Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Preparation
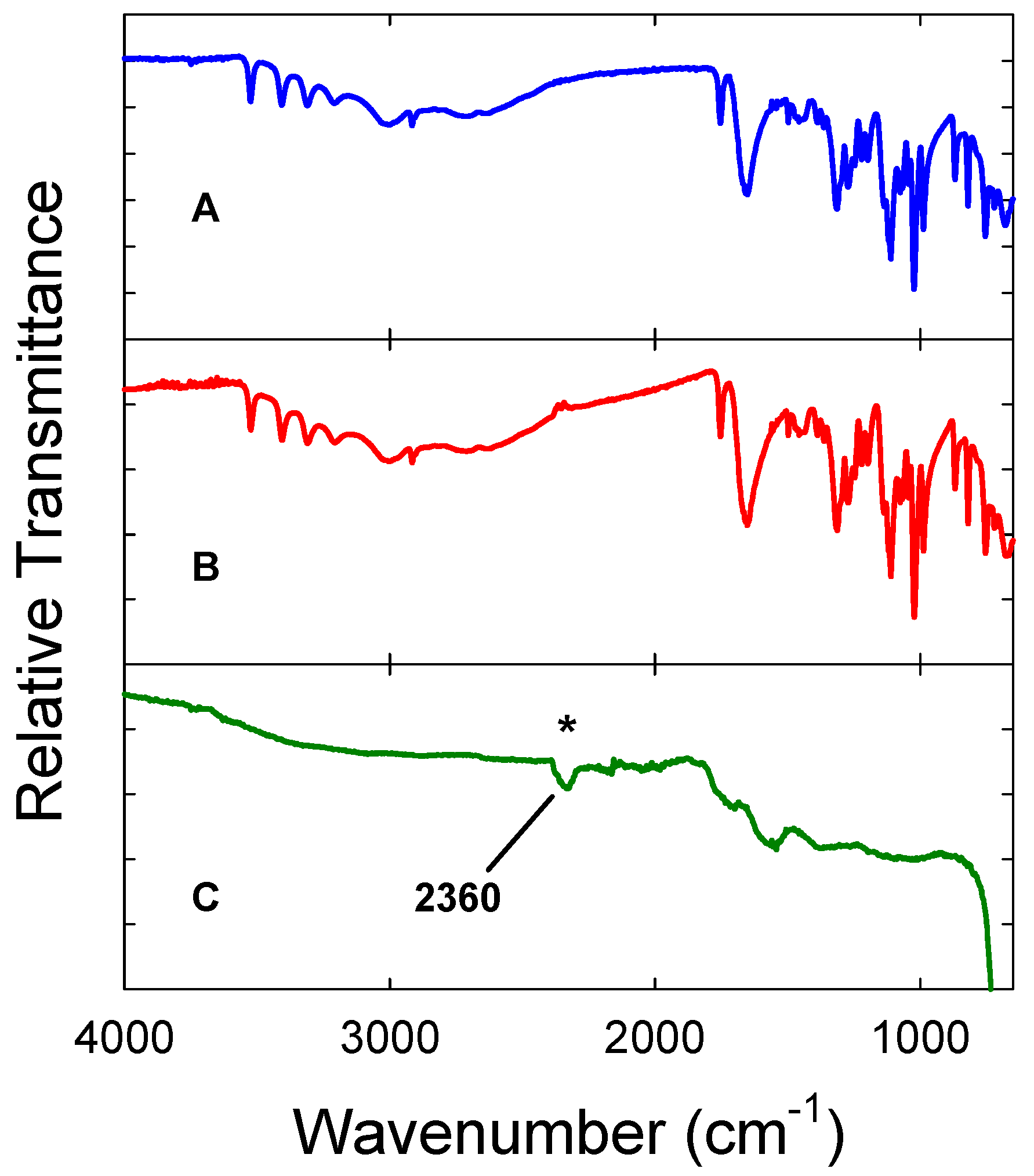
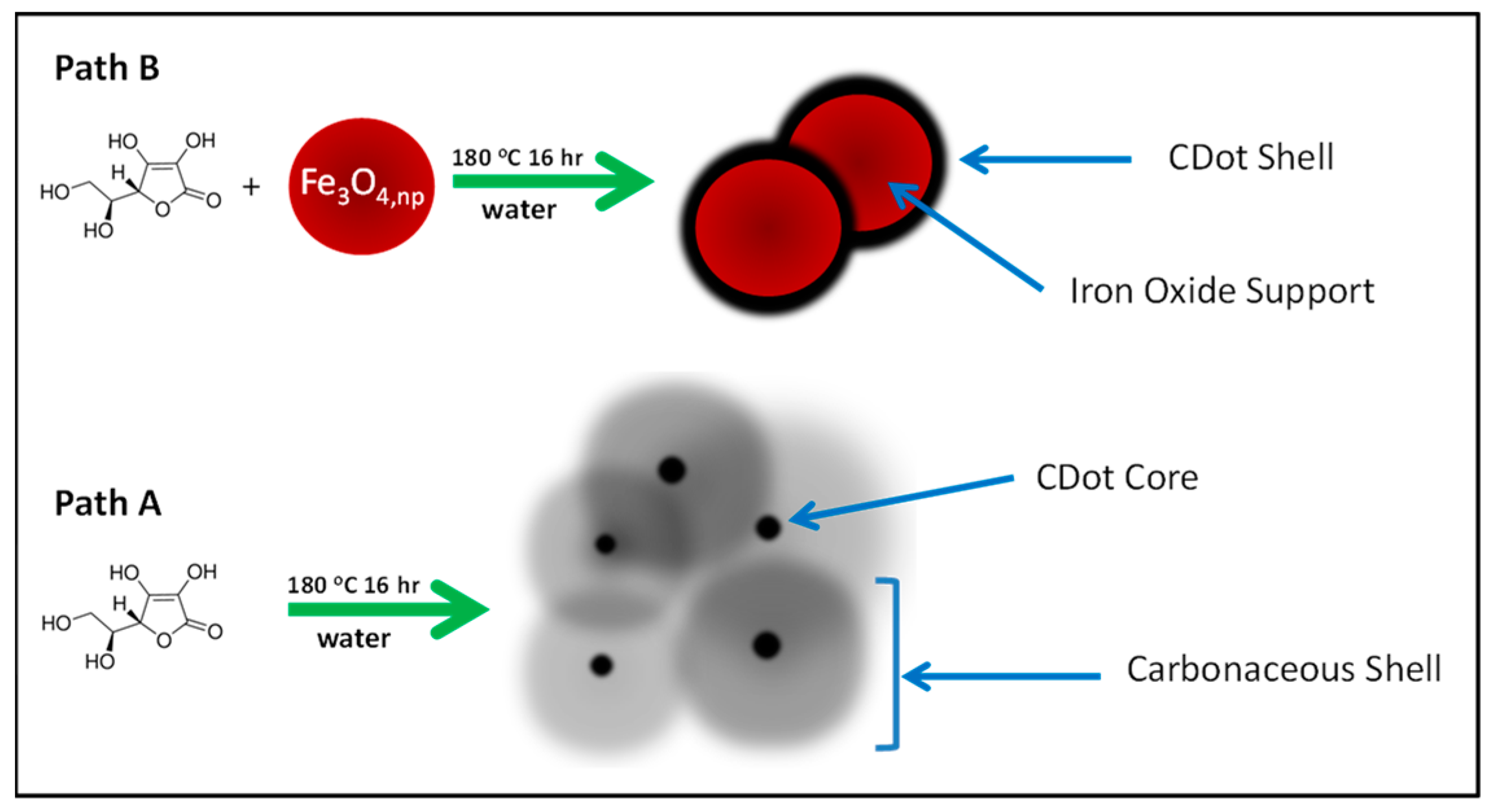
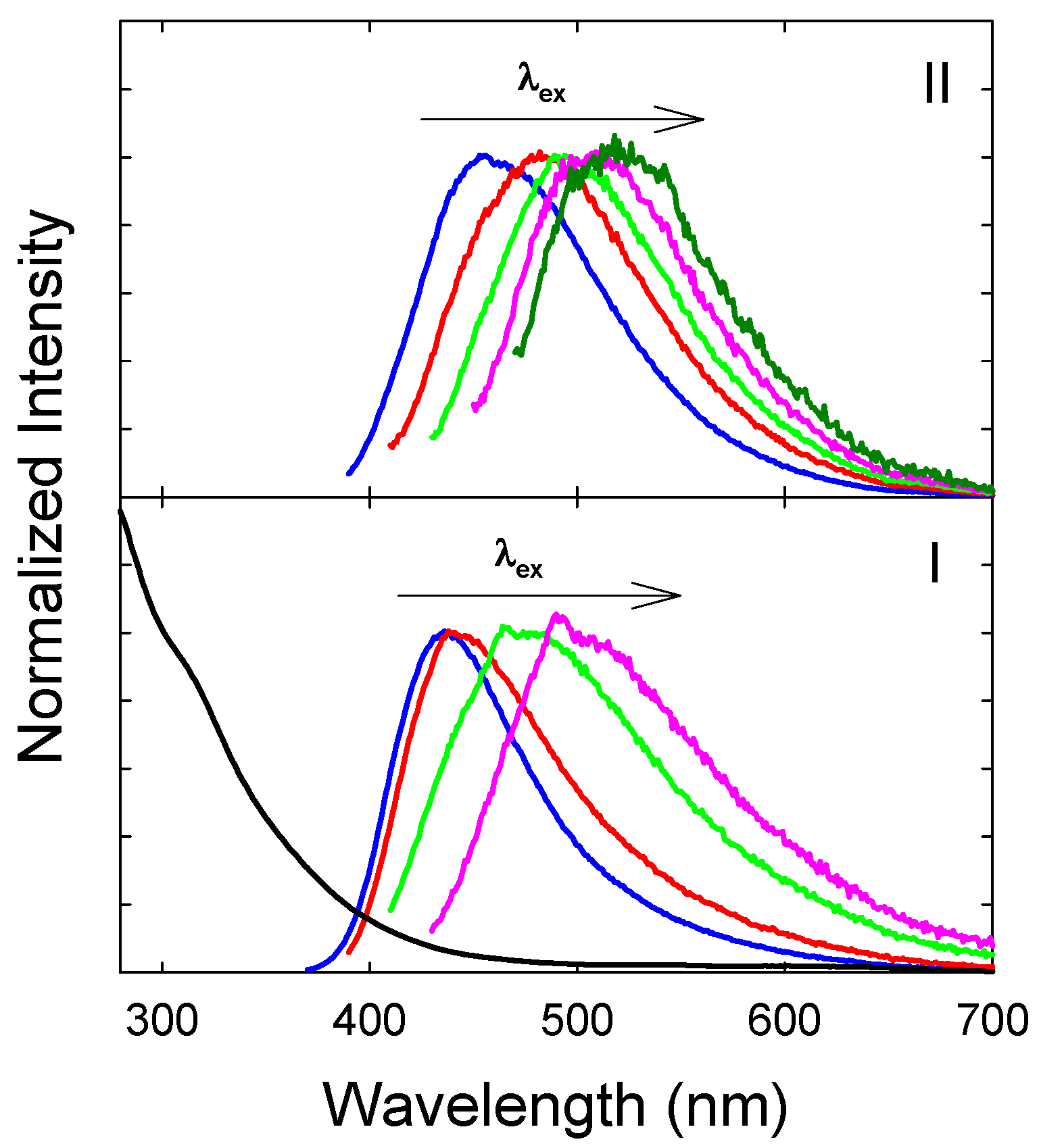
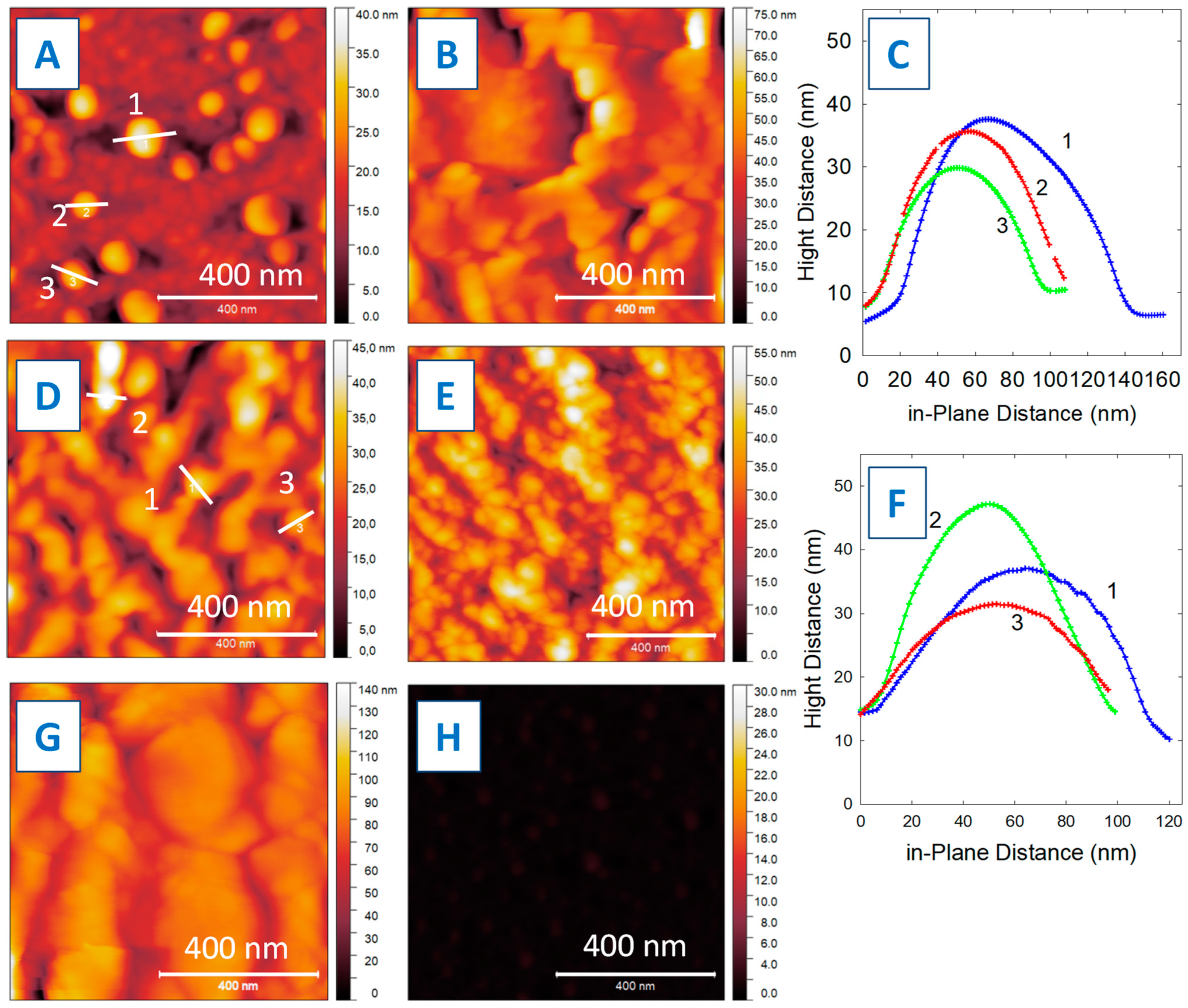
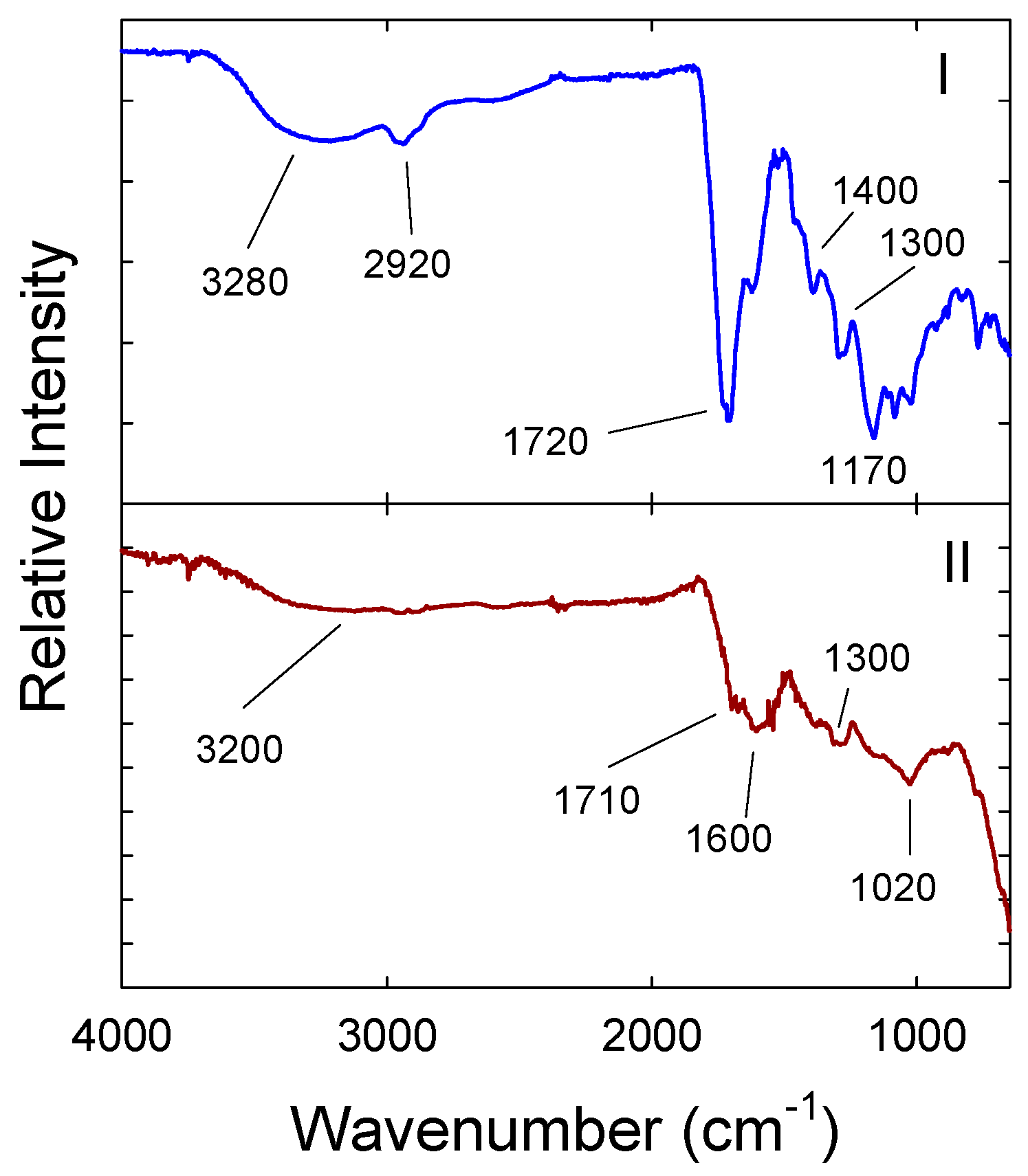
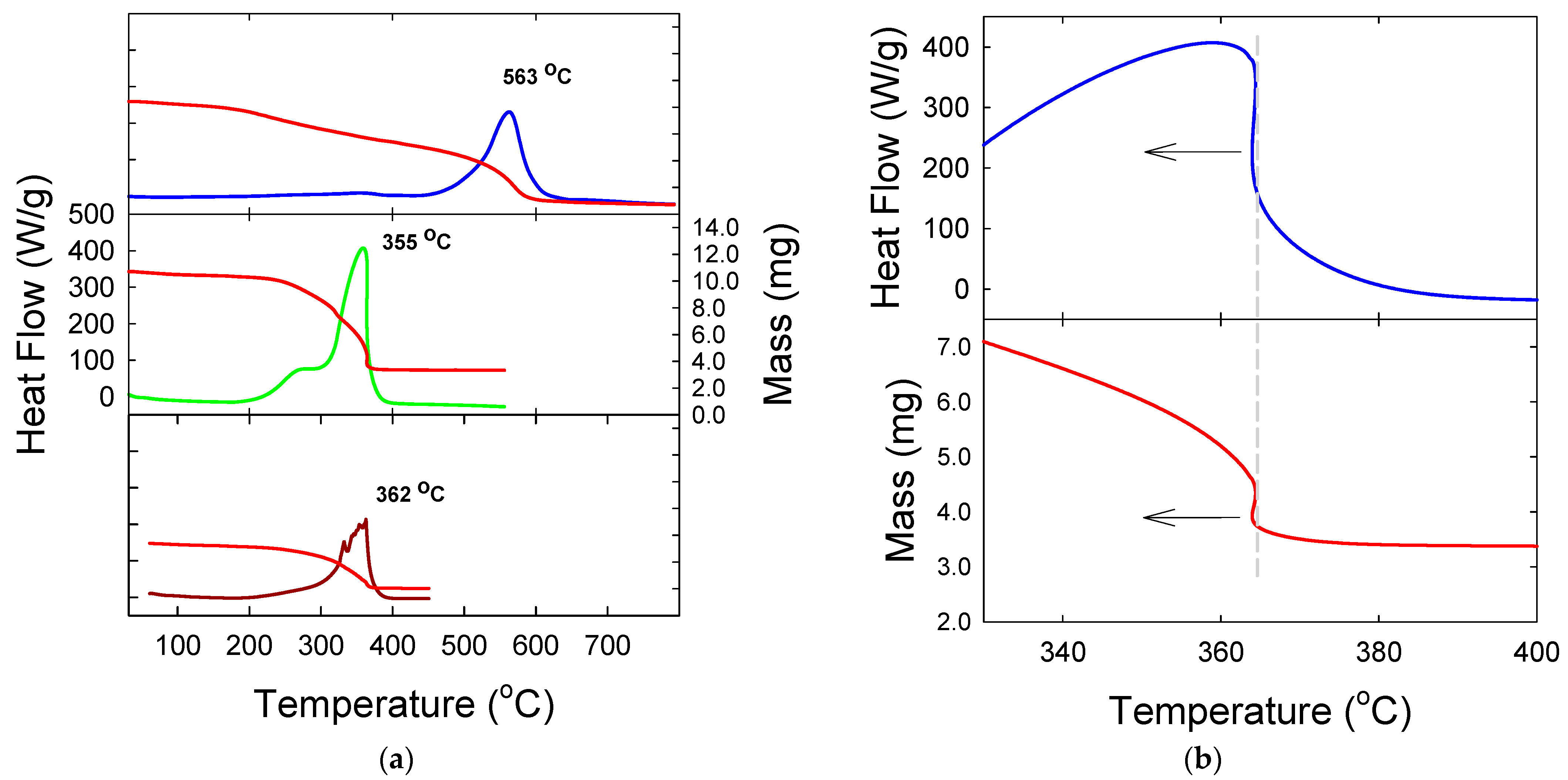
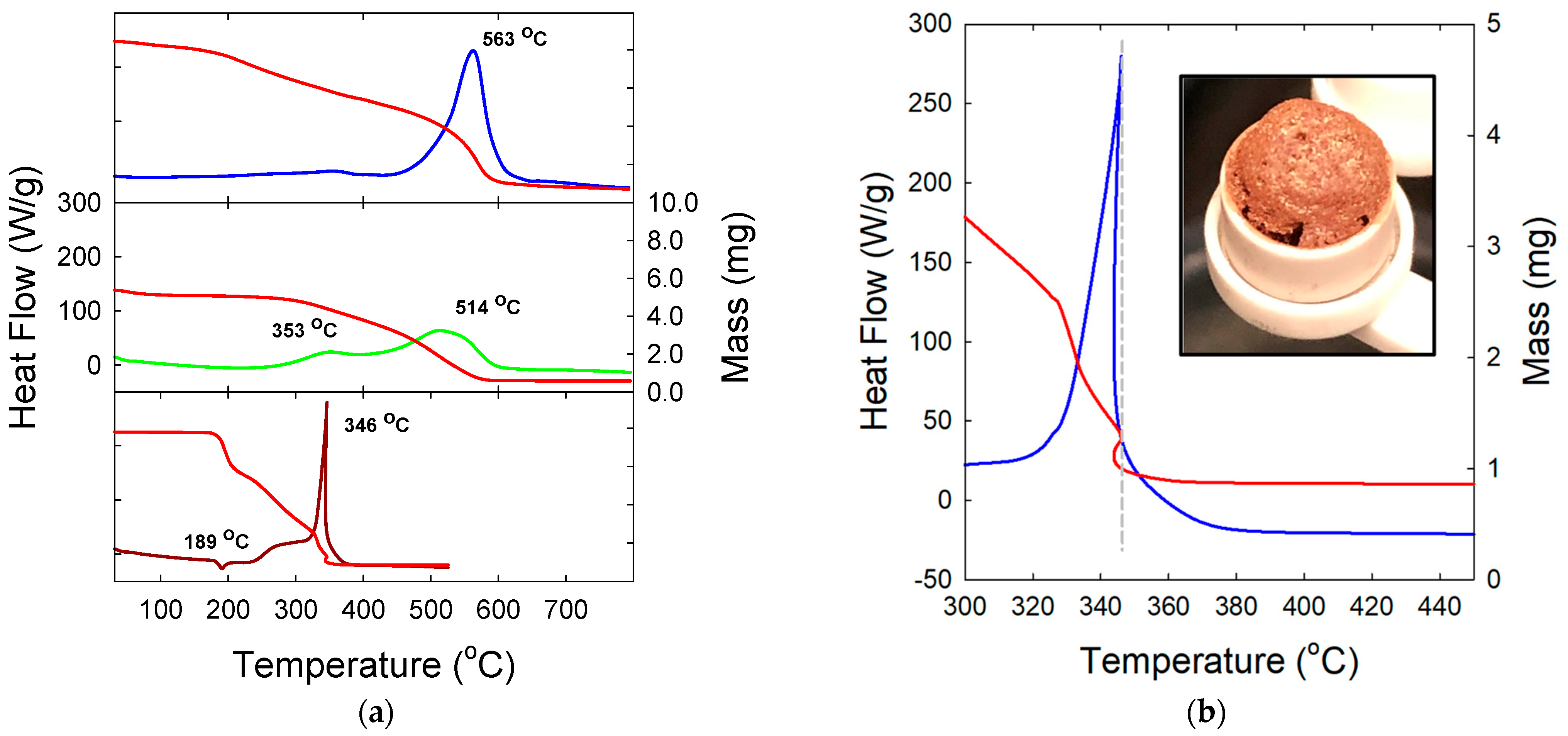
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wand, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Cao, L.; Fernando, K.A.S.; Liang, W.; Seilkop, A.; Veca, L.M.; Sun, Y.-P.; Bunker, C.E. Carbon dots for energy conversion applications. J. Appl. Phys. 2019, 125, 220903. [Google Scholar] [CrossRef]
- Wang, P.; Meziani, M.J.; Fu, Y.; Bunker, C.E.; Hou, X.; Yang, L.; Msellek, H.; Zaharias, M.; Darby, J.P.; Sun, Y.-P. Carbon dots versus nano-carbon/organic hybrids—Dramatically different behaviors in fluorescence sensing of metal cations with structural and mechanistic implications. Nanoscale Adv. 2021, 3, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Bunker, C.E.; Sun, Y.-P. Carbon Dots: Zero-Dimensional Carbon Allotrope with Unique Photoinduced Redox Characteristics. ACS Omega 2020, 5, 965–971. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Sonkar, S.K.; Yang, F.; Veca, L.M.; Wang, P.; Tackett, K.N., II; Yu, J.-J.; Vasile, E.; Qian, H.; Liu, Y.; et al. Toward Structurally Defined Carbon Dots as Ultracompact Fluorescent Probes. ACS Nano 2014, 8, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Ullal, N.; Muthamma, K.; Sunil, D. Carbon dots from eco-friendly precursors for optical sensing application: An up-to-date review. Chem. Pap. 2022, 76, 6097–6127. [Google Scholar] [CrossRef]
- Kumar, P.; Dua, S.; Kaur, R.; Kumar, M.; Bhatt, G. A review on advancements in carbon quantum dots and their application in photovoltaics. RSC Adv. 2022, 12, 4714–4759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, S.; Zhang, Y.; Li, Y.; Liu, Y.; Li, W.; Wang, Y.; Yan, X.; Luo, D. Carbon Dots in Biomedicine: A Review. ACS Appl. Bio Mater. 2022, 5, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Irmawati, R.; Hashim, H.S.; Ramdzan, N.S.M.; Fauzi, N.I.M. A Review on Carbon Dots: Synthesis, Characterization and Its Application in Optical Sensor for Environmental Monitoring. Nanomaterials 2022, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’aid, M.S.; Bakar, N.F.A. A Review on Multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 686549. [Google Scholar]
- Zhou, Y.; Zhang, W.; Leblanc, R.M. Structure−Property−Activity Relationships in Carbon Dots. J. Phys. Chem. B 2022, 126, 10777–10796. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, L.; Yu, S.; Yang, Y.; Liu, X. Advances in Magnetic Carbon Dots: A Theranostics Platform for Fluorescence/Magnetic Resonance Bimodal Imaging and Therapy for Tumors. ACS Biomater. Sci. Eng. 2023, 9, 6548–6566. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Koduru, J.R. Perspectives of magnetic nature carbon dots in analytical chemistry: From separation to detection and bioimaging. Trends Environ. Anal. Chem. 2022, 33, e00153. [Google Scholar] [CrossRef]
- Klekotka, U.; Zambrzycka-Szelewa, E.; Satula, D.; Kalska-Szostko, B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials 2021, 14, 5069. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, P.; Bunker, C.E.; Teisl, L.R.; Reibold, M.; Yan, S.; Qian, H.; He, D.; Sun, Y.-P. Preparation and optical properties of magnetic carbon/iron oxide hybrid dots. RSC Adv. 2017, 7, 41304–41310. [Google Scholar] [CrossRef]
- Stachowska, J.D.; Gamza, M.B.; Mellor, C.; Gibbons, E.N.; Krysmann, M.J.; Kelarakis, A.; Gumieniczek-Chlopek, E.; Straczek, T.; Kapusta, C.; Szwajca, A. Carbon dots/iron oxide nanoparticles with tuneable composition and properties. Nanomaterials 2022, 12, 674. [Google Scholar] [CrossRef]
- Xie, R.; Qu, Y.; Tang, M.; Zhao, J.; Chua, S.; Li, T.; Zhang, F.; Wheatley, A.E.H.; Chai, F. Carbon dots-magnetic nanocomposites for the detection and removal of Hg2+. Food Chem. 2021, 364, 130366. [Google Scholar] [CrossRef]
- Perelshtein, I.; Perkas, N.; Rahimipour, S.; Gedanken, A. Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications. Nanomaterials 2020, 10, 1384. [Google Scholar] [CrossRef]
- Stepanova, M.; Dubavik, A.; Efimova, A.; Konovalova, M.; Svirshchevskaya, E.; Zakharov, V.; Orlova, A. Magneto-Luminescent Nanocomposites Based on Carbon Dots and Ferrite with Potential for Bioapplication. Nanomaterials 2022, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-Y.; Gedda, G.; Girma, W.M.; Yen, C.-L.; Ling, Y.-C.; Chang, J.-Y. Magnetofluorescent Carbon Dots Derived from Crab Shell for Targeted Dual-Modality Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Li, Y.; Wei, Z.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Magnetic iron oxide–fluorescent carbon dots integrated nanoparticles for dual-modal imaging, near-infrared light-responsive drug carrier and photothermal therapy. Biomater. Sci. 2014, 2, 915–923. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, H.; Ye, J.; Zhao, C.; Gao, S.; Wu, C.; Li, C.; Li, J.; Wang, X. Nitrogen-Doped Carbon Quantum Dot Stabilized Magnetic Iron Oxide Nanoprobe for Fluorescence, Magnetic Resonance, and Computed Tomography Triple-Modal In Vivo Bioimaging. Adv. Funct. Mater. 2016, 26, 8694–8706. [Google Scholar] [CrossRef]
- Cao, L.; Anilkumar, P.; Wang, X.; Liu, J.H.; Sahu, S.; Meziani, M.J.; Myers, E.; Sun, Y.-P. Reverse Stern–Volmer Behavior for Luminescence Quenching in Carbon Nanoparticles. Can. J. Chem. 2011, 89, 104–109. [Google Scholar] [CrossRef]
- Jessup, R.S. Heats of Combustion of Diamond and of Graphite. J. Res. Nat. Bur. Stand. 1938, 21, 475. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameter. C 2021, 7, 41. [Google Scholar] [CrossRef]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural Magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef] [PubMed]
- Trimm, D.L. Catalytic Combustion (Review). Appl. Catal. 1983, 7, 249–282. [Google Scholar] [CrossRef]
- Jones, R.L. Catalytic Combustion Effects in Internal Combustion Engines. Combust. Sci. Tech. 1997, 129, 185–195. [Google Scholar] [CrossRef]
- Forzatti, P.; Groppi, G. Catalytic combustion for the production of energy. Catal. Today 1999, 54, 165–180. [Google Scholar] [CrossRef]
- Dey, S.; Sun, S.; Mehta, N.S. Carbon monoxide catalytic oxidation over various iron-based nanoparticles at ambient conditions: A Review. Carbon Capture Sci. Technol. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of Magnetite (Fe3O4) Oxidation to Hematite (Fe2O3) in Air for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2014, 53, 13320. [Google Scholar] [CrossRef]
- Smiltens, J. The Standard Free Energy of Oxidation of Magnetite to Hematite at Temperatures above 1000°. J. Am. Chem. Soc. 1957, 79, 4877–4880. [Google Scholar] [CrossRef]
- Zheng, H.; Schenk, J.; Spreitzer, D.; Wolfinger, T.; Daghagheleh, O. Review on the Oxidation Behaviors and Kinetics of Magnetite in Particle Scale. Steel Res. Int. 2021, 92, 2000687. [Google Scholar] [CrossRef]
- Jingyan, S.; Yuwen, L.; Zhiyong, W.; Cunxin, W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. Anal. 2013, 77, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Chakib, S.; Rogacheva, S.M.; Obretenov, T.D.; Parkanyi, C. Thermal decomposition of ascorbic acid. Carbohydr. Res. 1997, 305, 1–15. [Google Scholar] [CrossRef]
- Rahmawati, S.; Bundjali, B. Kinetics of the Oxidation of Vitamin C. Indo. J. Chem. 2012, 12, 291. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Lerdkanchanaporn, S.; Dollimore, D.; Alexander, K.S. A Thermal Analysis Study of Ascorbic Acid and Its Pharmaceutical Formulations, A Consideration of Time-Temperature Plots. J. Therm. Anal. 1997, 49, 887–896. [Google Scholar] [CrossRef]
- Yadav, R.A.; Rani, P.; Kumar, M.; Singh, R.; Singh, P.; Singh, N.P. Experimental IR and Raman spectra and quantum chemical studies of molecular structures, conformers and vibrational characteristics of L-ascorbic acid and its anion and cation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guihong, X.; He, D.; Zhang, L. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling. Appl. Catal. B Environ. 2020, 267, 118383. [Google Scholar] [CrossRef]
- Usman, M.; Monfort, O.; Haderlein, S.; Hanna, K. Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System. Catalysts 2021, 11, 331. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Li, M.; Zhang, Y.; Yuan, S.; Ai, Z.; Zhao, J.; Zhang, L. Fenton oxidation of organic contaminants with aquifer sediment activated by ascorbic acid. Chem. Eng. J. 2018, 348, 255–262. [Google Scholar] [CrossRef]
- Hakim, A.; Marliza, T.S.; Tahari, N.M.A.; Isahak, R.W.N.W.; Yusop, T.M.; Hisham, W.M.M.; Yarmo, A.M. Studies on CO2 Adsorption and Desorption Properties from Various Types of Iron Oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888. [Google Scholar] [CrossRef]
- Kumar, S.; Drozd, V.; Durygin, A.; Saxena, S.K. Capturing CO2 Emissions in the Iron Industries using a Magnetite–Iron Mixture. Energy Technol. 2016, 4, 560. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; Lopez, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Mirabella, F.; Zaki, E.; Ivars-Barcelo, F.; Schauermann, S.; Shaikhutdinov, S. CO2 Adsorption on Magnetite Fe3O4(111). J. Phys. Chem. C 2018, 122, 27433. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Roldan, A.; Dzade, N.Y.; de Leeuw, N.H. Reactivity of CO2 on the surfaces of magnetite (Fe3O4), greigite (Fe3S4) and mackinawite (FeS). Phil. Trans. R. Soc. A 2018, 376, 20170065. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corr. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corr. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Lewis, W.K.; Rumchik, C.G.; Smith, M.J.; Fernando, K.S.; Crouse, C.A.; Spowart, J.E.; Guliants, E.A.; Bunker, C.E. Comparison of post-detonation combustion in explosives incorporating aluminum nanoparticles: Influence of the passivation layer. J. Appl. Phys. 2013, 113, 044907. [Google Scholar] [CrossRef]
- Fernando, K.S.; Smith, M.J.; Harruff, B.A.; Lewis, W.K.; Guliants, E.A.; Bunker, C.E. Sonochemically assisted thermal decomposition of alane N, N-dimethylethylamine with titanium (IV) isopropoxide in the presence of oleic acid to yield air-stable and size-selective aluminum core−shell nanoparticles. J. Phys. Chem. C 2009, 113, 500. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouhri, K.; Snyder, L.J.; McFarland, M.; Laubie, P.O.; Fernando, K.A.S.; Bunker, C.E. Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate. Crystals 2024, 14, 520. https://doi.org/10.3390/cryst14060520
Zouhri K, Snyder LJ, McFarland M, Laubie PO, Fernando KAS, Bunker CE. Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate. Crystals. 2024; 14(6):520. https://doi.org/10.3390/cryst14060520
Chicago/Turabian StyleZouhri, Khalid, Luke J. Snyder, Michael McFarland, Parker O. Laubie, K. A. Shiral Fernando, and Christopher E. Bunker. 2024. "Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate" Crystals 14, no. 6: 520. https://doi.org/10.3390/cryst14060520
APA StyleZouhri, K., Snyder, L. J., McFarland, M., Laubie, P. O., Fernando, K. A. S., & Bunker, C. E. (2024). Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate. Crystals, 14(6), 520. https://doi.org/10.3390/cryst14060520





