Abstract
In this study, we explore the synthesis and solid-state characterization of four coumarin-3-carboxylic acid esters, each modified at the C-3 position with different cycloalkyl groups: cyclohexyl, menthyl, and iso-pulegyl. We conducted a detailed analysis of these compounds utilizing a variety of techniques such as a single-crystal X-ray diffraction, nuclear magnetic resonance (NMR), and Fourier-transform infrared (FTIR) spectroscopy. Additionally, we calculated the dipole moments for these molecules. Our findings include a thorough structural assessment, highlighting the role of noncovalent interactions through Full Interaction Maps and Hirshfeld surface analysis. This study reveals the critical influence of the weak C-H…O hydrogen bonds in determining the solid-state architecture of these esters, whereas π-π stacking interactions appear to be negligible among the studied derivatives.
1. Introduction
Coumarins, a class of naturally occurring phenolic compounds, have garnered significant interest in medicinal chemistry due to their diverse pharmacological properties. The recent literature on the therapeutic effects of coumarins reveals a range of potential medical applications for these compounds. The studies included the investigation of the pharmacological and biological impacts of coumarins, encompassing anti-inflammatory, anticoagulant, antihypertensive, anticonvulsant, antioxidant, antimicrobial, and neuroprotective activities [1,2,3,4,5,6,7,8]. This diverse range of activities makes coumarins promising candidates for the development of new therapeutic agents. Beyond their biological properties, coumarins find applications in various other fields. Due to their fluorescence properties, coumarins are used in the development of fluorescent dyes and sensors for detecting metal ions, pH changes, and biomolecules [9,10,11]. In the textile and paper industries, coumarins are used as optical brighteners to enhance the appearance of materials by absorbing UV light and emitting blue light. Coumarin compounds are often used in fragrances and cosmetic products for their sweet, pleasant odor [12]. Some coumarin derivatives are utilized in the development of pesticides and herbicides [13,14].
Terpenes are often used in organic chemistry to modify and enhance the chemical and biological properties of various compounds [15]. Terpenoid coumarins are not as common as other types of coumarins, but they are studied for their unique properties. Researchers are interested in these compounds because they may offer new therapeutic possibilities due to their specific pharmacological properties arising from the combination of coumarin and terpene moieties. Recently, prenylated and geranylated coumarins have been presented with a wide range of biological activities [16,17]. Studies delving into the chemistry of these compounds highlight their medicinal properties and bioactivities, which range from analgesic and anticoagulant to anti-inflammatory and antimicrobial effects. Also, the promising coumarin with two isobornyl substituents on the benzopyran ring was synthesized and was distinguished by its strong antioxidant activity [18]. In addition to the previously mentioned terpenoid coumarins, there are other more prominent examples with a broader spectrum of action (Figure 1). Auraptene, a prenylated coumarin found in citrus peels, is particularly noted for its anti-inflammatory, anti-tumor, and antioxidant properties [19,20]. Osthole is derived from plants such as Cnidium monnieri and is known for its anti-inflammatory, anti-cancer, and liver-protective properties [21,22,23]. Imperatorin, on the other hand, found in Angelica species, has anti-inflammatory and antimicrobial vasorelaxant effects [24].
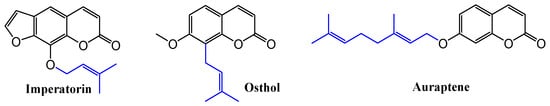
Figure 1.
Examples of terpene coumarins that demonstrate a number of biological activities.
In this preliminary study, we focused on the synthesis and characterization of coumarin derivatives derived from coumarin-3-carboxylic acid, containing ester groups such as cyclohexyl, menthyl, and isopulegyl, along with their subsequent structural analysis using X-ray crystallography. Cyclohexyl [25] and menthyl coumarin-3-carboxylic acid esters [26] are already known in the literature, but they were used as the building blocks for the syntheses of other compounds. Herein, we present a comprehensive structural and spectroscopic analysis of these compounds, which, in our opinion, will enrich the understanding of their potential biological activity. Up to now, considerable attention has been focused on the structural examination of basic coumarins, especially those bearing alkyl substitutions [27,28,29,30,31,32]. These studies have employed X-ray crystallography as a key methodological tool, providing insights into the molecular conformations, intermolecular interactions, and crystal packing. However, despite research on alkyl-substituted coumarins, there remains a notable gap in the structural characterization of coumarin derivatives featuring an ester substituent at the C-3 position, especially those with cyclohexyl ester moieties. Moreover, substitution with menthyl and isopulegyl groups turns the molecule into a chiral system, with a larger hydrophobic part. This gap is significant, considering the potential these compounds hold in terms of their unique chemical, physical, and biological properties, which could lead to novel applications. The solid-state analysis will allow for a deeper understanding of the intermolecular forces between these molecules and their biological targets. It may also contribute to a better understanding of the structure–activity relationship in this class of coumarin esters. It should be noted that the biological evaluation of the coumarins presented by us is currently ongoing. The results of biological studies could extend beyond the scope of this article and will be presented in future publications.
2. Materials and Methods
2.1. Instrumentation
2.1.1. General
Chemicals procured commercially from Sigma-Aldrich were utilized without further purification. NMR spectra were acquired employing a Bruker AV500 spectrometer (1H 500 MHz, 13C NMR 126 MHz), with recordings carried out in CDCl3 solutions. Chemical shifts (δ) were referenced to TMS, and coupling constants (J) were provided in Hz. Signal abbreviations included s for singlet, d for doublet, t for triplet, q for quartet, m for multiplet, and b for broad. Elemental analyses were conducted using a Perkin-Elmer CHN 2400 system. FTIR spectra were obtained via attenuated total (internal) reflection (ATR/FTIR) using a Bruker TENSOR 27 spectrophotometer equipped with a PIKE measuring cell featuring crystalline diamond embedded in zinc selenide. The spectra were recorded in the range of 4000 to 600 cm−1, with 64 scans per sample, at a resolution of 2 cm−1 in absorption mode. Optical rotations were measured using a Perkin-Elmer 341LC digital polarimeter. Melting points were determined on a Buchi 510 apparatus. Thin-layer chromatography (TLC) was performed on silica gel (Kieselgel 60, F254 on aluminum sheets, Merck KGaA, Darmstadt, Germany under UV light (254 nm). Column chromatographic separations and purifications were carried out using Merck silica gel 60 (230–400 mesh).
2.1.2. X-ray Crystallography
The single crystal diffraction data were collected at room temperature with a SuperNova diffractometer (Oxford Diffraction; Agilent [33]) with the graphite monochromated CuKα radiation (λ = 1.54184 Å). The CrysAlisPro program system [34] was used for data collection, cell refinement, and data reduction. The intensities were corrected for Lorentz and polarization effects, and the multi-scan absorption corrections were applied. The crystal structure was solved by direct methods using the SHELXT program and refined by the full-matrix least squares method on F2 using the SHELXL-2018/3 program [35,36]. The non-hydrogen atoms were refined with anisotropic displacement parameters, H-atoms were positioned at calculated positions and refined using the riding model. The experimental details and final atomic parameters for the analyzed crystals were deposited with the Cambridge Crystallographic Data Centre as Supplementary Material (CCDC Nos 2329676–2329679).
The powder diffraction data were collected at room temperature using an Empyrean diffractometer with a PIXcel3D area detector (PANalytical, Almelo, The Netherlands) and monochromated Cu-Kα radiation (λ = 1.54184 Å), in the 2θ range of 3–50° and a θ step of 0.0131°.
2.2. General Procedure for Synthesis of Coumarins CMR 1–4
To a solution of coumarin-3-carboxylic acid (1.0 g, 5.26 mmol) in dry dichloromethane (DCM, 15 mL) N,N′-dicyclohexylcarbodiimide (DCC, 5.78 mmol), 4-dimethyl-aminopyridine (DMAP, 0.26 mmol), and the appropriate alcohol (5.78 mmol, cyclohexanol, menthol, isopulegol) were added. The reaction mixture was stirred at room temperature for 12–48 h. The resultant by-product DCU (N,N’-dicyclohexylurea) precipitate was eliminated from the reaction mixture by filtration, and the filtrate was concentrated. The resultant coumarin esters were purified by column chromatography (hexane/ethyl acetate 15:1) and/or by recrystallization with ethanol.
Cyclohexyl 2-oxo-2H-chromene-3-carboxylate (CMR-1). The reaction of 2-oxo-2H-chromene-3-carboxylic acid (1) (1.0 g, 5.26 mmol) with cyclohexanol (0.57 g, 5.78 mmol) in the presence of DCC (1.19 g, 5.78 mmol) and DMAP (32.0 mg, 0.26 mmol) for 12 h gave a white solid of CMR-1 (620 mg, 45%). Rf = 0.5 (hexane/ethyl acetate 5:1). Mp. 107–108 °C. 1H NMR (500 MHz, CDCl3): δ 2.02–1.28 (m, 10 H, CH2), 5.07 (ddd, J = 12.9, 8.9, 3.9 Hz, 1 H, CH), 7.40–7.36 (m, 2 H, CHarom), 7.68–7.63 (m, 2 H, CHarom), 8.49 (s, 1 H, CH), 13C NMR (126 MHz, CDCl3): δ 23.6, 25.4, 31.5, 56.9, 74.4, 116.8, 117.9, 118.9, 124.7, 129.4, 134.1, 147.9, 155.1, 156.7, 162.4. ATR-FTIR (υ/cm−1): 3108, 3056, 2856, 1763, 1747, 1722, 1700, 1618, 1216, 794, 759. Anal. Calcd. for C16H16O4; C, 70.57; H, 5.92; Found C, 70.49; H, 5.60.
Crystal data for CMR-1: crystal system triclinic, space group P-1, unit cell dimensions a = 6.1358 (8) Å, b = 9.6241 (14) Å, c = 12.1824 (17) Å, α = 95.904 (12)°, β = 94.563 (11)°, γ = 103.632 (12)°, V = 691.39 (17) Å3, Z = 2, density (calcd) = 1.308 g/cm3, absorption coeff. 0.771 mm−1, F(000) = 288. Reflections collected/independent 4488/2639 [R(int) = 0.0256], data/parameters 2639/182, goodness-of-fit on F2 1.076, final R indices [I > 2σ(I)] R1 = 0.0589, wR2 = 0.1743, Δρ max/min 0.33, and −0.20 e. Å−3. CCDC No. 2329676.
(1R,2S,5R)-2-isopropyl-5-methylcyclohexyl 2-oxo-2H-chromene-3-carboxylate (CMR-2). The reaction of 2-oxo-2H-chromene-3-carboxylic acid (1.0 g, 5.26 mmol) with L-(-)-menthol (0.90 g, 5.78 mmol) in the presence of DCC (1.19 g, 5.78 mmol) and DMAP (32.0 mg, 0.26 mmol) for 48 h gave a white solid of CMR-2 (480 mg, 30%). [α]D20 −58.5 (c 0.55, CH2Cl2). Rf = 0.6 (hexane/ethyl acetate 5:1). Mp. 144–146 °C. 1H NMR (500 MHz, CDCl3): δ 0.83 (d, J = 6.9 Hz, 3 H, CH3), 0.95 (d, J = 3.8 Hz, 3 H, CH3), 0.97 (d, J = 3.4 Hz, 3 H, CH3), 1.29–1.26 (m, 2 H, CH2), 1.56–1.64 (m, 3 H, CH2, CH), 1.73–1.79 (m, 2 H, CH2), 2.04 (dtd, J = 13.9, 7.0, 2.7 Hz, 1 H, CH), 2.20–2.14 (m, 1 H, CH2), 4.99 (td, J = 10.9, 4.4 Hz, 1 H, CH), 7.41–7.33 (m, 2 H, CHarom), 7.69–7.62 (m, 2 H, CHarom), 8.49 (s, 1 H, CH). 13C NMR (126 MHz, CDCl3): δ 16.2, 20.8, 22.0, 23.3, 26.1, 31.4, 34.1, 40.7, 46.9, 76.0, 116.7, 117.9, 118.7, 124.7, 129.4, 134.1, 147.9, 155.1, 156.6, 162.5. ATR-FTIR (υ/cm−1): 3093, 3059, 3056, 2956, 2937, 2897, 2867, 1743, 1701, 1606, 1451, 1379, 1242, 1015, 769. Anal. Calcd. for C20H24O4; C, 73.15; H, 7.37; Found C, 73.20; H, 7.41.
Crystal data for CMR-2: crystal system orthorhombic, space group P212121, unit cell dimensions a = 11.0514 (3) Å, b = 12.3778 (4) Å, c = 13.4983 (4) Å, V = 1846.46 (10) Å3, Z = 4, Density (calcd) 1.181 g/cm3, absorption coeff. 0.656 mm−1, F(000) = 704. Reflections collected/independent 4914/3240 [R(int) = 0.0196], data/parameters 3240/218. Goodness-of-fit on F2 1.055, final R indices [I > 2σ (I)] R1 = 0.038, wR2 = 0.097, absolute structure parameter x = 0.2 (2), Δρ max/min 0.14 and −0.12 e Å−3. CCDC No. 2329677.
(1S,2R,5S)-2-isopropyl-5-methylcyclohexyl 2-oxo-2H-chromene-3-carboxylate (CMR-3). The reaction of 2-oxo-2H-chromene-3-carboxylic acid (1) (1.0 g, 5.26 mmol) with D-(+)-menthol (0.90 g, 5.78 mmol) in the presence of DCC (1.19 g, 5.78 mmol) and DMAP (32.0 mg, 0.26 mmol) for 48 h gave a white solid of CMR-3 (500 mg, 31%). [α]D20 +59.3 (c 0.60, CH2Cl2). Rf = 0.6 (hexane/ethyl acetate 5:1). Mp. 145–146 °C. 1H NMR (500 MHz, CDCl3): δ 0.83 (d, J = 6.9 Hz, 3 H, CH3), 0.96 (d, J = 3.9 Hz, 3H, CH3), 0.97 (d, J = 3.4 Hz, 3 H, CH3), 1.10–1.21 (m, 2 H, CH2), 1.57–1.62 (m, 3 H, CH, CH2), 1.73–1.79 (m, 2 H, CH2), 2.01–2.07 (m, 1 H, CH), 2.15–2.19 (m, 1 H, CH2), 4.99 (td, J = 10.9, 4.4 Hz, 1 H, CH), 7.33–7.39 (m, 2 H, CHarom), 7.61–7.77 (m, 2 H, CHarom), 8.49 (s, 1 H, CH). 13C NMR (126 MHz, CDCl3): δ 16.2, 20.8, 22.0, 23.3, 26.1, 31.4, 34.1, 40.7, 46.9, 76.0, 116.6, 117.9, 118.7, 124.7, 129.4, 134.1, 147.9, 155.0, 156.5, 162.5. ATR-FTIR (υ/cm−1): 3094, 3060, 3040, 2956, 2937, 2897, 2867, 1743, 1702, 1606, 1451, 1379, 1260, 1015, 769. Anal. Calcd. for C20H24O4; C, 73.15; H, 7.37; Found C, 73.16; H, 7.33.
Crystal data for CMR-3: crystal system orthorhombic, space group P212121, unit cell dimensions a = 11.0516 (7) Å, b = 12.3932 (9) Å, c = 13.5021 (9) Å, V = 1849.3 (2) Å3, Z = 4, density (calcd) 1.179 g/cm3, absorption coeff. 0.655 mm−1, F(000) = 704. Reflections collected/independent 4800, 3179 [R(int) = 0.0245], data/parameters 3179/218. Goodness-of-fit on F2 1.039, final R indices [I > 2σ(I)] R1 = 0.0459, wR2 = 0.1249, absolute structure parameter x = 0.3(3), Δρ max/min 0.18 and −0.14 e. Å−3. CCDC No. 2329678.
(1R,2S,5R)-5-methyl-2-(prop-1-en-2-yl)cyclohexyl 2-oxo-2H-chromene-3-carboxylate (CMR-4). The reaction of 2-oxo-2H-chromene-3-carboxylic acid (1) (1.0 g, 5.26 mmol) with (-)-isopulegol (0.92 g, 5.78 mmol) in the presence of DCC (1.19 g, 5.78 mmol) and DMAP (32.0 mg, 0.26 mmol) for 48 h gave a white solid of CMR-4 (630 mg, 37%). [α]D20 −49.5 (c 0.55, CH2Cl2). Rf = 0.55 (hexane/ethyl acetate 5:1). Mp. 123–124 °C. 1H NMR (500 MHz, CDCl3): δ 0.99 (d, J = 6.6 Hz, 3H, CH3), 1.01–1.07 (m, 1 H, CH), 1.22 (dd, J = 23.3, 12.1 Hz, 1 H, CH), 1.42–1.53 (m, 1 H, CH2), 1.61–1.69 (m, 1 H, CH2), 1.74 (s, 3H, CH3), 1.82–1.86 (m, 1 H, CH2), 2.16–2.23 (m, 1 H, CH), 2.30–2.36 (m, 1H, CH2), 4.74–4.75 (m, 2 H, CH=CH2), 5.08 (td, J = 10.9, 4.5 Hz, 1 H, CH), 7.39–7.30 (m, 2 H, CHarom), 7.68–7.55 (m, 2 H, CHarom), 8.44 (s, 1 H, CH). 13C NMR (126 MHz, CDCl3): δ 19.45, 22.04, 30.36, 31.45, 34.04, 40.26, 50.84, 75.13, 112.08, 116.78, 117.95, 118.66, 124.69, 129.42, 134.12, 146.24, 147.95, 155.12, 156.62, 162.07. ATR-FTIR (υ/cm−1): 3081, 3040, 2956, 2914, 2934, 2846, 2360, 1751, 1705, 1607, 1451, 1373, 1243, 1205, 1006, 769. Anal. Calcd. for C20H22O4; C, 73.60; H, 6.79; Found C, 73.52; H, 6.70.
Crystal data for CMR-4: crystal system orthorhombic, space group P212121, unit cell dimensions a = 9.8116 (3) Å, b = 12.8626 (3) Å, c = 14.2305 (4) Å, V = 1795.93 (9) Å3, Z = 4, density (calcd) 1.207 g/cm3, absorption coeff. 0.675 mm−1, F(000) = 696. Reflections collected/independent 4426/3212 [R(int) = 0.0151], data/parameters 3212/219. Goodness-of-fit on F2 1.013, final R indices [I > 2σ(I)] R1 = 0.0409, wR2 = 0.1286, absolute structure parameter x = 0.02 (17), Δρ max/min 0.14 and −0.14 e. Å−3. CCDC No. 2329679.
3. Results and Discussion
3.1. Synthesis of Coumarins
The synthesis of ester derivatives of coumarin-3-carboxylic acid is achievable through several routes. These include condensation reactions involving salicylaldehyde and compounds featuring an active methylene group [37], as well as a FeCl3-catalyzed multicomponent reaction employing salicylaldehydes, Meldrum acid, and alcohols [38]. Additionally, these compounds can be prepared via esterification reactions between coumarin-3-carboxylic acid and alcohols, employing either DCC (N,N’-dicyclohexyl-carbodiimide) with DMAP (Steglich esterification) [39] or KPF6 (potassium hexafluorophosphate) [40].
In the synthesis of cyclohexyl derivatives of coumarins (CRM 1–4), we employed the Steglich esterification which is a more efficient and mild alternative to traditional esterification methods, Scheme 1 [41,42]. The substrate used in the esterification reaction, coumarin-3-carboxylic acid (1), was synthesized from Meldrum’s acid and salicylaldehyde. This synthesis employed potassium carbonate to facilitate the Knoevenagel condensation, followed by intramolecular cyclization. The procedure was carried out according to the method described by Brahmachari [43].
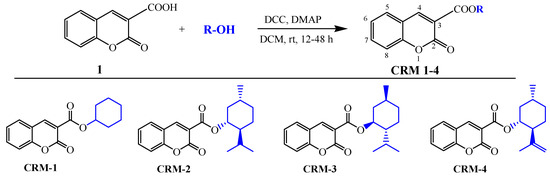
Scheme 1.
The synthetic approaches towards coumarins CRM 1–4.
The esterification of coumarin-3-carboxylic acid (1) with various alcohols, including cyclohexanol, L-menthol, D-menthol, and (-)-isopulegol, was conducted on a one-gram scale. This reaction was performed in the presence of 1.1 equivalents of DCC and 0.05 equivalents of DMAP, using dichloromethane (DCM) as the solvent. The reactions were maintained at room temperature for a duration ranging from 12 to 48 h. The resultant products were initially purified using column chromatography, employing a hexane/ethyl acetate mixture. The subsequent recrystallization from ethyl alcohol yielded coumarins CMR 1–4 with yields of 30–42%. The synthesis of compound CMR-1 was presented already in the publication by Kuang et al. [44] The authors utilized a condensation reaction of salicylaldehyde with dicyclohexyl malonate for this purpose. The publication did not provide the reaction yield, and there is also a lack of complete spectroscopic data for the compound (13C NMR, IR). The synthesis of methyl esters of coumarins was described in the work by Ichikawa et al. [26]. The authors successfully employed the Steglich esterification reaction for their preparation. Additionally, Xu et al. [27] applied the reaction between 2-oxo-2H-chromene-3-acyl chloride and L-menthol in the presence of a base for the synthesis of CMR-2. Unfortunately, the yield of this reaction was not determined, and spectroscopic analysis of this coumarin was also not provided.
3.2. NMR and FTIR Spectra of Coumarins
1H NMR and 13C NMR spectral data for CMR 1–4 were recorded in CDCl3 and are presented in the Supplementary Materials (Figures S1–S8). In the coumarin derivatives, signals from the proton CH4 group were observed at 8.44–8.49 ppm as a singlet. The signals from cyclohexyl protons appeared in the range of 2.02–1.80 ppm as multiplets. The CH group in the cyclohexyl ring manifests as a multiplet in the region of 5.09–5.06 ppm, whereas for coumarins CRM 2–4, it appears as a doublet of triplets in the region of 4.99–5.08 ppm. The CH3 protons of the menthol moiety displayed signals at 0.97 ppm, 0.95 ppm, and 0.83 ppm as doublets. The two protons of the CH=CH2 group of CMR-4 were located in the range of 4.85–4.74 ppm as a multiplet. The aromatic protons for all coumarins were presented as multiplets around 7.40–7.62 ppm.
For all the examined compounds CMR 1–4, in the FTIR spectra (Figure 2) characteristic bands for stretching vibrations of C-H groups of the coumarin and cyclohexyl rings can be distinguished in the range of 3100–3000 cm−1. The range of 3000–2800 cm−1 is characteristic for stretching vibrations of CH3 groups. The region from 1800 to 1200 cm−1 is associated with stretching vibrations of C=C, C-C, and C-O bonds, as well as deformations of CH2 and CH3 groups. The sharp bands at around 1740 cm−1 and 1700 cm−1 are characteristic of the carbonyl group C=O. In the case of coumarin with a cyclohexyl substituent (CMR-1), in the region characteristic of the carbonyl group C=O, besides the two sharp bands at 1747 cm−1 and 1700 cm−1, two less intense bands at 1763 cm−1 and 1722 cm−1 are clearly visible, which may indicate the formation of the C-H…O hydrogen bonds of different geometry than those in CMR-2–4 crystals.
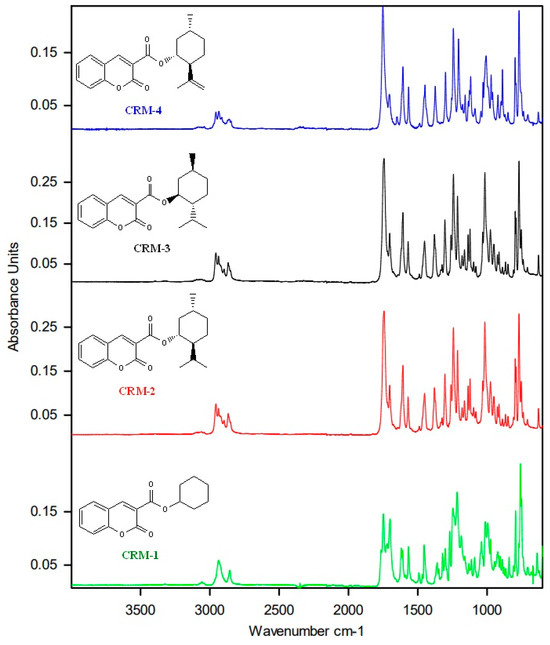
Figure 2.
FTIR spectra of coumarins CMR 1–4.
3.3. Molecular Structure
The structure of the molecules CMR 1–4 is shown in Figure 3. The conformers in the solid phase are diverse, the molecules containing chiral L- and D-menthyl groups (CMR-2 and CMR-3) are mirror images of each other (see Figure S9a), which is expected. Moreover, substitution with menthyl and isopulegyl groups turns the molecule CMR-1 into a chiral system, with a larger hydrophobic part. The fitting of the molecules through the coumarin system shows different orientations of the cycloalkyl substituent (Figure 4) due to the rotation around the C3-C11 bond. In the molecule with the simplest basic structure, CMR-1, the orientation of the C=O bonds of the lactone and ester groups is anti; the value of the torsion angle C2-C3-C11-O4 is −156.1 (3)°. However, in the remaining molecules with a more extensive cycloalkyl part, the C=O2 and C=O4 bonds are in the syn positions (Figure 3 and Figure 4a,b). In addition, the molecular dipol moments calculated for the solid-state conformers and the optimized ones show significant differences between their values (Figure 3) [45].
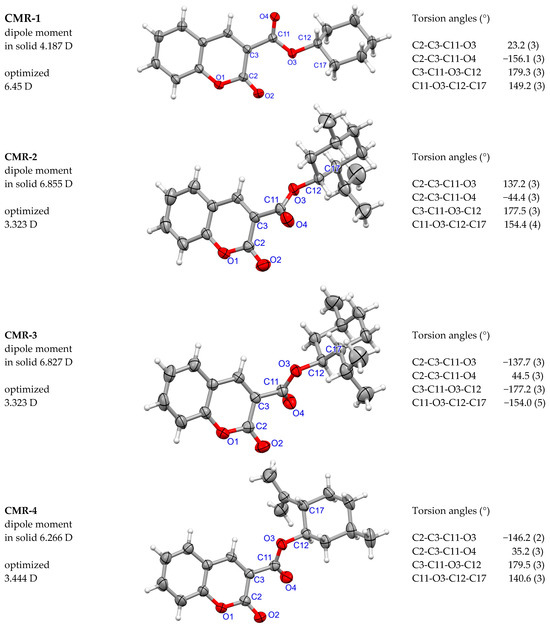
Figure 3.
Molecular structure of coumarin esters CMR 1–4 illustrated using the ORTEP program. Ellipsoids are shown with a 30% probability. The values (Debye) of dipole moments calculated for the solid-state conformers and the molecules with optimized geometry are given. The torsion angles describing the conformation around the ester group are given.
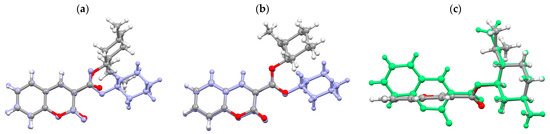
Figure 4.
Molecular fitting of conformers: (a) CMR-1 (light blue) and CMR-2 (atoms marked in standard colors). fitted through the coumarin rings; (b) CMR-1 (light blue) and CMR-4 (atoms marked in standard colors) fitted through the coumarin rings; (c) CMR-2 (atoms marked in standard colors) and CMR-4 (green) the cyclohexane rings are fitted.
The observation resulting from the comparison of the conformations of the ester molecules with the L-menthyl (CMR-2) and (-)-isopulegyl (CMR-4) group is puzzling. Despite the identical configuration at the chiral centers and the only difference in the molecular structure, which is the presence of the isopropyl (CH3-CH-CH3) vs. isopropenyl (CH3-C=CH2) group, the molecules have significantly different conformations. This is visible in the fitting of the molecules through the cyclohexyl ring (Figure 4c); the position of the coumarin rings in the molecules varies by 60°.
3.4. Crystal Structure and Intermolecular Contacts
The analysis of the packing of the molecules in the crystals (Figure S12) shows the centrosymmetric arrangement of CMR-1. These molecules (having extended conformation) form catemeric associates stabilized by the C-H…O intermolecular hydrogen bonds (Figure 5a). In contrast, three chiral esters CMR 2–4, crystallize in the chiral P212121 space group. As a consequence, these molecules are transformed around the 21 screw axes.
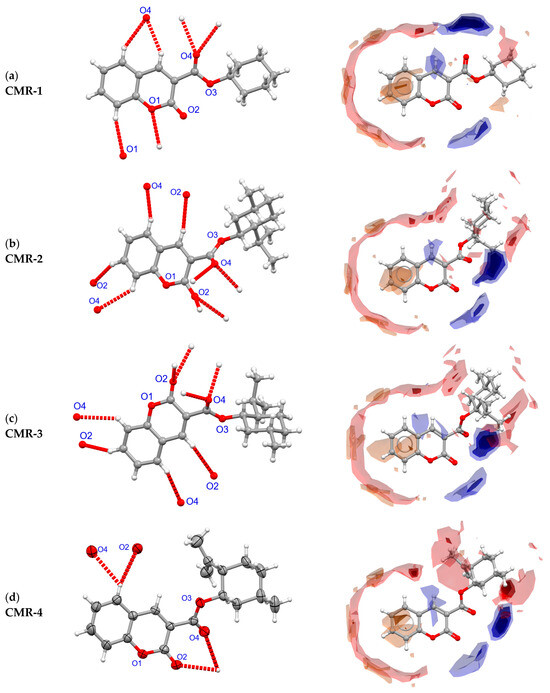
Figure 5.
The C-H…O hydrogen bonds involving molecules in crystals (left column) and Full Interaction Maps (right).
Intermolecular forces depend on the chemical composition of the compound; in the case of the tested group of derivatives, the chemical composition is C, H, O. As a consequence, in the solid-state structures of coumarin and the studied C-3 alkyl ester derivatives, CMR 1–4, the strongest interactions will be C-H…O hydrogen bonds. Despite there being the same number of acceptors in these molecules, i.e., oxygen atoms, the number of hydrogen bonds formed in the crystals is different. Three hydrogen bonds are formed between achiral CMR-1 molecules and the acceptors are the O1 and O4 atoms (Figure 5a). However, in chiral crystals, the enantiomeric molecules CMR-2 and CMR-3 form four bonds, and the crystal packings are mirror images; in each crystal, the O2 and O4 atoms are bifurcated acceptors (Figure 5b,c). In the crystal CMR-4, the pattern of two C-H...O hydrogen bonds is different with the O2 and O4 atoms involved as acceptors (Figure 5d). The only hydrogen bond donors (i.e., C-H bonds) are aromatic fragments of all molecules CMR 1–4.
Two methods were used to interpret and illustrate the environment of molecules in the three-dimensional framework; Hirshfeld surfaces (Figure S10) and Full Interaction Maps (FIMs) (Figure 5 and Figure S11) were calculated using CrystalExplorer [46] and Mercury software [47], respectively. It seems that the second way of interpretation is more useful for this group of compounds. The FIM illustrates hydrogen bond donors (C-H) with red zones and hydrogen bond acceptors (O) with blue zones. The intensity of these color zones correlates with the likelihood of the respective interactions occurring.
It can be seen in these maps that the interactions are concentrated around the edges of the molecules, and there is no π-stacking. Interestingly, both chiral molecules transformed around the two-fold screw axes do not stack through the coumarin system as in the centrosymmetric CMR-1 crystals (Figure S12). Comparing these associates to the associations of coumarin molecules themselves shows the absence of significant π-π interactions in these crystals (Figure S11).
Using FIMs, one can easily see the correlation between the conformation of the ester group, resulting from the rotation around the C3-C11 bond, and the location of hydrogen bond acceptors (Figure 5 and Figure S11). Molecules with an anti C=O bond orientation (CMR-1) can interact in two opposite directions. However, the remaining molecules have acceptors located in the syn positions, on the same side of the molecules. It can therefore be assumed that increasing the size of the hydrophobic group results in a specific localization of the C-H…O interactions in solid, which makes the molecule more polar. It is supported by the calculated dipole moments for the molecular conformations in solid which are: 4.187 D for CMR-1, 6.855 D for CMR-2, 6.827 D for CMR-3, and 6.266 D for CMR-4, respectively. This can be used to design subsequent substitutions for coumarin-3-carboxylic acid.
4. Conclusions
Four ester derivatives of coumarin-3-carboxylic acid featuring cyclohexyl groups have been synthesized and their structures elucidated using X-ray crystallography. Our research centered on examining the crystal structures, exploring the intermolecular interactions, and identifying diverse associations patterns. Previously described structures of coumarins in the literature, featuring simple alkyl ester substituents, have demonstrated that the coumarin skeleton was coplanar with the alkyl substituents. However, our study of coumarins with cyclohexyl substituents has clearly shown a change in the molecular conformations and association. This is evident in the altered orientation of the C=O ester groups relative to the coumarin ring, indicating a significant deviation from the planar alignment observed in simpler alkyl-substituted coumarins. Despite the presence of an aromatic ring in the structure of CMR 1–4 coumarins, no π-π interactions were detected. The molecular structures of the examined coumarins were stabilized by the intermolecular C-H…O hydrogen bonds. Introducing the bulky cyclohexyl substituents, such as menthyl or isopulegyl, significantly increased the localized character of the H-bond acceptors of the coumarin molecule. Moreover, the conformations of molecules, resulting from the rotation of the coumarin and ester fragments, have different dipole moments when comparing the optimized and the observed in crystal. These aspects may be significant in the future for the interaction of CMR 1–4 coumarins with biological molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14020196/s1, Supplementary material includes 1HNMR, 13CNMR, and FTIR spectra of isolated compounds CMR 1–4 (Figures S1–S8); Figure S9. Molecular fitting of conformers; Figure S10. Hirshfeld surfaces for molecules of coumarin and esters CMR 1–4; Figure S11. Full Interaction Maps for the molecules of coumarin and esters CMR 1–4; Figure S12. Crystal packing, and Figures S13–S16. X-Ray powder diffraction patterns.
Author Contributions
Conceptualization, K.S. and A.E.K.; methodology, K.S., D.M.K. and A.E.K.; investigation, K.S., D.M.K. and A.E.K.; writing—original draft preparation, K.S. and A.E.K.; writing—review and editing, K.S., D.M.K. and A.E.K.; visualization, K.S. and A.E.K.; supervision, A.E.K., syntheses K.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research was carried out with the facilities purchased thanks to the financial support of the European Regional Development Fund in the framework of the Operational Program Development of Eastern Poland 2007–2013 (Contract No. POPW.01.03.00-06-009/11-00), equipping the laboratories of the Faculties of Biology and Biotechnology, Mathematics, Physics and Informatics and Chemistry for studies of biologically active substances and environmental samples.
Data Availability Statement
Crystallographic data for the structures presented in this paper have been deposited in the Cambridge Crystallographic Data Center as a Supplementary Publication (CCDC Nos 2329676–2329679). These data are provided free of charge by the Cambridge Crystallographic Data Centre Access Structures service www.ccdc.cam.ac.uk/structures/.
Acknowledgments
We thank Andrzej Puszka for providing FTIR spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, P.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms, Oxidative Medicine and Cellular Longevity. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Patil, S.A.; Kandathil, V.; Sobha, A.; Somappa, S.B.; Feldman, M.R.; Bugarin, A.; Patil, S.A. Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes. Molecules 2022, 27, 5220. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Seboletswe, P.; Gundla, R.; Kushwaha, N.D.; Kumar, V.; Singh, P.; Karpoormath, R.; Bala, M.D. Recent Literature Review on Coumarin Hybrids as Potential Anticancer Agents. Anticancer Agents Med. Chem. 2023, 23, 142–163. [Google Scholar] [CrossRef]
- Toan, D.N.; Thanh, N.D.; Truong, M.X.; Van, D.T.; Thanh, N.N. Design, synthesis, molecular docking study and molecular dynamics simulation of new coumarin-pyrimidine hybrid compounds having anticancer and antidiabetic activity. Med. Chem. Res. 2023, 32, 1143–1162. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.S.; Kumar, A.; Kaur, K.; Jaitak, V. Recent Developments in Coumarin Derivatives as Neuroprotective Agents. Curr. Med. Chem. 2023; in press. [Google Scholar] [CrossRef]
- Shefali, S.; Pragya, G.; Ameya, K.; Arush, A.; Sharda, P. Exploring Coumarin and Chalcone Analogues as Potential Antimycobacterial Agents. Anti-Infect. Agents 2017, 15, 69–86. [Google Scholar] [CrossRef]
- Sarmah, M.; Chutia, K.; Dutta, D.; Gogoi, P. Overview of coumarin-fused-coumarins: Synthesis, photophysical properties and their applications. Org. Biomol. Chem. 2022, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Mirmahalleh, S.-A.; Golshan, M.; Gheitarani, B.; Hosseini, M.S.; Salami-Kalajahi, M. A review on applications of coumarin and its derivatives in preparation of photo-responsive polymers. Eur. Polym. J. 2023, 198, 112430. [Google Scholar] [CrossRef]
- Szwaczko, K. Fluorescent Coumarin-based Probe for Detection of Biological Thiols. Curr. Org. Chem. 2023, 27, 1329–1335. [Google Scholar] [CrossRef]
- Foroozesh, M.; Sridhar, J.; Goyal, N.; Liu, J. Coumarins and P450s, Studies Reported to-Date. Molecules 2019, 24, 1620. [Google Scholar] [CrossRef]
- Berestetskiy, A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-X.; Wang, Z.-X.; Peng, J.-F.; Zou, Y.-L.; Hui, Y.-Z.; Chen, Y.-Z.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel phenoxypyridine derivatives containing natural product coumarin. Pest Manag. Sci. 2021, 77, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. Progress in the Chemistry of Naturally Occurring Coumarins. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 106, pp. 241–304. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yan, G.; Xie, Y.T.; Lin, T.-C.; Zhang, W.; Li, J.; Wu, Y.-J.; Zhou, Y.-J.; Fu, Y.-H. Bioactive prenylated coumarins as potential anti-inflammatory and anti-HIV agents from Clausena lenis. Bioorg. Chem. 2020, 97, 103699. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Luo, F.; Manse, Y.; Sugita, H.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Ninomiya, K. Geranylated Coumarins From Thai Medicinal Plant Mammea siamensis with Testosterone 5α-Reductase Inhibitory Activity. Front. Chem. 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.A.; Shevchenko, O.G.; Chukicheva, I.Y.; Kutchin, A.V. Synthesis and biological evaluation of novel coumarins with tert-butyl and terpene substituents. Chem. Biodivers. 2019, 16, e1800317. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021, 12, 698352. [Google Scholar] [CrossRef] [PubMed]
- Bibak, B.; Shakeri, F.; Barreto, G.E.; Keshavarzi, Z.; Sathyapalan, T.; Sahebkar, A. A Review of the Pharmacological and Therapeutic Effects of Auraptene. BioFactors 2019, 45, 867–879. [Google Scholar] [CrossRef]
- Zafar, S.; Sarfraz, I.; Rasul, A.; Shah, M.A.; Hussain, G.; Zahoor, M.K.; Shafiq, N.; Riaz, A.; Selamoglu, Z.; Sarker, S.D. Osthole: A Multifunctional Natural Compound with Potential Anticancer, Antioxidant and Anti-inflammatory Activities. Mini Rev. Med. Chem. 2021, 21, 2747–2763. [Google Scholar] [CrossRef]
- Chen, J.; Liao, X.; Gan, J. Review on the protective activity of osthole against the pathogenesis of osteoporosis. Front. Pharmacol. 2023, 14, 1236893. [Google Scholar] [CrossRef]
- Zili, R.; Min, L.; Hui, X. Osthole: Synthesis, Structural Modifications, and Biological Properties. Mini Rev. Med. Chem. 2022, 22, 2124–2137. [Google Scholar] [CrossRef]
- Kozioł, E.; Skalicka-Woźniak, K. Imperatorin–pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef]
- Neel, M.; Gouin, J.; Voituriez, A.; Marinetti, A. Phosphine-catalyzed [3+2] cyclizations: Applications to the enantioselective synthesis of cyclopentene-fused chromanones and dihydroquinolinones. Synthesis 2011, 12, 2003–2009. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Harada, N. Stereochemical Studies of Chiral Resolving Agents, M9PP and H9PP Acids. Chirality 2004, 16, 559–567. [Google Scholar] [CrossRef]
- Xu, C.L.; Liu, S.Y.; Chen, G.; Yang, G.Y.; Zhao, M.Q. Menthyl 2-oxo-2H-chromene-3-carboxyl-ate. Acta Cryst. 2009, E65, o2431. [Google Scholar] [CrossRef]
- Škoch, K.; Císařová, I.; Štěpnička, P. Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4. Z. Kristallogr. NCS 2016, 231, 609–611. [Google Scholar] [CrossRef][Green Version]
- Saeed, A.; Ibrar, A.; Arshad, M.; Bolte, M. Methyl 2-oxo-2H-chromene-3-carboxylate. Acta Cryst. 2012, E68, o3024. [Google Scholar] [CrossRef]
- Nowatschin, V.; Nather, C.; Luning, U. Synthesis and crystal structure of allyl 7-(diethyl-amino)-2-oxo-2H-chromene-3-carboxylate. Acta Cryst. 2021, 77, 331–334. [Google Scholar] [CrossRef]
- Yavari, I.; Djahaniani, H.; Nasiri, F. The crystal structure of tert-butyl coumarin-3-carboxylate. Iran. Chem. Soc. 2006, 3, 46–50. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.L.; Wang, J.; Zhong, L.; Nie, X.-Y.; Peng, D.-Y. Synthesis, crystal structure, spectroscopic characterization and anti-fungal activity of Ethyl 2-Oxo-2H-chromene-3-carboxylate Derivatives. J. Mol. Struct. 2022, 1257, 132576. [Google Scholar] [CrossRef]
- User Manual Agilent Technologies Inc.: Yarnton, UK, 2014.
- CrysAlisPro 1.171.42.79a; Rigaku Oxford Diffraction: Tokyo, Japan, 2022.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dinparast, L.; Hemmati, S.; Zengin, G.; Alizadeh, A.A.; Bahadori, M.B.; Kafil, H.S.; Dastmalchi, S. Rapid, efficient, and green synthesis of coumarin derivatives via Knoevenagel condensation and investigating their biological effects. Chem. Sel. 2019, 4, 9211–9215. [Google Scholar] [CrossRef]
- He, X.; Shang, Y.; Zhou, Y.; Yu, Z.; Han, G.; Jin, W.; Chen, J. Synthesis of coumarin-3-carboxylic esters via FeCl3-catalyzed multicomponent reaction of salicylaldehydes, Meldrum’s acid and alcohols. Tetrahedron 2015, 71, 863–868. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Shinde, S.V.N.; Kumar, A. KPF6-Mediated esterification and amidation of carboxylic acids. J. Org. Chem. 2022, 87, 2651–2661. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2024, 10, e23416. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A solvent-reagent selection guide for Steglich-type esterification of carboxylic acids. Green Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Brahmachari, G. Room temperature one-pot green synthesis of coumarin-3-carboxylic acids in water: A practical method for the large-scale synthesis. ACS Sustain. Chem. Eng. 2015, 3, 2350–2358. [Google Scholar] [CrossRef]
- Kuang, Y.; Liu, X.; Chang, L.; Wang, M.; Lin, L.; Feng, X. Catalytic Asymmetric Conjugate Allylation of Coumarins. Org. Lett. 2011, 13, 3814–3817. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).