Pressure-Driven Responses in Cd2SiO4 and Hg2GeO4 Minerals: A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
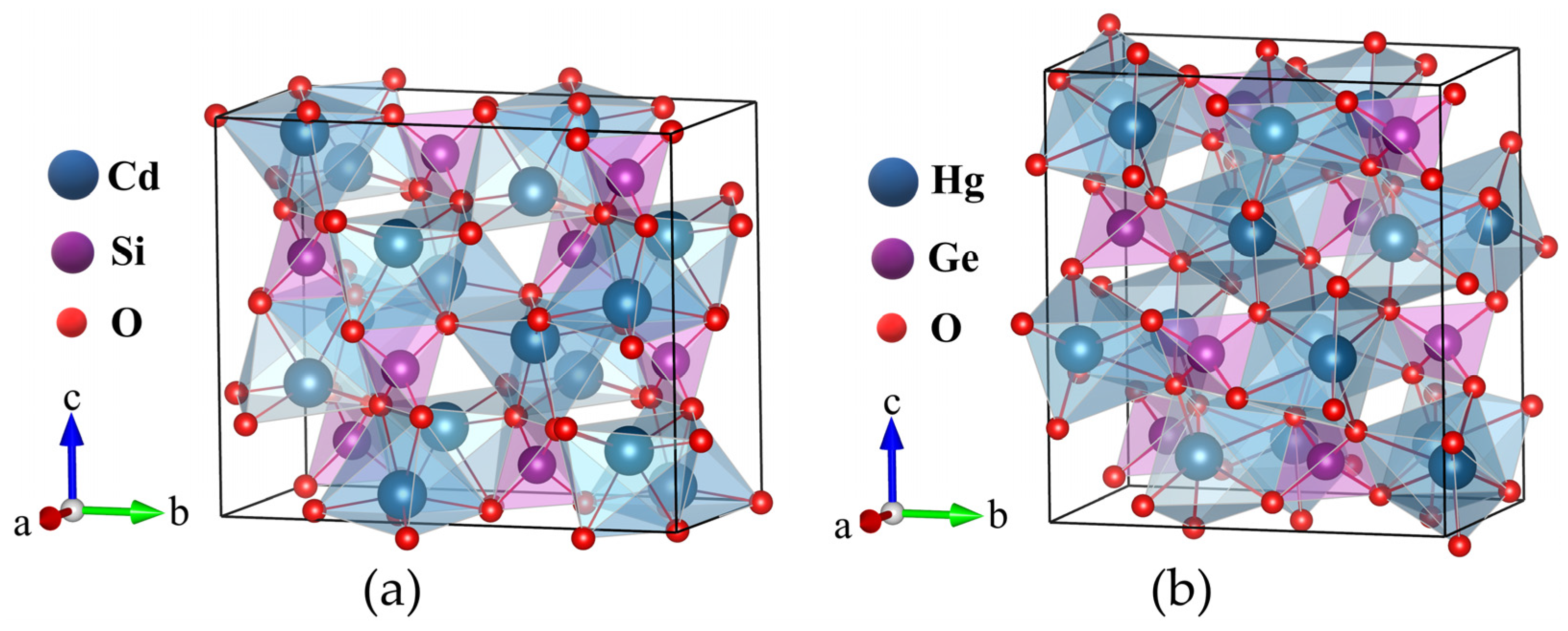
3.1. Structural Properties
3.2. Elastic and Mechanical Properties
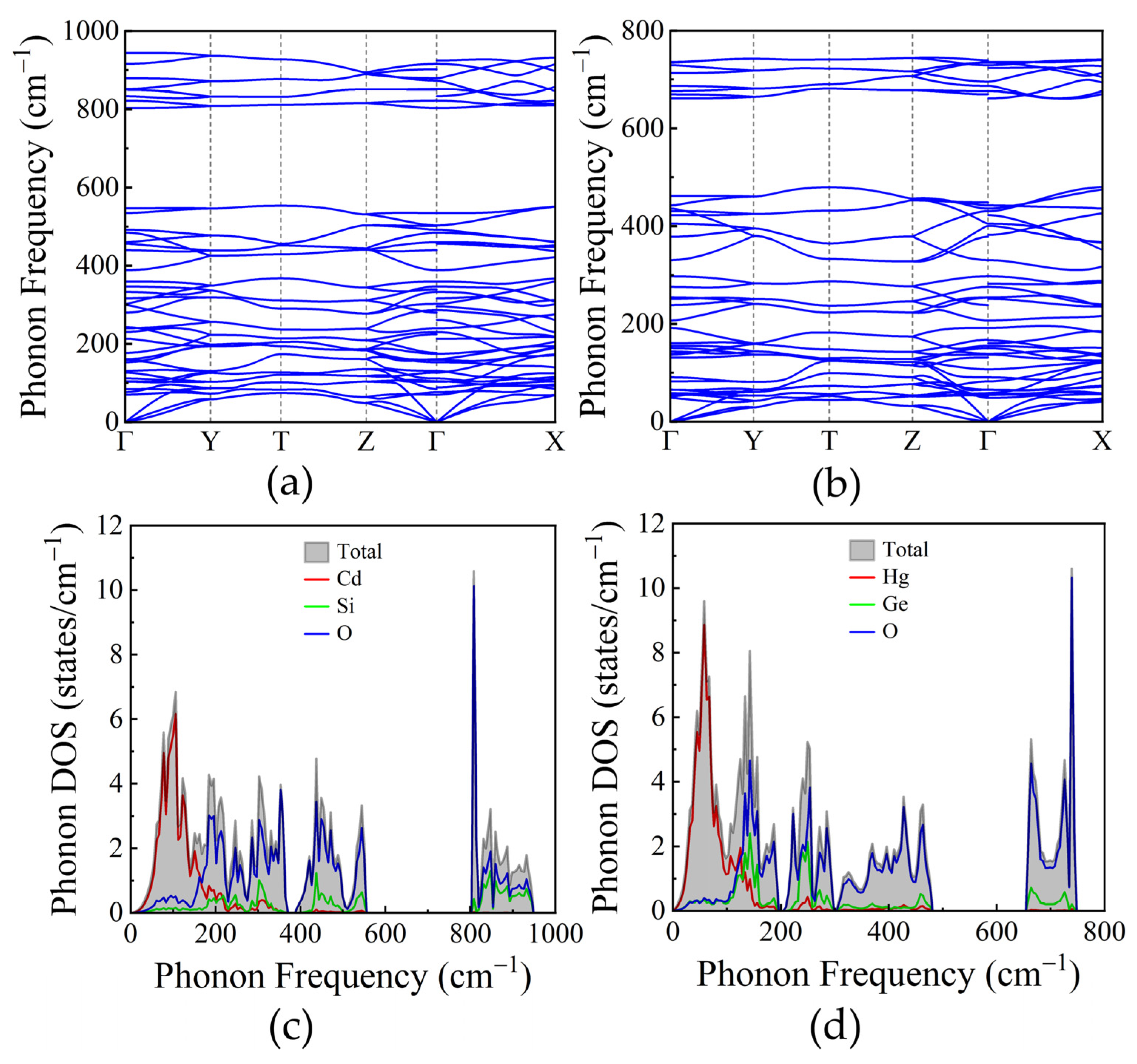
3.3. Lattice Dynamics
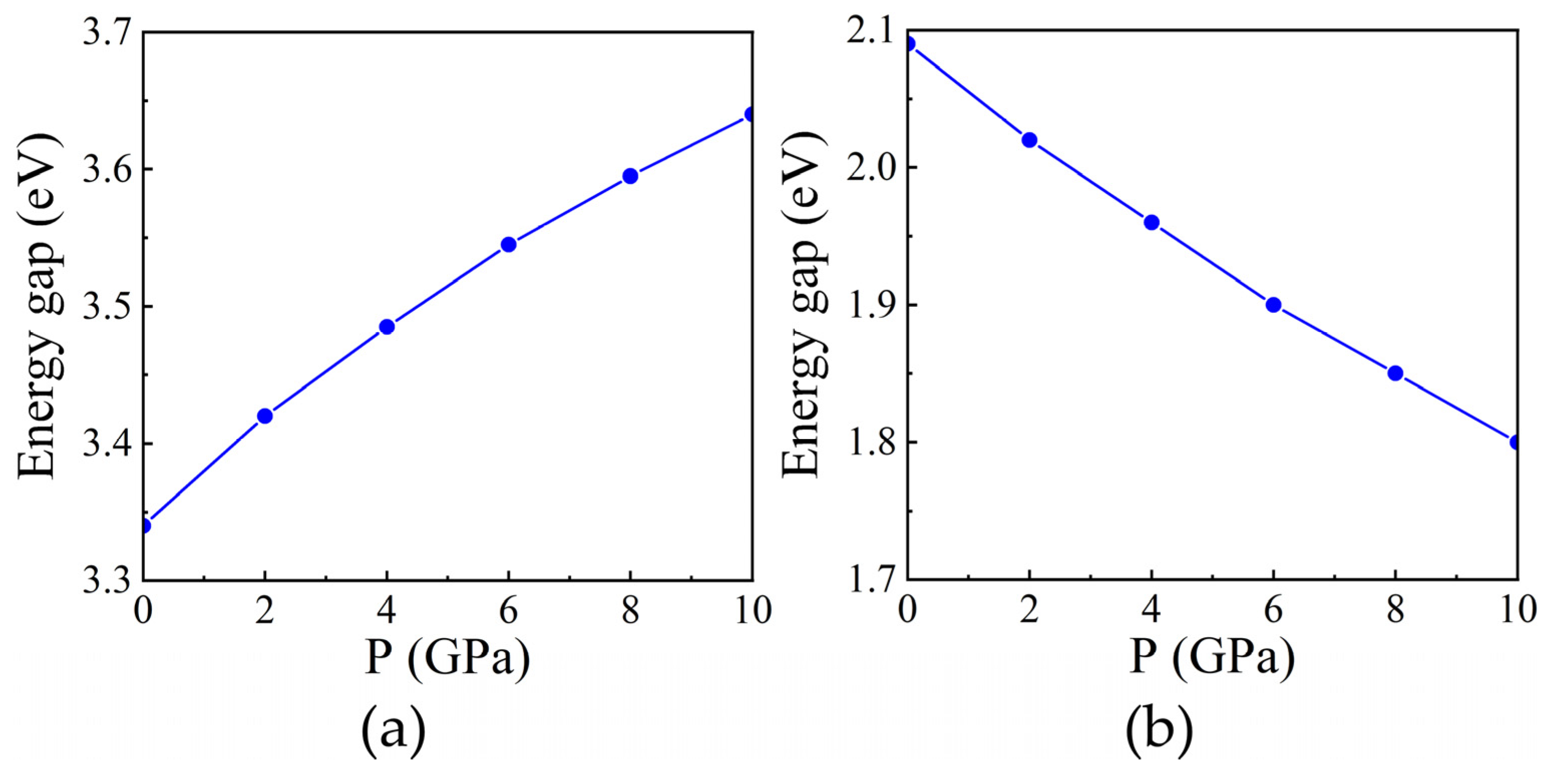
3.4. Electronic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gmelin, L. Gmelin Handbook Der Anorganischen Chemie; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Muller, O.; Roy, R. The Major Ternary Structural Families; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1974. [Google Scholar]
- Dollase, W.A.; Seifert, F.; O’Neill, H.S.C. Structure of Cr2SiO4 and Possible Metal-Metal Interactions in Crystal and Melt. Phys. Chem. Miner. 1994, 21, 104–109. [Google Scholar] [CrossRef]
- Zachariasen, W.H.; Ziegler, G.E. The Crystal Structure of Anhydrous Sodium Sulfate Na2SO4. Z. Kristall.-Cryst. Mater. 1932, 81, 92–101. [Google Scholar] [CrossRef]
- Mehrotra, B.N.; Th, H.; Eysel, W.; Röpke, H.; Illguth, A. Crystal chemistry of compounds with thenardite (Na2SO4V) structure. Neues Jahrb. Mineral. Monatshefte 1978, 408–421. [Google Scholar]
- Hesse, K.F.; Eysel, W. Structure of Dimercury (II) Germanate (IV). Acta Crystallogr. B 1981, 37, 429–431. [Google Scholar] [CrossRef]
- Glasser, L.S.D.; Glasser, F.P. The Preparation and Crystal Data of the Cadmium Silicates CdSiO3, Cd2SiO4, and Cd3SiO5. Inorg. Chem. 1964, 3, 1228–1230. [Google Scholar] [CrossRef]
- Weil, M. Parawollastonite-Type Cd3[Si3O9]. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, i102–i104. [Google Scholar] [CrossRef]
- Lei, B.; Liu, Y.; Ye, Z.; Shi, C. Luminescence Properties of CdSiO3: Mn2+ Phosphor. J. Lumin. 2004, 109, 215–219. [Google Scholar] [CrossRef]
- den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Aitasalo, T.; Hölsä, J.; Jungner, H.; Lastusaari, M.; Niittykoski, J. Thermoluminescence Study of Persistent Luminescence Materials: Eu2+-and R3+-Doped Calcium Aluminates, CaAl2O4: Eu2+, R3+. J. Phys. Chem. B 2006, 110, 4589–4598. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, B.; Shi, C. Luminescent Properties of a White Afterglow Phosphor CdSiO3: Dy3+. Chem. Mater. 2005, 17, 2108–2113. [Google Scholar] [CrossRef]
- Eysel, W. Kristallchemie von Oxyorthoverbindungen A30[B04]. Neues Jahrb. Mineral. Monatshefte 1970, 1970, 534–547. [Google Scholar]
- Wedepohl, K.H.; Correns, C.W.; Shaw, D.M.; Turekian, K.K.; Zemann, J. Handbook of Geochemistry; Springer: Berlin/Heidelberg, Germany, 1969; Volume 2. [Google Scholar]
- Miletich, R.; Seifert, F.; Angel, R.J. Compression of Cadmium Orthosilicate, Cd2SiO4: A High-Pressure Single-Crystal Diffraction Study. Z. Kristall.-Cryst. Mater. 1998, 213, 288–295. [Google Scholar] [CrossRef]
- Miletich, R.; Nowak, M.; Seifert, F.; Angel, R.J.; Brandstätter, G. High-Pressure Crystal Chemistry of Chromous Orthosilicate, Cr2SiO4. A Single-Crystal X-Ray Diffraction and Electronic Absorption Spectroscopy Study. Phys. Chem. Miner. 1999, 26, 446–459. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Gonze, X.; Lee, C. Dynamical Matrices, Born Effective Charges, Dielectric Permittivity Tensors, and Interatomic Force Constants from Density-Functional Perturbation Theory. Phys. Rev. B Condens. Matter Mater. Phys. 1997, 55, 10355–10368. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Drickamer, H.G.; Lynch, R.W.; Clendenen, R.L.; Perez-Albueene, E.A. X-Ray Diffraction Studies of the Lattice Parameters of Solids under Very High Pressure. In Solid State Physics; Elsevier: Amsterdam, The Netherlands, 1967; Volume 19, pp. 135–228. [Google Scholar]
- Ruiz-Fuertes, J.; Friedrich, A.; Errandonea, D.; Segura, A.; Morgenroth, W.; Rodríiguez-Hernández, P.; Muñoz, A.; Meng, Y. Optical and Structural Study of the Pressure-Induced Phase Transition of CdWO4. Phys. Rev. B 2017, 95, 174105. [Google Scholar] [CrossRef]
- Isaak, D.G.; Graham, E.K.; Bass, J.D.; Wang, H. The Elastic Properties of Single-Crystal Fayalite as Determined by Dynamical Measurement Techniques. Pure Appl. Geophys. 1993, 141, 393–414. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Hazen, R.M.; Finger, L.W. High-Pressure Crystal Chemistry of Monticellite, CaMgSiO4. Am. Mineral. 1987, 72, 748–755. [Google Scholar]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Mouhat, F.; Coudert, F.-X. Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Reuß, A. Berechnung Der Fließgrenze von Mischkristallen Auf Grund Der Plastizitätsbedingung Für Einkristalle. ZAMM-J. Appl. Math. Mech./Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, V.K.; Kanchana, V.; Vaitheeswaran, G.; Errandonea, D. High-Pressure Structural, Lattice Dynamics, and Electronic Properties of Beryllium Aluminate Studied from First-Principles Theory. Mater. Today Commun. 2021, 26, 101801. [Google Scholar] [CrossRef]
- Singh, J.; Errandonea, D.; Kanchana, V.; Vaitheeswaran, G. Deep Earth Chronicles: High-Pressure Investigation of Phenakite Mineral Be2SiO4. ChemPhysChem 2024, 25, e202300901. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Ma, C.-G.; Brik, M.G.; Akbudak, S. Vibrational and Elastic Properties of Silicate Spinels A2SiO4 (A = Mg, Fe, Ni, and Co). J. Phys. Chem. Solids 2018, 117, 167–172. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the Elastic Moduli and the Plastic Properties of Polycrystalline Pure Metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic Plasticity or Brittleness of Metallic Glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar] [CrossRef]
- Köster, W.; Franz, H. Poisson’s Ratio for Metals and Alloys. Metall. Rev. 1961, 6, 1–56. [Google Scholar] [CrossRef]
- Ledbetter, M.H. Materials at Low Temperatures; Reed, R.P., Clark, A.F., Eds.; American Society for Metals: Metals Park, OH, USA, 1983; pp. 1–45. [Google Scholar]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density Functional Theory for Calculation of Elastic Properties of Orthorhombic Crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Chung, D.H.; Buessem, W.R. The Elastic Anisotropy of Crystals. J. Appl. Phys. 1967, 38, 2010–2012. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 55504. [Google Scholar] [CrossRef]
- Brugger, K. Determination of Third-Order Elastic Coefficients in Crystals. J. Appl. Phys. 1965, 36, 768–773. [Google Scholar] [CrossRef]
- Music, D.; Houben, A.; Dronskowski, R.; Schneider, J.M. Ab Initio Study of Ductility in M2AlC(M = Ti, V, Cr). Phys. Rev. B 2007, 75, 174102. [Google Scholar] [CrossRef]
- Vines, F.; Lamiel-García, O.; Chul Ko, K.; Yong Lee, J.; Illas, F. Systematic Study of the Effect of HSE Functional Internal Parameters on the Electronic Structure and Band Gap of a Representative Set of Metal Oxides. J. Comput. Chem. 2017, 38, 781–789. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, Q. Investigation on the Electrical and Optical Properties of Forsterite Mg2SiO4 under Pressure up to 30 GPa. Mol. Simul. 2020, 46, 805–811. [Google Scholar] [CrossRef]
- Zhang, Y. Electronic and Optical Properties of Mg2GeO4 under Pressure Effect: Ab Initio Study. ECS J. Solid. State Sci. Technol. 2022, 11, 16002. [Google Scholar] [CrossRef]
- Sampath, S.K.; Cordaro, J.F. Optical Properties of Zinc Aluminate, Zinc Gallate, and Zinc Aluminogallate Spinels. J. Am. Ceram. Soc. 1998, 81, 649–654. [Google Scholar] [CrossRef]
- Errandonea, D.; Martinez-Garcia, D.; Lacomba-Perales, R.; Ruiz-Fuertes, J.; Segura, A. Effects of High Pressure on the Optical Absorption Spectrum of Scintillating PbWO4 Crystals. Appl. Phys. Lett. 2006, 89, 091913. [Google Scholar] [CrossRef]
- Ruiz-Fuertes, J.; López-Moreno, S.; López-Solano, J.; Errandonea, D.; Segura, A.; Lacomba-Perales, R.; Muñoz, A.; Radescu, S.; Rodríiguez-Hernández, P.; Gospodinov, M.; et al. Pressure Effects on the Electronic and Optical Properties of AWO4 Wolframites (A= Cd, Mg, Mn, and Zn): The Distinctive Behavior of Multiferroic MnWO4. Phys. Rev. B 2012, 86, 125202. [Google Scholar] [CrossRef]
- Liang, A.; Shi, L.-T.; Turnbull, R.; Manjón, F.J.; Ibáñez, J.; Popescu, C.; Jasmin, M.; Singh, J.; Venkatakrishnan, K.; Vaitheeswaran, G.; et al. Pressure-Induced Band-Gap Energy Increase in a Metal Iodate. Phys. Rev. B 2022, 106, 235203. [Google Scholar] [CrossRef]
- Monteseguro, V.; Ruiz-Fuertes, J.; Contreras-García, J.; Rodríguez-Hernández, P.; Muñoz, A.; Errandonea, D. High Pressure Theoretical and Experimental Analysis of the Bandgap of BaMoO4, PbMoO4, and CdMoO4. Appl. Phys. Lett. 2019, 115, 012102. [Google Scholar] [CrossRef]





| Compound | PBE | PBEsol | Expt. [6,15] | |
|---|---|---|---|---|
| Cd2SiO4 | a (Å) | 6.100 | 6.008 | 6.011 |
| b (Å) | 12.005 | 11.883 | 11.805 | |
| c (Å) | 10.015 | 9.834 | 9.802 | |
| V (Å3) | 733.44 | 701.97 | 695.60 | |
| Hg2GeO4 | a (Å) | 6.728 | 6.552 | 6.603 |
| b (Å) | 11.305 | 10.621 | 10.596 | |
| c (Å) | 11.661 | 11.667 | 11.485 | |
| V (Å3) | 886.88 | 811.93 | 803.55 |
| Compound | Atom | x | y | z |
|---|---|---|---|---|
| Cd2SiO4 | Cd(1) | 0.1250 | 0.1250 | 0.4401 |
| Cd(2) | 0.8750 | 0.8750 | 0.5599 | |
| Cd(3) | 0.3750 | 0.8750 | 0.6901 | |
| Si(1) | 0.1250 | 0.1250 | 0.1250 | |
| Si(2) | 0.8750 | 0.8750 | 0.8750 | |
| O(1) | 0.9689 | 0.0508 | 0.2301 | |
| O(2) | 0.0311 | 0.9492 | 0.7699 | |
| O(3) | 0.7811 | 0.6992 | 0.2301 | |
| O(4) | 0.2189 | 0.3008 | 0.7699 | |
| O(5) | 0.7811 | 0.0508 | 0.5199 | |
| O(6) | 0.2189 | 0.9492 | 0.4801 | |
| O(7) | 0.9689 | 0.6992 | 0.5199 | |
| O(8) | 0.0310 | 0.3008 | 0.4801 | |
| Hg2GeO4 | Hg(1) | 0.1250 | 0.4454 | 0.1250 |
| Hg(2) | 0.8750 | 0.5546 | 0.8750 | |
| Hg(3) | 0.3750 | 0.6954 | 0.8750 | |
| Ge(1) | 0.1250 | 0.1250 | 0.1250 | |
| Ge(2) | 0.8750 | 0.8750 | 0.8750 | |
| O(1) | 0.9783 | 0.2319 | 0.0425 | |
| O(2) | 0.0217 | 0.7681 | 0.9574 | |
| O(3) | 0.7717 | 0.5181 | 0.0425 | |
| O(4) | 0.2283 | 0.4819 | 0.9574 | |
| O(5) | 0.7717 | 0.2319 | 0.7074 | |
| O(6) | 0.2283 | 0.7681 | 0.2925 | |
| O(7) | 0.9783 | 0.5181 | 0.7074 | |
| O(8) | 0.0217 | 0.4819 | 0.2925 |
| Compound | P (GPa) | A1 | A2 | A3 | AB | AG | AU |
|---|---|---|---|---|---|---|---|
| Cd2SiO4 | 0 | 0.4375 | 0.7863 | 0.7614 | 0.0568 | 0.0779 | 0.9658 |
| 2 | 0.3958 | 0.7686 | 0.7643 | 0.0520 | 0.0869 | 1.0613 | |
| 4 | 0.3598 | 0.7486 | 0.7603 | 0.0505 | 0.0977 | 1.1889 | |
| 6 | 0.3276 | 0.7254 | 0.7500 | 0.0498 | 0.1101 | 1.3420 | |
| 8 | 0.2997 | 0.7016 | 0.7367 | 0.0498 | 0.1230 | 1.5077 | |
| 10 | 0.2731 | 0.6760 | 0.7188 | 0.0499 | 0.1380 | 1.7055 | |
| Hg2GeO4 | 0 | 0.3260 | 0.6662 | 0.7101 | 0.3245 | 0.2199 | 3.7801 |
| 2 | 0.3371 | 0.6137 | 0.7262 | 0.2639 | 0.1972 | 3.1736 | |
| 4 | 0.3410 | 0.5733 | 0.7764 | 0.2193 | 0.1852 | 2.8348 | |
| 6 | 0.3409 | 0.5246 | 0.7973 | 0.1772 | 0.1808 | 2.6384 | |
| 8 | 0.3310 | 0.4933 | 0.8050 | 0.1459 | 0.1870 | 2.6420 | |
| 10 | 0.3104 | 0.4591 | 0.7787 | 0.1191 | 0.1975 | 2.732 |
| Compound | P (GPa) | ρ (gm/cc) | vl (km/s) | vt (km/s) | vm (km/s) | θD |
|---|---|---|---|---|---|---|
| Cd2SiO4 | 0 | 5.9973 | 5.4857 | 2.6479 | 2.9763 | 381.45 |
| 2 | 6.0949 | 5.5822 | 2.6569 | 2.9887 | 385.11 | |
| 4 | 6.1848 | 5.6551 | 2.6550 | 2.9885 | 386.98 | |
| 6 | 6.2744 | 5.7198 | 2.6466 | 2.9812 | 387.88 | |
| 8 | 6.3578 | 5.7737 | 2.6346 | 2.9696 | 388.07 | |
| 10 | 6.4383 | 5.8208 | 2.6172 | 2.9518 | 387.37 | |
| Hg2GeO4 | 0 | 8.7991 | 3.4010 | 1.5646 | 1.7628 | 215.23 |
| 2 | 9.0717 | 3.5619 | 1.5657 | 1.7677 | 218.03 | |
| 4 | 9.3188 | 3.6957 | 1.5632 | 1.7674 | 219.96 | |
| 6 | 9.5266 | 3.8229 | 1.5628 | 1.7690 | 221.78 | |
| 8 | 9.7168 | 3.9256 | 1.5749 | 1.7838 | 225.11 | |
| 10 | 9.8873 | 4.0251 | 1.5853 | 1.7966 | 228.06 |
| Cd2SiO4 | Hg2GeO4 | ||||
|---|---|---|---|---|---|
| Mode | Raman/IR Active | Frequency (cm−1) | Mode | Raman/IR Active | Frequency (cm−1) |
| B3g | Raman | 70.48 | B1g | Raman | 48.66 |
| B2g | Raman | 77.06 | B3u | IR | 52.15 |
| B3u | IR | 84.64 | B2g | Raman | 54.79 |
| Ag | Raman | 103.24 | B3g | Raman | 55.31 |
| B1g | Raman | 104.33 | Ag | Raman | 58.19 |
| Au | IR | 111.70 | Au | IR | 65.15 |
| B3g | Raman | 126.47 | B1u | IR | 65.44 |
| B1u | IR | 130.56 | B3g | Raman | 82.04 |
| B1g | Raman | 153.06 | B1g | Raman | 90.45 |
| B1g | Raman | 159.24 | B2u | IR | 106.81 |
| B2g | Raman | 160.85 | B3u | IR | 131.11 |
| B2u | IR | 174.60 | B1g | Raman | 137.11 |
| B3u | IR | 176.64 | B2g | Raman | 138.72 |
| B1u | IR | 213.17 | B3g | Raman | 150.48 |
| B3g | Raman | 230.41 | B2g | Raman | 155.27 |
| B2g | Raman | 240.23 | B1u | IR | 160.18 |
| B3u | IR | 280.10 | B2u | IR | 191.72 |
| B2u | IR | 286.03 | Ag | Raman | 207.15 |
| Ag | Raman | 302.00 | B3u | IR | 238.31 |
| B1u | IR | 316.32 | B1u | IR | 250.96 |
| B1g | Raman | 332.81 | Au | IR | 254.10 |
| B3g | Raman | 346.75 | B1g | Raman | 275.48 |
| Au | IR | 359.30 | B3g | Raman | 297.23 |
| Ag | Raman | 388.13 | Ag | Raman | 330.62 |
| B3u | IR | 439.61 | B3u | IR | 377.96 |
| B1u | IR | 456.44 | B2u | IR | 400.32 |
| B3g | Raman | 460.04 | B3g | Raman | 404.92 |
| Au | IR | 484.22 | B1u | IR | 422.41 |
| B1g | Raman | 491.78 | B1g | Raman | 430.53 |
| B2u | IR | 502.84 | B2g | Raman | 435.91 |
| B2g | Raman | 534.46 | Au | IR | 442.38 |
| Au | IR | 803.04 | B1u | IR | 661.33 |
| Ag | Raman | 822.00 | B3u | IR | 669.70 |
| B1u | IR | 833.21 | B3g | Raman | 676.43 |
| B3u | IR | 849.23 | B1g | Raman | 686.86 |
| B3g | Raman | 851.31 | B2u | IR | 695.40 |
| B2u | IR | 872.81 | Au | IR | 729.19 |
| B1g | Raman | 878.83 | B2g | Raman | 734.43 |
| B2g | Raman | 915.79 | Ag | Raman | 735.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Errandonea, D.; Kanchana, V.; Vaitheeswaran, G. Pressure-Driven Responses in Cd2SiO4 and Hg2GeO4 Minerals: A Comparative Study. Crystals 2024, 14, 538. https://doi.org/10.3390/cryst14060538
Singh J, Errandonea D, Kanchana V, Vaitheeswaran G. Pressure-Driven Responses in Cd2SiO4 and Hg2GeO4 Minerals: A Comparative Study. Crystals. 2024; 14(6):538. https://doi.org/10.3390/cryst14060538
Chicago/Turabian StyleSingh, Jaspreet, Daniel Errandonea, Venkatakrishnan Kanchana, and Ganapathy Vaitheeswaran. 2024. "Pressure-Driven Responses in Cd2SiO4 and Hg2GeO4 Minerals: A Comparative Study" Crystals 14, no. 6: 538. https://doi.org/10.3390/cryst14060538
APA StyleSingh, J., Errandonea, D., Kanchana, V., & Vaitheeswaran, G. (2024). Pressure-Driven Responses in Cd2SiO4 and Hg2GeO4 Minerals: A Comparative Study. Crystals, 14(6), 538. https://doi.org/10.3390/cryst14060538






