Structural Characterization of B-DNA d(CGTGAATTCACG)2 in Complex with the Specific Minor Groove Binding Fluorescent Marker Hoechst 33342
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
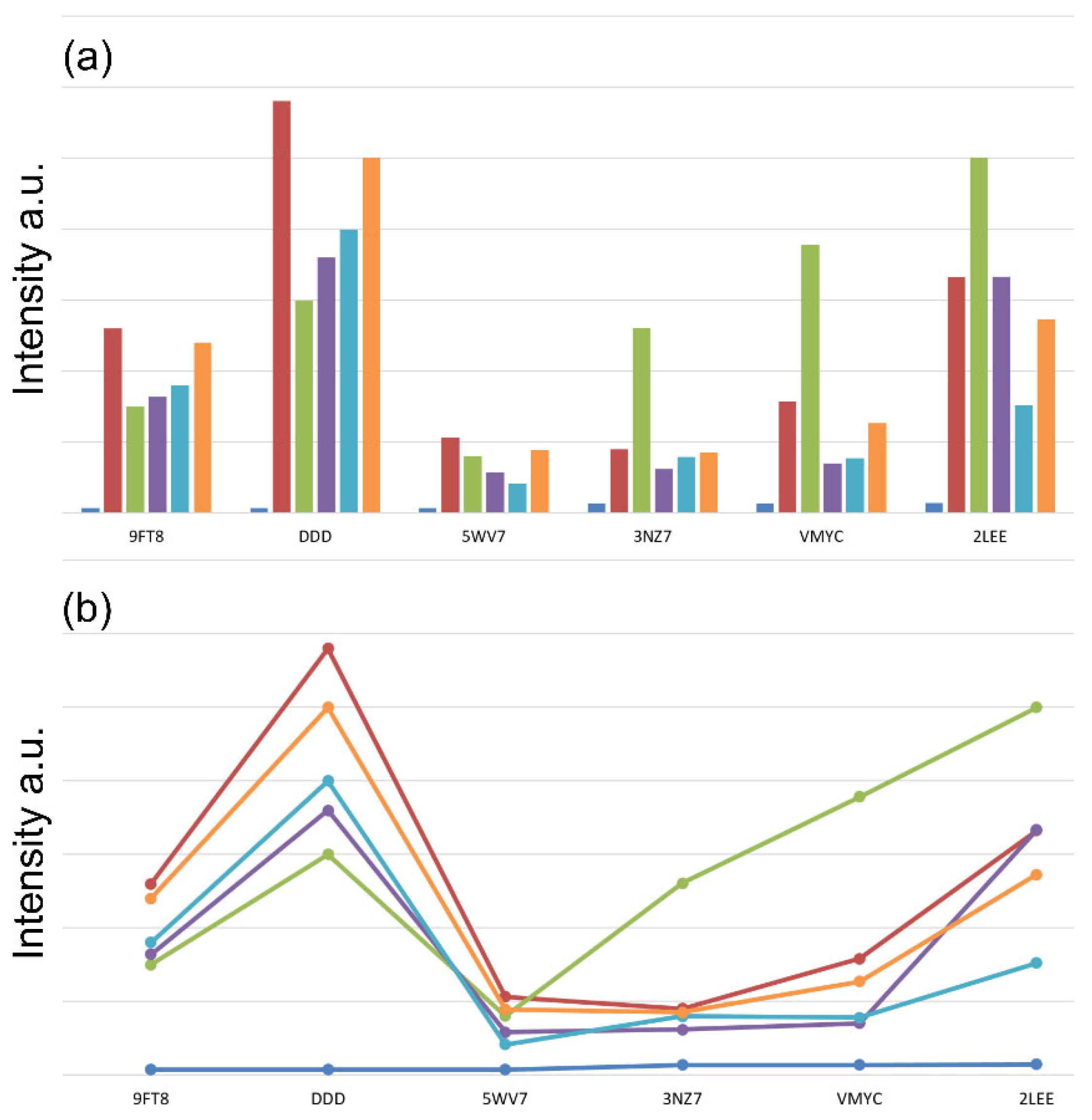
2.2. Fluorescent Intercalator Displacement (FID) Assay
2.3. Samples Crystallization
2.4. Single Crystal X-Ray Diffraction (SCXRD), Data Collection and Processing
2.5. Macromolecules Structure Solution and Refinement
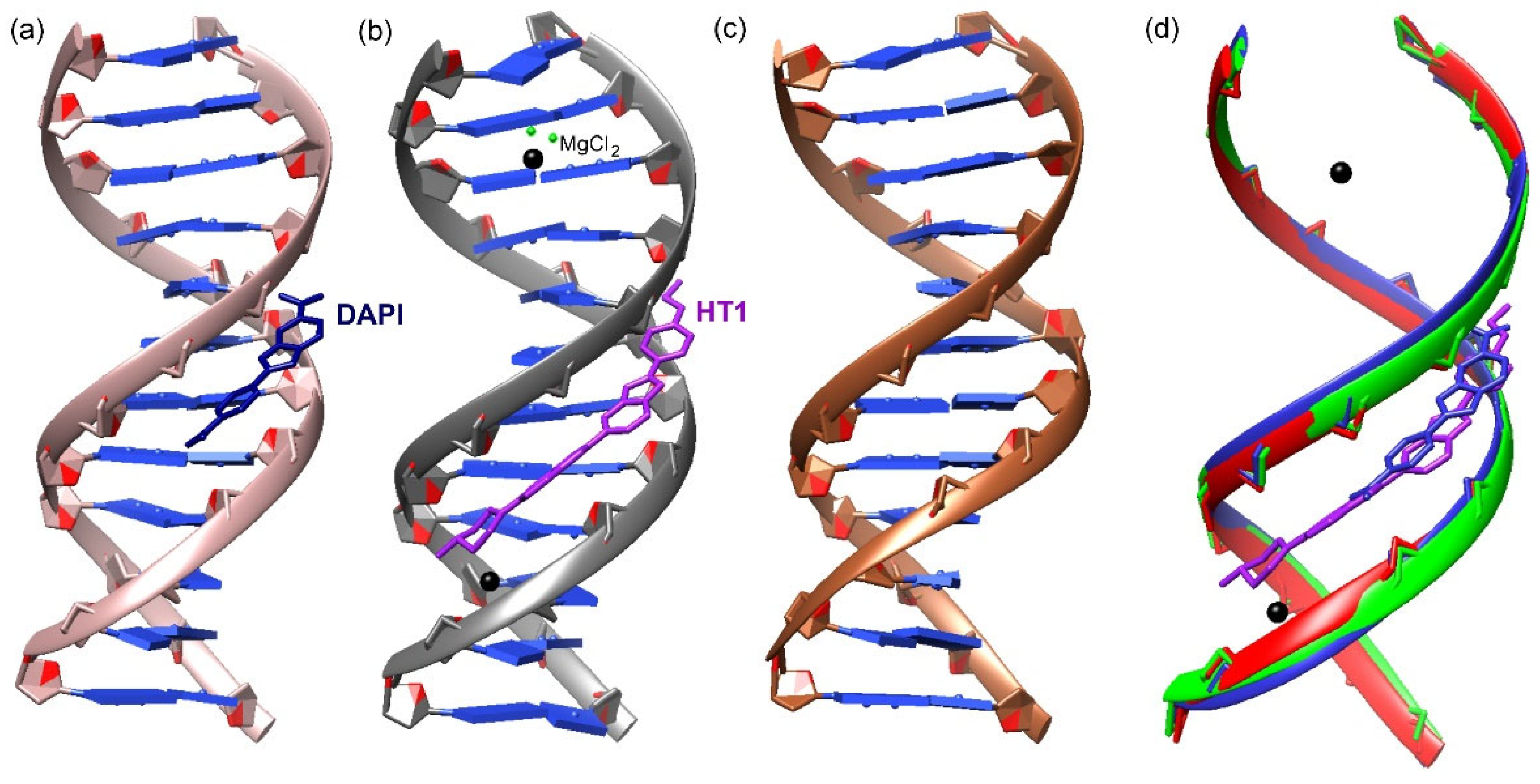
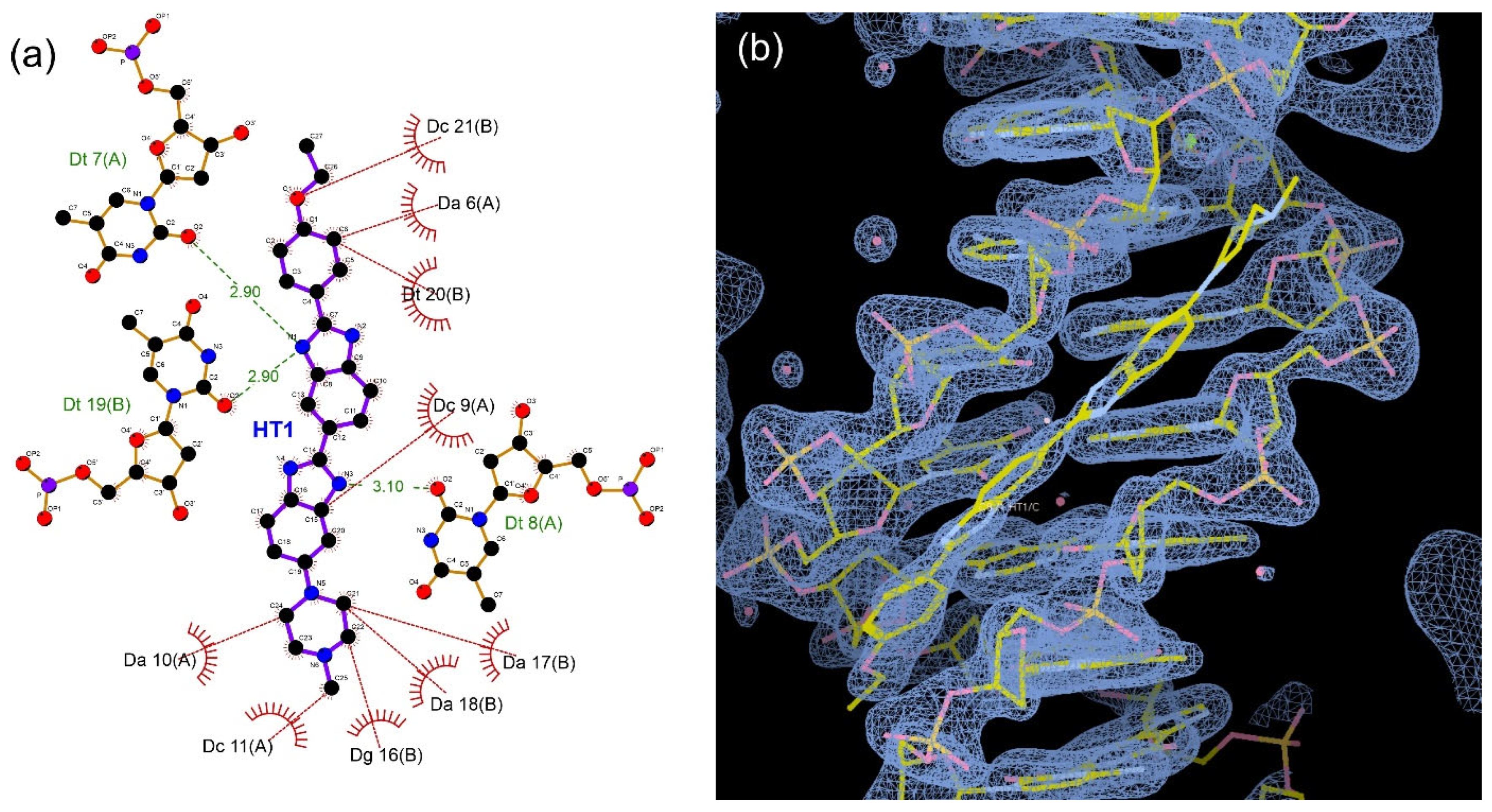
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhang, S.C.; Sun, D.; Hu, J.; Wali, A.; Pass, H.; Fernandez-Madrid, F.; Harbut, M.R.; Tang, N. New insight into the molecular mechanisms of the biological effects of DNA minor groove binders. PLoS ONE 2011, 6, e25822. [Google Scholar] [CrossRef] [PubMed]
- Sharga, B.M.; Chromiak, U.I.; Pylypiv, D.B.; Feketa, V.P. Molecular biology practicals. 2022. [Google Scholar]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The use of hoechst dyes for DNA staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Latt, S.A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc. Natl. Acad. Sci. USA 1973, 70, 3395–3399. [Google Scholar] [CrossRef] [PubMed]
- Purschke, M.; Rubio, N.; Held, K.D.; Redmond, R.W. Phototoxicity of Hoechst 33342 in time-lapse fluorescence microscopy. Photochem. Photobiol. Sci. 2010, 9, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Charousset, C.; Grimberg, J.; Duranthon, L.D.; Bellaïche, L.; Petrover, D.; Kalra, K. The time for functional recovery after arthroscopic rotator cuff repair: Correlation with tendon healing controlled by computed tomography arthrography. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 25–33. [Google Scholar] [CrossRef]
- Suss, O.; Motiei, L.; Margulies, D. Broad applications of thiazole orange in fluorescent sensing of biomolecules and ions. Molecules 2021, 26, 2828. [Google Scholar] [CrossRef]
- Andrade, N.M.; Arismendi, N.L. DAPI staining and fluorescence microscopy techniques for phytoplasmas. In Phytoplasma: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2013; pp. 115–121. [Google Scholar]
- Fuchs, H.; Jahn, K.; Hu, X.; Meister, R.; Binter, M.; Framme, C. Breaking a Dogma: High-Throughput Live-Cell Imaging in Real-Time with Hoechst 33342. Adv. Healthc. Mater. 2023, 12, 2300230. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016, prot087205. [Google Scholar] [CrossRef]
- Shapiro, M.; Brumer, P. Principles of the Quantum Control of Molecular Processes; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Selvaraj, C.; Singh, S.K. Computational and experimental binding mechanism of DNA-drug interactions. Curr. Pharm. Des. 2018, 24, 3739–3757. [Google Scholar] [CrossRef]
- Tse, W.C.; Boger, D.L. A fluorescent intercalator displacement assay for establishing DNA binding selectivity and affinity. Acc. Chem. Res. 2004, 37, 61–69. [Google Scholar] [CrossRef]
- Sheng, Q.; Neaverson, J.C.; Mahmoud, T.; Stevenson, C.E.; Matthews, S.E.; Waller, Z.A. Identification of new DNA i-motif binding ligands through a fluorescent intercalator displacement assay. Org. Biomol. Chem. 2017, 15, 5669–5673. [Google Scholar] [CrossRef] [PubMed]
- Karg, T.J.; Golic, K.G. Photoconversion of DAPI and Hoechst dyes to green and red-emitting forms after exposure to UV excitation. Chromosoma 2018, 127, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Jež, M.; Bas, T.; Veber, M.; Košir, A.; Dominko, T.; Page, R.; Rožman, P. The hazards of DAPI photoconversion: Effects of dye, mounting media and fixative, and how to minimize the problem. Histochem. Cell Biol. 2013, 139, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Sbirkova-Dimitrova, H.; Rusew, R.; Kuvandjiev, N.; Heroux, A.; Doukov, T.; Shivachev, B.L. Structural Characterization of Alzheimer DNA Promoter Sequences from the Amyloid Precursor Gene in the Presence of Thioflavin T and Analogs. Crystals 2022, 12, 1717. [Google Scholar] [CrossRef]
- Sibirtsev, V.; Garabadzhiu, A. Spectral study of the interaction of DNA with benzothiazolyl-benz-α-chromene. Biochemistry 2007, 72, 901–909. [Google Scholar] [CrossRef]
- Ikeda, S.; Kubota, T.; Kino, K.; Okamoto, A. Sequence dependence of fluorescence emission and quenching of doubly thiazole orange labeled DNA: Effective design of a hybridization-sensitive probe. Bioconjugate Chem. 2008, 19, 1719–1725. [Google Scholar] [CrossRef]
- Lubitz, I.; Zikich, D.; Kotlyar, A. Specific high-affinity binding of thiazole orange to triplex and G-quadruplex DNA. Biochemistry 2010, 49, 3567–3574. [Google Scholar] [CrossRef]
- Giegé, R.; McPherson, A. Vapour diffusion methods. In International Tables for Crystallography, Vol. F; Springer: Berlin/Heidelberg, Germany, 2006; pp. 82–84. [Google Scholar]
- Sbirkova-Dimitrova, H.I.; Shivachev, B. Crystal structure of the DNA sequence d (CGTGAATTCACG)2 with DAPI. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 500–504. [Google Scholar] [CrossRef]
- Kabsch, W. xds. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 1993, 26, 795–800. [Google Scholar] [CrossRef]
- McCoy, A.J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 32–41. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Zheng, G.; Lu, X.-J.; Olson, W.K. Web 3DNA—A web server for the analysis, reconstruction, and visualization of three-dimensional nucleic-acid structures. Nucleic Acids Res. 2009, 37, W240–W246. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Kricka, L.J.; Fortina, P. Analytical ancestry:“Firsts” in fluorescent labeling of nucleosides, nucleotides, and nucleic acids. Clin. Chem. 2009, 55, 670–683. [Google Scholar] [CrossRef]
- Waring, M. Complex formation between ethidium bromide and nucleic acids. J. Mol. Biol. 1965, 13, 269–282. [Google Scholar] [CrossRef]
- Campbell, N.H.; Smith, D.L.; Reszka, A.P.; Neidle, S.; O’Hagan, D. Fluorine in medicinal chemistry: β-fluorination of peripheral pyrrolidines attached to acridine ligands affects their interactions with G-quadruplex DNA. Org. Biomol. Chem. 2011, 9, 1328–1331. [Google Scholar] [CrossRef]
- Trajkovski, M.; Webba da Silva, M.; Plavec, J. Unique structural features of interconverting monomeric and dimeric G-quadruplexes adopted by a sequence from the intron of the N-myc gene. J. Am. Chem. Soc. 2012, 134, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Y.; Afrin, S.; Husain, M.A.; Sarwar, T.; Ali, A.; Tabish, M. Unravelling the interaction of pirenzepine, a gastrointestinal disorder drug, with calf thymus DNA: An in vitro and molecular modelling study. Arch. Biochem. Biophys. 2017, 625, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Rahman, Y.; Sarwar, T.; Husain, M.A.; Ali, A.; Tabish, M. Molecular spectroscopic and thermodynamic studies on the interaction of anti-platelet drug ticlopidine with calf thymus DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 66–75. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Olives, A.I.; Martín, M.A.; Ribelles, P.; Ramos, M.T.; Menéndez, J.C. An overview of analytical techniques employed to evidence drug-DNA interactions. Applications to the design of genosensors. Biomed. Eng. Trends Res. Technol. 2011, 32, 215–219. [Google Scholar]
- Larsen, T.A.; Kopka, M.L.; Dickerson, R.E. Crystal structure analysis of the B-DNA dodecamer CGTGAATTCACG. Biochemistry 1991, 30, 4443–4449. [Google Scholar] [CrossRef]
- Manivelan, V.; Kavyashree, B.; Sadanandan, B.; Vaidya, S.; Acharya, K.K. Comparative Analysis of DNA Structural Parameters and the Corresponding Computational Tools to Differentiate Regulatory DNA Motifs and Promoters. bioRxiv 2024. bioRxiv: 2026.584893. [Google Scholar]
- Li, S.; Olson, W.K.; Lu, X.-J. Web 3DNA 2.0 for the analysis, visualization, and modeling of 3D nucleic acid structures. Nucleic Acids Res. 2019, 47, W26–W34. [Google Scholar] [CrossRef]
| Name | Sequence (5′->3′) | MW (g/mol) | GC-Content (%) | Extinction Coefficient (mol.cm) | Tm [°C] | Bases |
|---|---|---|---|---|---|---|
| 9FT8 | 5′-CGTGAATTCACG-3′ | 3645 | 50 | 131,400 | 36 | 12 |
| DDD | 5′-CGCGAATTCGCG-3′ | 3646 | 67 | 110,700 | 40 | 12 |
| 5WV7 | 5′-CCGGGGTACCCCGG-3′ | 3679 | 86 | 112,200 | 58 | 14 |
| 3NZ7 | 5′-GGGGTTTTGGGG-3′ | 3427 | 67 | 104,500 | 40 | 12 |
| VMYC | 5′-GGGAGGCGTGGGGGTG-GGACGGTGGGG-3′ | 7787 | 82 | 237,500 | 59 | 27 |
| 2LEE | 5′-TAGGGCGGAGGGAGGG-AA-3′ | 5453 | 69 | 166,300 | 50 | 19 |
| PDB Code | 9FT8 |
|---|---|
| Space group | P212121 |
| Cell dimensions | |
| a, b, c, Å | 24.59, 40.41, 65.03 |
| α, β, γ, ° | 90, 90, 90 |
| Independent molecules | 1 |
| Diffraction data | |
| Wavelength, Å | 0.99 |
| Resolution, Å | 1.90 |
| Reflections | 5451 |
| Completeness, % | 99.8 |
| I/σ(I) | 4.36 |
| Redundancy | 7.0 (5.9) |
| Rmerge % | 4.6 (25) |
| Refinement statistics | |
| Reflections used | 5200 |
| Resolution, Å | 1.90 |
| R (Rfree) % | 21.9 (24.6) |
| No. of atoms | 542 |
| DNA | 488 |
| HT1 | 33 |
| Average B fatcor, Å2 | 37.1 |
| r.m.s.d. | |
| Bond lengths, Å | 0.72 |
| Bond angles, ° | 1.60 |
| Pair | Shear | Stretch | Stagger | Buckle | Propeller | Opening | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9FT8 | 5T4W | 9FT8 | 5T4W | 9FT8 | 5T4W | 9FT8 | 5T4W | 9FT8 | 5T4W | 9FT8 | 5T4W | ||
| 1 | C–G | 0.41 | 0.05 | −0.23 | −0.05 | −0.02 | −0.13 | 6.53 | −8.49 | −14.17 | −11.90 | 0.7 | −2.10 |
| 2 | G–C | −0.3 | −0.04 | −0.2 | −0.23 | −0.18 | 0.07 | −5.51 | 1.25 | −9.89 | −18.61 | −3.6 | −3.28 |
| 3 | T–A | −0.31 | 0.20 | −0.16 | −0.32 | −0.09 | −0.17 | −0.24 | 1.6 | −8.83 | −5.78 | −1.35 | 0.62 |
| 4 | G–C | −0.37 | −0.45 | −0.18 | −0.34 | −0.16 | −0.15 | 11.96 | −4.95 | −8.83 | −5.93 | −0.3 | 3.04 |
| 5 | A–T | 0 | −0.38 | −0.12 | −0.15 | −0.13 | −0.25 | 8.35 | −6.5 | −14.98 | −14.74 | 2.25 | 5.33 |
| 6 | A–T | 0.04 | 0.28 | −0.08 | −0.11 | 0.06 | −0.18 | 5.48 | −11.92 | −17.17 | −21.83 | 4.76 | 3.85 |
| 7 | T–A | −0.04 | 0.12 | −0.12 | 0.01 | 0.15 | 0.15 | −2.03 | 0.88 | −19.44 | −22.16 | 4.23 | 9.28 |
| 8 | T–A | −0.01 | 0.43 | −0.21 | −0.34 | −0.01 | 0.03 | −4.77 | 11.26 | −15.4 | −14.96 | 1.02 | 5.61 |
| 9 | C–G | 0.31 | 0.18 | −0.06 | −0.16 | −0.14 | −0.39 | −10.35 | 14.94 | −11.58 | −7.94 | 0.36 | 1.83 |
| 10 | A–T | 0.02 | −0.29 | −0.17 | −0.25 | 0.13 | 0.1 | 5.97 | −4.85 | −11.01 | −6.41 | 4.29 | −1.43 |
| 11 | C–G | 0.09 | −0.17 | −0.12 | 0.05 | 0.21 | −0.08 | 4.31 | 3.25 | −19.18 | −21.55 | −0.79 | −8.78 |
| 12 | G–C | −0.4 | 0.12 | −0.2 | −0.12 | −0.01 | −0.14 | 11.78 | 4.63 | 19.06 | −6.21 | −4.49 | −6.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbirkova-Dimitrova, H.; Rusew, R.; Gerginov, H.; Heroux, A.; Shivachev, B.L. Structural Characterization of B-DNA d(CGTGAATTCACG)2 in Complex with the Specific Minor Groove Binding Fluorescent Marker Hoechst 33342. Crystals 2025, 15, 20. https://doi.org/10.3390/cryst15010020
Sbirkova-Dimitrova H, Rusew R, Gerginov H, Heroux A, Shivachev BL. Structural Characterization of B-DNA d(CGTGAATTCACG)2 in Complex with the Specific Minor Groove Binding Fluorescent Marker Hoechst 33342. Crystals. 2025; 15(1):20. https://doi.org/10.3390/cryst15010020
Chicago/Turabian StyleSbirkova-Dimitrova, Hristina, Rusi Rusew, Hristo Gerginov, Annie Heroux, and Boris L. Shivachev. 2025. "Structural Characterization of B-DNA d(CGTGAATTCACG)2 in Complex with the Specific Minor Groove Binding Fluorescent Marker Hoechst 33342" Crystals 15, no. 1: 20. https://doi.org/10.3390/cryst15010020
APA StyleSbirkova-Dimitrova, H., Rusew, R., Gerginov, H., Heroux, A., & Shivachev, B. L. (2025). Structural Characterization of B-DNA d(CGTGAATTCACG)2 in Complex with the Specific Minor Groove Binding Fluorescent Marker Hoechst 33342. Crystals, 15(1), 20. https://doi.org/10.3390/cryst15010020





