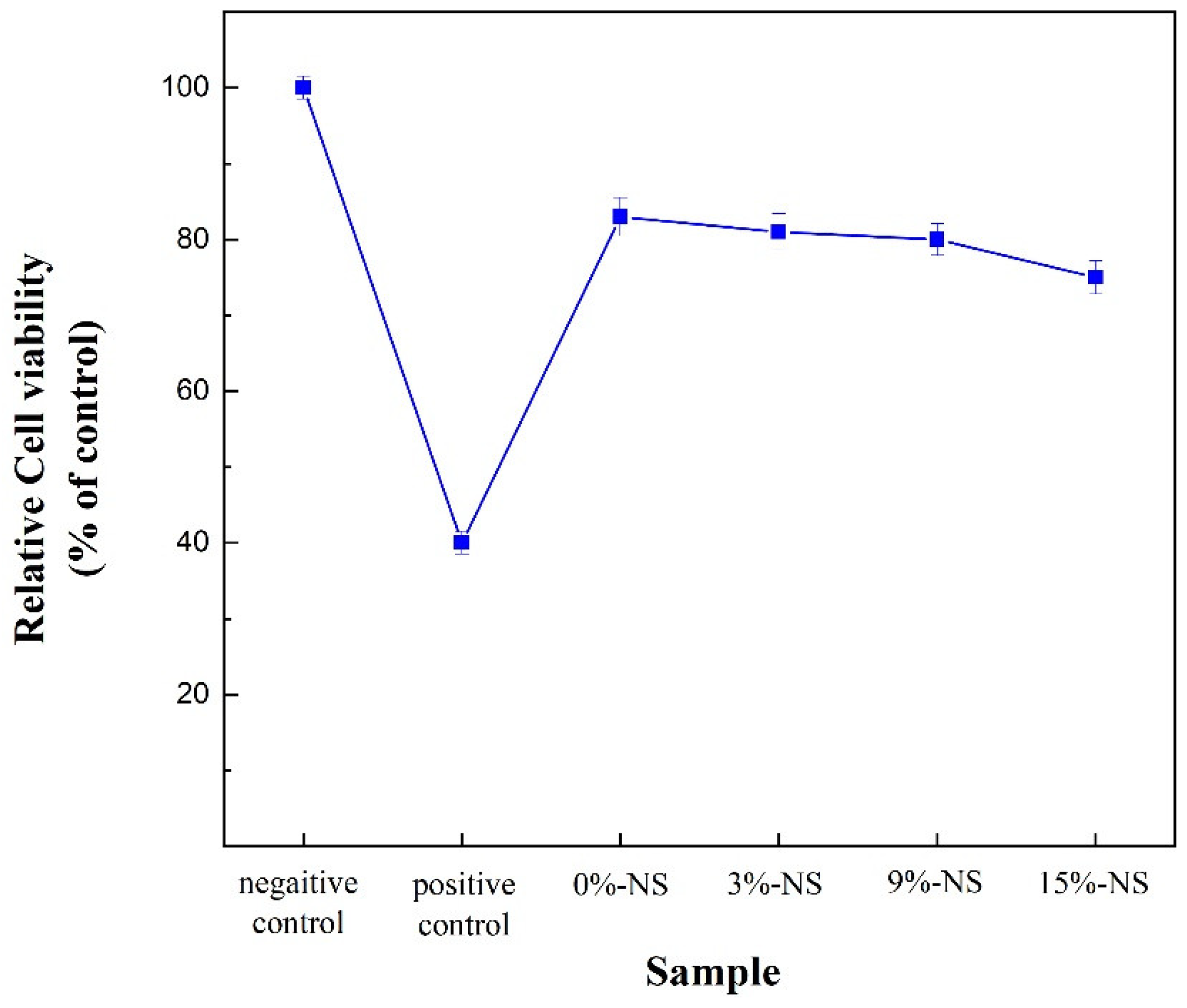
4.3. Effect of NS Content on the Cytotoxicity of the Material
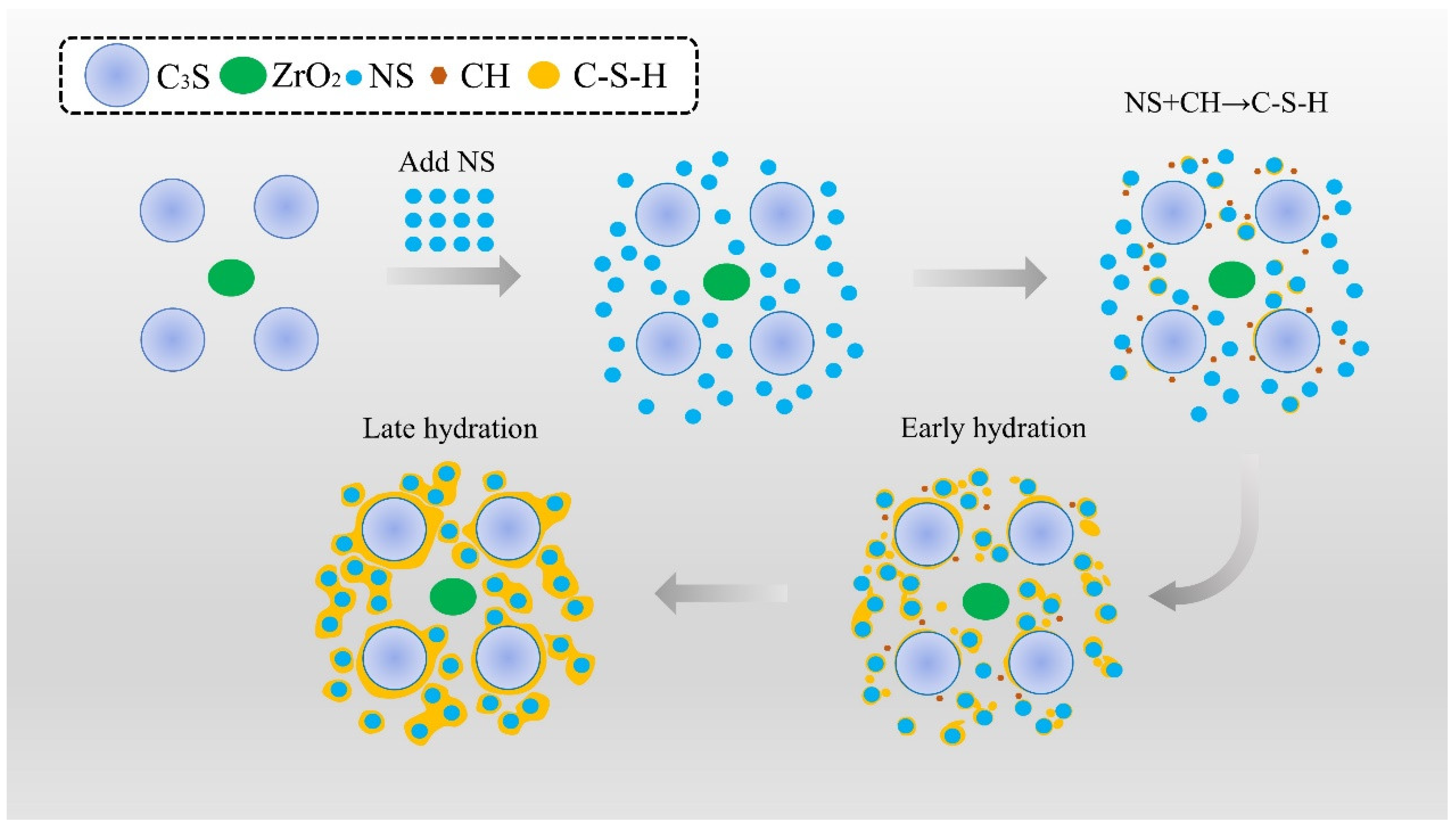
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olcay, K.; Eyüboglu, T.F.; Özcan, M. Clinical outcomes of non-surgical multiple-visit root canal retreatment: A retrospective cohort study. Odontology 2019, 107, 536–545. [Google Scholar] [CrossRef]
- Xiao, S.; Sun, G.; Huang, S.; Lin, C.; Li, Y. Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections. Pharmaceutics 2024, 16, 759. [Google Scholar] [CrossRef]
- Song, W.C.; Sun, W.; Chen, L.L.; Yuan, Z.L. In vivo biocompatibility and bioactivity of calcium silicate-based bioceramics in endodontics. Front. Bioeng. Biotechnol. 2020, 8, 580954. [Google Scholar] [CrossRef] [PubMed]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, T.; Rosen, E.; Blau-Venezia, N.; Tamse, A.; Littner, D. Pathognomonic combination of clinical signs for diagnosis of vertical root fracture: Systematic review of the literature. Appl. Sci. 2021, 11, 10893. [Google Scholar] [CrossRef]
- Liao, W.C.; Chen, C.H.; Pan, Y.H.; Chang, M.C.; Jeng, J.H. Vertical Root Fracture in Non-Endodontically and Endodontically Treated Teeth: Current Understanding and Future Challenge. J. Pers. Med. 2021, 11, 1375. [Google Scholar] [CrossRef]
- Sairaman, S.; Solete, P.; Jeevanandan, G.; Antony, S.D.P.; Kavoor, S.; Adimulapu, H.S. Comparative analysis of novel heat-treated retreatment file system on the removal of obturating material using nano-computed tomography. J. Conserv. Dent. Endod. 2024, 27, 82–86. [Google Scholar] [CrossRef]
- Grinkevičiūtė, P.; Leknickė, G.; Lodienė, G.; Kriūkienė, R. Effects of irrigation solutions on root canal dentin. Int. J. Appl. Sci. Eng. Technol. 2019, 9, 4. [Google Scholar] [CrossRef]
- Oliveira Tavares, S.J.; Pintor, A.V.B.; Caetano, S.K.; Dos Santos, N.C.A.; Pistoia, B.M.; de Carvalho Camilo, M.R.; Scelza, P.; Scelza, M.F.Z. Is There a Relationship between Laser Therapy and Root Canal Cracks Formation? A Systematic Review. Iran. Endod. J. 2023, 18, 2–14. [Google Scholar] [PubMed]
- Dimitriu, B.; Suciu, I.; Amza, O.E.; Ciocârdel, M.; Bodnar, D.; Țâncu, A.M.C.; Tanase, M.; Branescu, M.S.; Chirilă, M. Optical microscopy evaluation of root canal filling removal by beginner operators in posterior teeth. J. Med. Life 2024, 17, 555–563. [Google Scholar] [PubMed]
- D’souza, J.M.; de Ataide, I.N.; Lambor, R. Effect of Newer Intraorifice Barriers on the Fracture Resistance of Endodontically Treated Teeth: An In Vitro Study. Cureus 2024, 16, e69463. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, S.; Koç, C. Effect of several nickel-titanium rotary systems on stress distribution in mandibular molars under occlusal forces: A finite element analysis. Odontology 2024. Advance online publication. [Google Scholar] [CrossRef]
- Abdel-Maksoud, H.B.; Eid, B.M.; Hamdy, M.; Abdelaal, H.M. Optimizing fracture resistance of endodontically treated maxillary premolars restored with preheated thermos-viscous composite post-thermocycling, a comparative study. Part I. BMC Oral Health 2024, 24, 295. [Google Scholar] [CrossRef] [PubMed]
- Mergulhão, V.A.; de Mendonça, L.S.; de Albuquerque, M.S.; Braz, R. Fracture Resistance of Endodontically Treated Maxillary Premolars Restored with Different Methods. Oper. Dent. 2019, 44, E1–E11. [Google Scholar] [CrossRef] [PubMed]
- Aslan, T.; Esim, E.; Üstün, Y. Finite element evaluation of dentin stress changes following different endodontic surgical approaches. Odontology 2024, 112, 798–810. [Google Scholar] [CrossRef]
- Uzunoglu Ozyurek, E.; Aktemur Turker, S. Evaluation of fracture resistance of roots-filled with various root canal sealers at different time periods. Eur. Oral Res. 2019, 53, 6–11. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Portnyagin, A.S.; Belov, A.A.; Maiorov, V.Y.; Skurikhina, Y.E.; Merkulov, E.B.; Glavinskaya, V.O.; Nomerovskii, A.D.; et al. Reactive Spark Plasma Synthesis of Porous Bioceramic Wollastonite. Russ. J. Inorg. Chem. 2020, 65, 263–270. [Google Scholar] [CrossRef]
- Issar, R.; Ranjan, S.; Saurav, S.; Kar, S.; Bera, S.; Singh, P. Comparison of Fracture Resistance of Two Resin-based Sealers to Root Canal Walls: An In Vitro Study. J. Contemp. Dent. Pract. 2020, 21, 1253–1257. [Google Scholar]
- Chew, S.T.; Eshak, Z.; Al-Haddad, A. Evaluation of interfacial adaptation and penetration of bioceramic-based sealers in oval root canals: A confocal laser scanning microscope study. Microsc. Res. Tech. 2023, 86, 754–761. [Google Scholar] [CrossRef]
- Turkyilmaz, A.; Erdemir, A. Comparison of dentin penetration ability of different root canal sealers used with different obturation methods. Microsc. Res. Tech. 2020, 83, 1544–1551. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [PubMed]
- Hachem, R.E.; Khalil, I.; Brun, G.L.; Pellen, F.; Jeune, B.L.; Daou, M.; Osta, N.E.; Naaman, A.; Abboud, M. Dentinal tubule penetration of AH Plus, BC Sealer and a novel tricalcium silicate sealer: A confocal laser scanning microscopy study. Clin. Oral Investig. J. 2019, 23, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Monasterio, M.; Zheng, D.P.; Cui, H.Z.; Tang, W.C.; Bao, X.H.; Chen, X.S. Effects of nano silica on the properties of cement-based materials: A comprehensive review. Constr. Build. Mater. 2021, 282, 122715. [Google Scholar] [CrossRef]
- Bai, S.; Guan, X.C.; Li, H.; Ou, J.P. Effect of the specific surface area of nano-silica particle on the properties of cement paste. Powder Technol. 2021, 392, 680–689. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, H.; Hu, Y.; Wang, J.; Hou, G.; Ma, J.; Jiang, R. Effect of Stöber Nano-SiO2 Particles on the Hydration Properties of Calcined Coal Gangue-Blended Cement. Materials 2024, 17, 4218. [Google Scholar] [CrossRef]
- Boris, R.; Wilińska, I.; Pacewska, B.; Antonovič, V. Investigations of the Influence of Nano-Admixtures on Early Hydration and Selected Properties of Calcium Aluminate Cement Paste. Materials 2022, 15, 4958. [Google Scholar] [CrossRef]
- Hisham Shah, M.H.; Lee, C.C.; Zamzuri, M.Z.; Vijayan, V. Colloidal Nanosilica Effect on the Properties and Durability of Coir Reinforced Cement Brick. Adv. Mater. Sci. Eng. 2022, 2022, 7610297. [Google Scholar] [CrossRef]
- Shahram, M.A.; Alireza, K.; Mohammad, H.B.; Mohammad, S.T.M. Performance of polypropylene fiber reinforced concrete-filled UPVC tube columns under axial compression. Constr. Build. Mater. 2020, 231, 117049. [Google Scholar]
- 1SO 6876: 2012[S/OL]; Dentistry—Root Canal Sealing Materials. ISO Technical Committees: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/45117.html (accessed on 28 December 2024).
- C266-21[S/OL]; Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. ISO Technical Committees: Geneva, Switzerland, 2021. Available online: https://www.astm.org/standards/c266 (accessed on 28 December 2024).
- Hall, C.; Hamilton, A. Porosity–density relations in stone and brick materials. Mater. Struct. J. 2015, 48, 1265–1271. [Google Scholar] [CrossRef]
- Shen, C.C. Preparation process and application research of calcium silicate-based materials. Master’s Thesis, Tongfang CNKI (Beijing) Technology Co., Ltd., Beijing, China, 2020. [Google Scholar] [CrossRef]
- GB/T 28629-2012[S/OL]; Methods for Chemical Analysis of Free Silicon Dioxide for Cement Clinker. National Technical Committee for Cement Standardization: Beijing, China, 2013. Available online: https://std.samr.gov.cn/gb/search/gbDetailed?id=173829859CF91AA5E06397BE0A0AA311 (accessed on 28 December 2024).
- ISO 7405: 2018[S/OL]; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. ISO Technical Committees: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71503.html (accessed on 28 December 2024).
- Jain, D.; Shetty, K.P.; Luke, A.M.; Ballal, N.V. Root canal irrigants effect on the compressive strength and calcium ion release of Biodentine. Heliyon 2024, 10, e38267. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Linares, M.; de Oliveira Fagundes, J.; Solana, C.; Baca, P.; Ferrer-Luque, C.M. Current status on antimicrobial activity of a tricalcium silicate cement. J. Oral Sci. 2022, 64, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, I.; AbuTarboush, B.J.; Zaazou, A.; Skienhe, H.; Özcan, M.; Zakhour, M.; Salameh, Z. Investigation of the structure and compressive strength of a bioceramic root canal sealer reinforced with nanomaterials. J. Appl. Biomater. 2021, 19, 22808000211014747. [Google Scholar] [CrossRef]
- Li, L.G.; Zheng, J.Y.; Ng, P.L.; Zhu, J.; Kwan, A.K.H. Cementing efficiencies and synergistic roles of silica fume and nano-silica in sulphate and chloride resistance of concrete. Constr. Build. Mater. 2019, 223, 965–975. [Google Scholar] [CrossRef]
- Saleh, A.N.; Attar, A.A.; Ahmed, O.K.; Mustafa, S.S. Improving the thermal insulation and mechanical properties of concrete using Nano-SiO2. Results Eng. 2021, 12, 100303. [Google Scholar] [CrossRef]
- Vineet, S. Performance evaluation of carbonated cement paste derived from hydrated Portland cement based binders as supplementary cementitious material. CEMENT 2024, 15, 100091. [Google Scholar]
- Gao, C.; Huang, L.; Yan, L.B.; Jin, R.Y.; Chen, H.Z. Mechanical properties of recycled aggregate concrete modified by nano-particles. Constr. Build. Mater. 2020, 241, 118030. [Google Scholar] [CrossRef]
- Ma, B.G.; Li, H.N.; Mei, J.P.; Li, X.G. Influence of Nano-SiO2 addition on properties of sulphoaluminate cement based material. J. Wuhan Univ. Technol. 2017, 32, 106–112. [Google Scholar] [CrossRef]
- Kooshafar, M.; Madani, H. An investigation on the influence of nano silica morphology on the characteristics of cement composites. J. Build. Eng. 2020, 30, 101293. [Google Scholar] [CrossRef]
- Anna, M.; Andrea, C.; Francesco, B.B.; Emiliano, B.; Fabio, P.; Andrea, S.; Fabio, I.; Giovanni, I.; Peter, Z.; Giovanni, C.; et al. Exceptional thermal strain reduction by a tilting pillar architecture: Suspended Ge layers on Si (001). Mater. Design 2017, 116, 144–151. [Google Scholar]
- Wang, D.; Shi, C.; Wu, Z.; Wu, L.; Xiang, S.; Pan, X. Effects of nanomaterials on hardening of cement–silica fume–fly ash-based ultra-high-strength concrete. Adv. Cem. Res. 2016, 28, 555–566. [Google Scholar] [CrossRef]
- Wang, L.G.; Zheng, D.P.; Zhang, S.P.; Cui, H.Z.; Li, D.X. Effect of Nano-SiO2 on the hydration and microstructure of portland cement. Nanomater. 2016, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.K.; Shi, J.; Prabakar, S.; Cheng, X.; Wang, K.J.; Zhou, X.M.; Surendra, P.S. Effects of mixing sequences of nanosilica on the hydration and hardening properties of cement-based materials. Constr. Build. Mater. 2020, 263, 120226. [Google Scholar] [CrossRef]
- Vivek, D.; Elango, K.S.; Saravanakumar, R.; Mohamed Rafek, B.; Ragavendra, P.; Kaviarasan, S.; Raguram, E. Effect of Nano-Silica in high performance concrete. Mater. Today Proc. 2021, 37, 1226–1229. [Google Scholar] [CrossRef]
- La Rosa, G.R.M.; Canova, F.S.; Leotta, M.L.; Pedullà, E. Effects of heating on bioceramic sealers: A scoping review of chemo-physical properties and clinical implications. Odontology 2024, 1–14. [Google Scholar] [CrossRef]
- Zhou, H.M.; Du, T.F.; Shen, Y.; Wang, Z.J.; Zheng, Y.F.; Haapasalo, M. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J. Endodont. 2015, 41, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.G.; Lee, D.; Kim, Y.M.; Song, D.; Kim, S.Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials 2019, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- López-García, S.; Myong-Hyun, B.; Lozano, A.; García-Bernal, D.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Murcia, L.; Rodríguez-Lozano, F.J. Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin. Oral Investig. 2020, 24, 1749–1759. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Peng, B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef]
- Mestieri, L.B.; Zaccara, I.M.; Pinheiro, L.S.; Barletta, F.B.; Kopper, P.M.P.; Grecca, F.S. Cytocompatibility and cell proliferation evaluation of calcium phosphate-based root canal sealers. Restor. Dent. Endod. 2019, 45, e2. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.; Carvalho, N.K.; Ronconi, C.T.; Deus, G.D.; Zuolo, M.L.; Zaia, A.A. Cytotoxicity profile of endodontic sealers provided by 3D cell culture experimental model. Braz. Dent. J. 2016, 27, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.; Tolar, M.; Peters, O.A. Cytokine Production and Cytotoxicity of Calcium Silicate-based Sealers in 2- and 3-dimensional Cell Culture Models. J. Endod. 2020, 46, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.; Coelho, F.H.; Moreira, M.S.; Marques, M.M.; Pilar, E.F.S.; Palo, R.M.; Silveira, F.M.; Kopper, P.M.P. Cytotoxicity, biocompatibility and osteoinductive profile of an MTA-hydrogel-based cement: An in vitro and animal study. Int. Endod. J. 2023, 56, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, T.; Zha, W.; Xiang, W.; Zheng, W.B. Effects of nano-SiO2 particles on physio-chemical properties of bioactive tricalcium silicate cements. J. Aust. Ceram. Soc. 2021, 57, 9–20. [Google Scholar] [CrossRef]
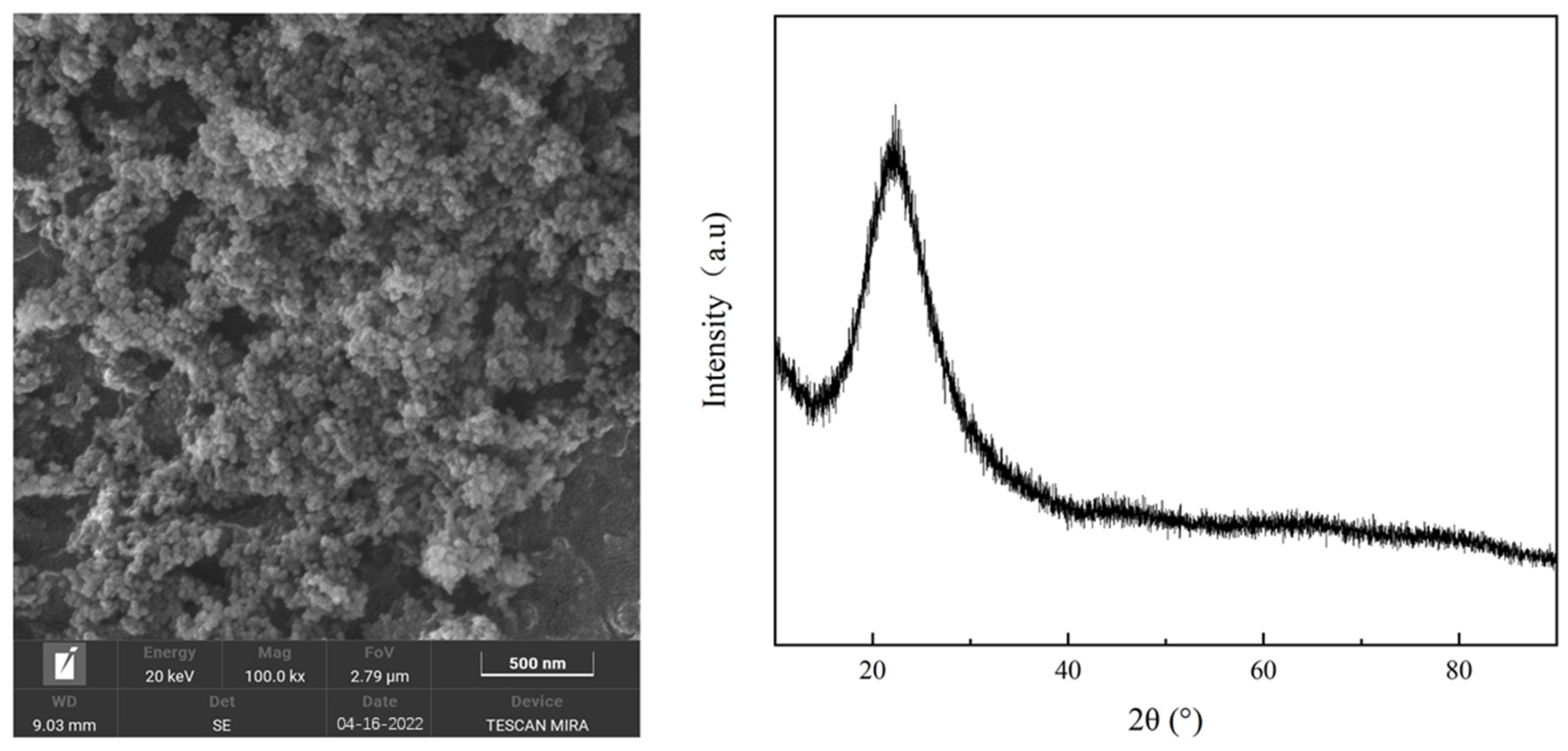







| Material | Density (g/mL) | Average Particle Size (Standard Deviation) | Purity | Manufacturer |
|---|---|---|---|---|
| Ca3SiO5 (C3S) | 3.74 | 12 ± 2 μm | ≥99.0% | Hubei Wande Chemical Co., Ltd. Hubei, China. |
| NS | 2.60 | 30 ± 5 nm | ≥99.5% | Shanghai Maclin Biochemical Technology Co., Ltd. Shanghai, China. |
| ZrO2 | 5.89 | 50 ± 10 nm | ≥99.9% | Shanghai Maclin Biochemical Technology Co., Ltd. Shanghai, China. |
| Type | Solid–Liquid Ratio | ZrO2 Content (Solid) | NS Content (Solid) |
|---|---|---|---|
| Paste 1 | 4:1 | 20% | 0% |
| Paste 2 | 4:1 | 20% | 3% |
| Paste 3 | 4:1 | 20% | 9% |
| NS Content (%) | Detection Method | Residual NS Content (%) | NS Involved in Reaction (%) |
|---|---|---|---|
| 3 | Spectrophotometer | 0.26 | 2.74 |
| 9 | Spectrophotometer | 4.14 | 4.86 |
| 15 | Potassium fluorosilicate | 5.56 | 9.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Hao, B.; Xiong, X.; Cheng, Y.; Lou, J.; He, Z.; Li, D.; Wang, Z.; Qin, J. Effect of Nano-Silica on Mechanical Properties and Cytotoxicity of Calcium-Silicate-Based Root Canal Filling Materials. Crystals 2025, 15, 55. https://doi.org/10.3390/cryst15010055
He H, Hao B, Xiong X, Cheng Y, Lou J, He Z, Li D, Wang Z, Qin J. Effect of Nano-Silica on Mechanical Properties and Cytotoxicity of Calcium-Silicate-Based Root Canal Filling Materials. Crystals. 2025; 15(1):55. https://doi.org/10.3390/cryst15010055
Chicago/Turabian StyleHe, Hao, Bolang Hao, Xiang Xiong, Yi Cheng, Jia Lou, Zheyu He, Dongyang Li, Zhihuan Wang, and Jian Qin. 2025. "Effect of Nano-Silica on Mechanical Properties and Cytotoxicity of Calcium-Silicate-Based Root Canal Filling Materials" Crystals 15, no. 1: 55. https://doi.org/10.3390/cryst15010055
APA StyleHe, H., Hao, B., Xiong, X., Cheng, Y., Lou, J., He, Z., Li, D., Wang, Z., & Qin, J. (2025). Effect of Nano-Silica on Mechanical Properties and Cytotoxicity of Calcium-Silicate-Based Root Canal Filling Materials. Crystals, 15(1), 55. https://doi.org/10.3390/cryst15010055







