A Fluorescence Sensor Based on Biphenolic Backbone for Metal Ion Detection: Synthesis and Crystal Structure
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. Apparatus
2.3. X-Ray Crystallography
2.4. Fluorescence Titration
2.5. Synthesis of 2′-(Hexyloxy)-[1,1′-Biphenyl]-2-yl 5-(Dimethylamino)Naphthalene-1-Sulfonate, KC1 Exhibited in Scheme 1
2.5.1. 2′-(Hexyloxy)-[1,1′-Biphenyl]-2-ol, (C18H22O2) Compound A
2.5.2. 2′-(Hexyloxy)-[1,1′-Biphenyl]-2-yl 5-(Dimethylamino)Naphthalene-1-Sulfonate, C30H33NO4S (KC1)
3. Results and Discussion
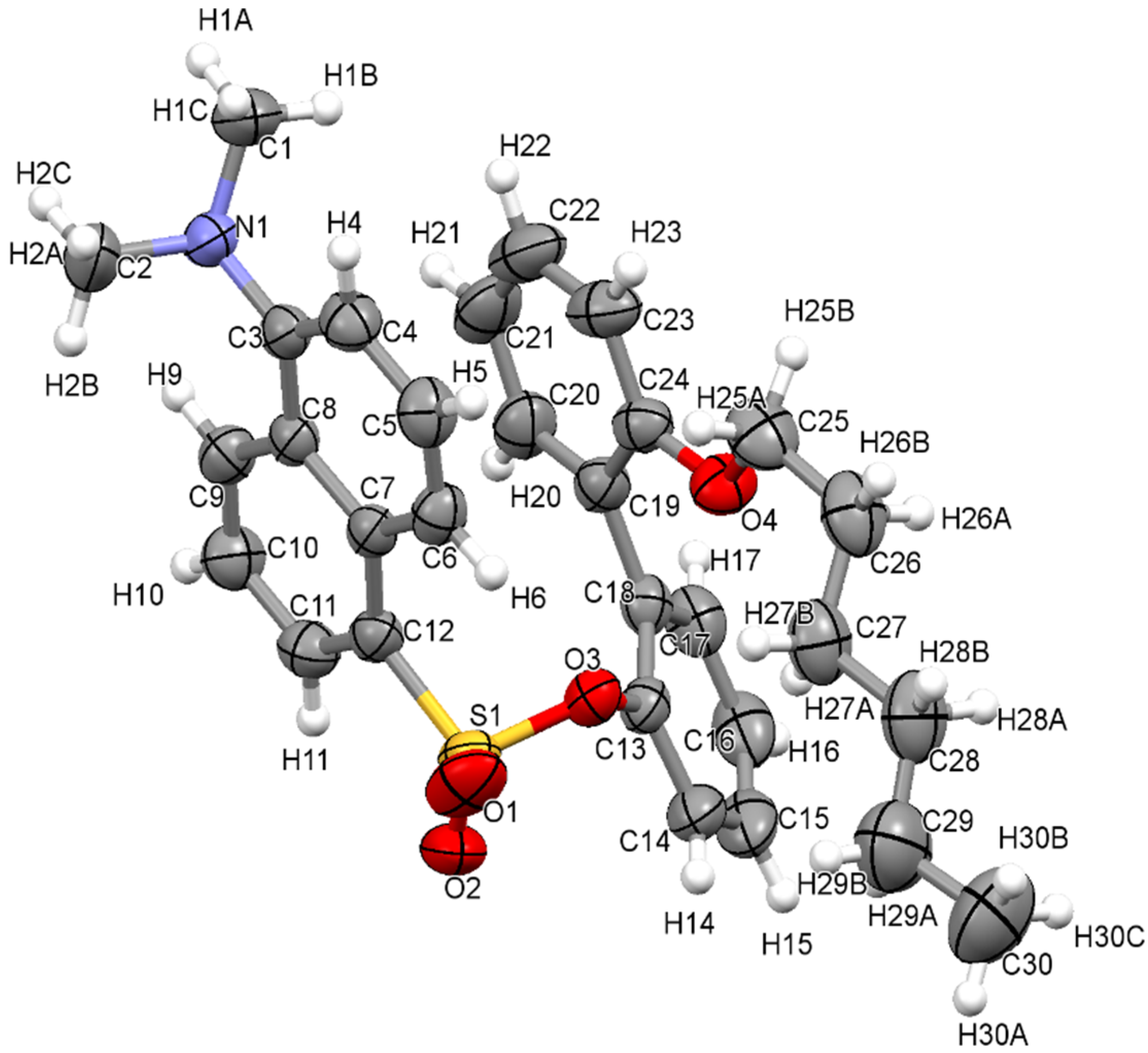
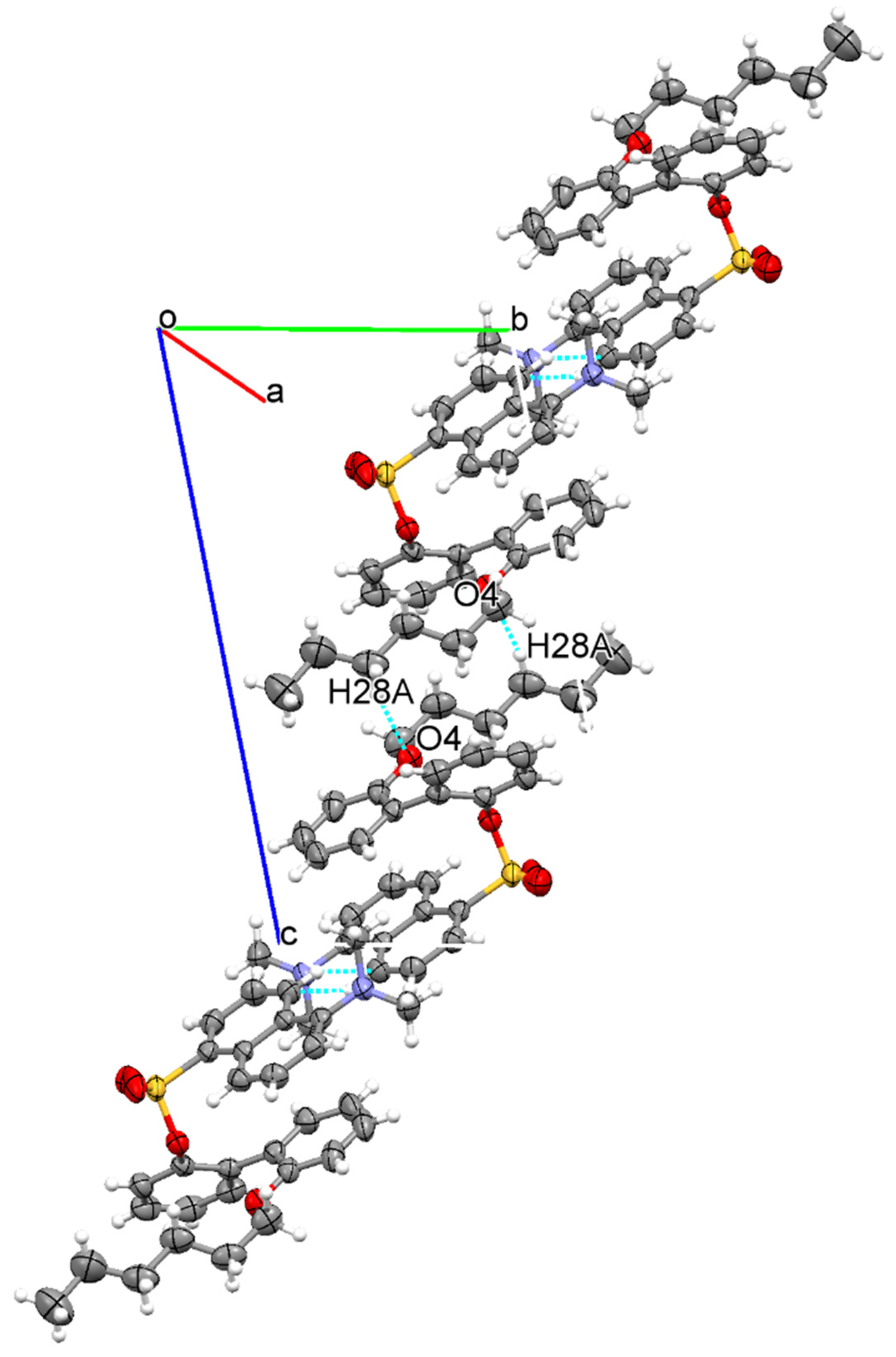
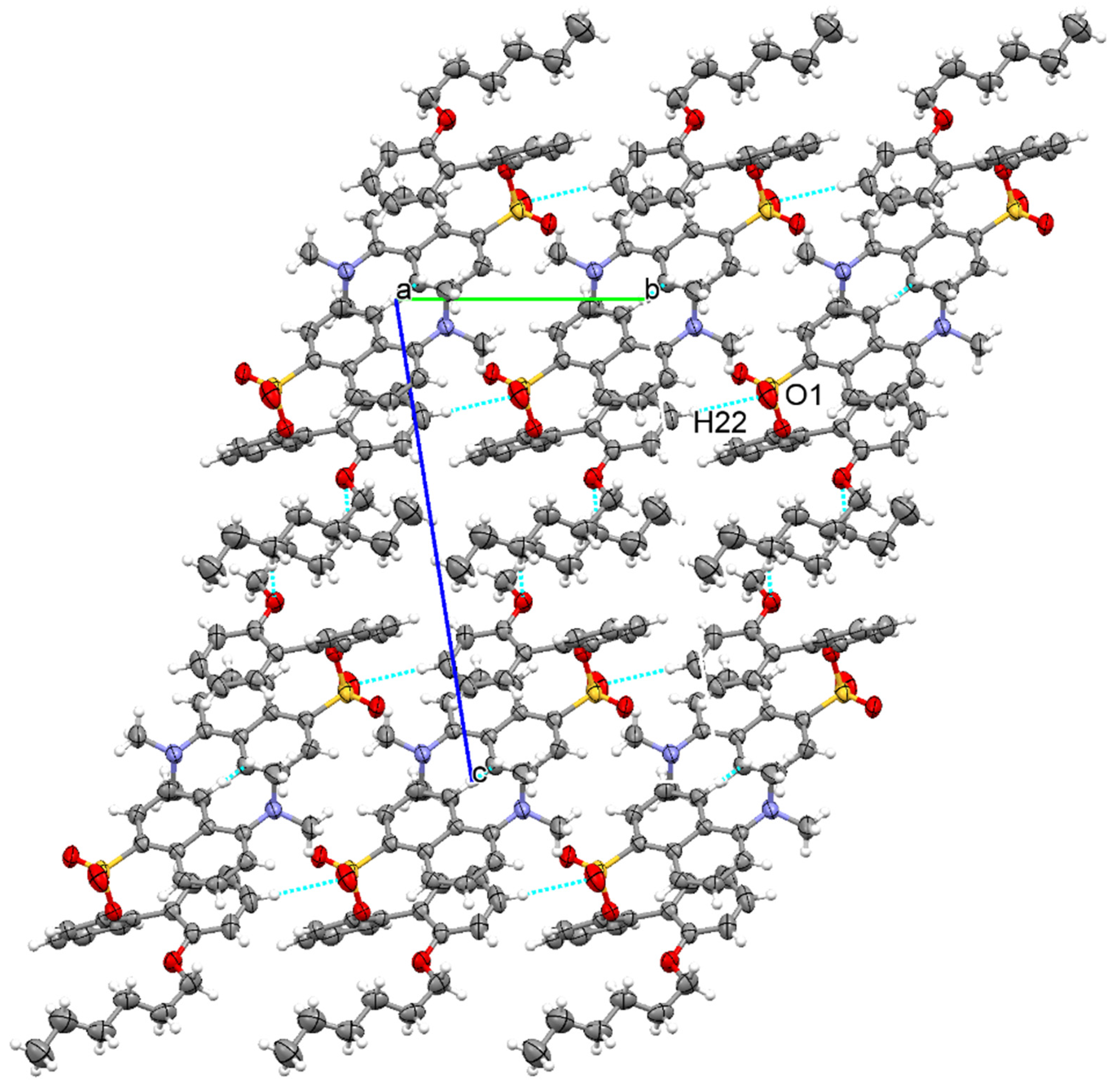
3.1. The Crystallographic Structure Detail of Sensor KC1
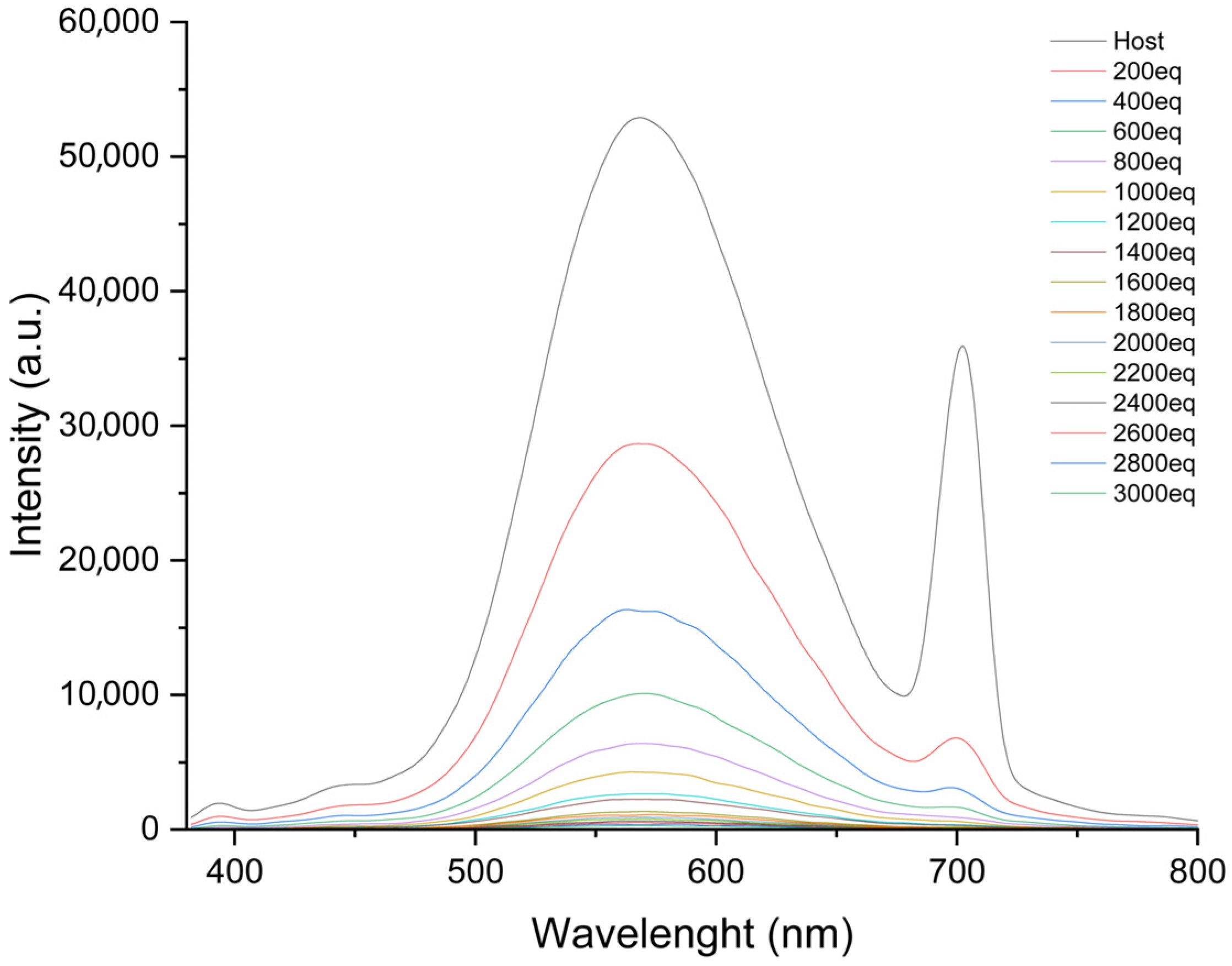
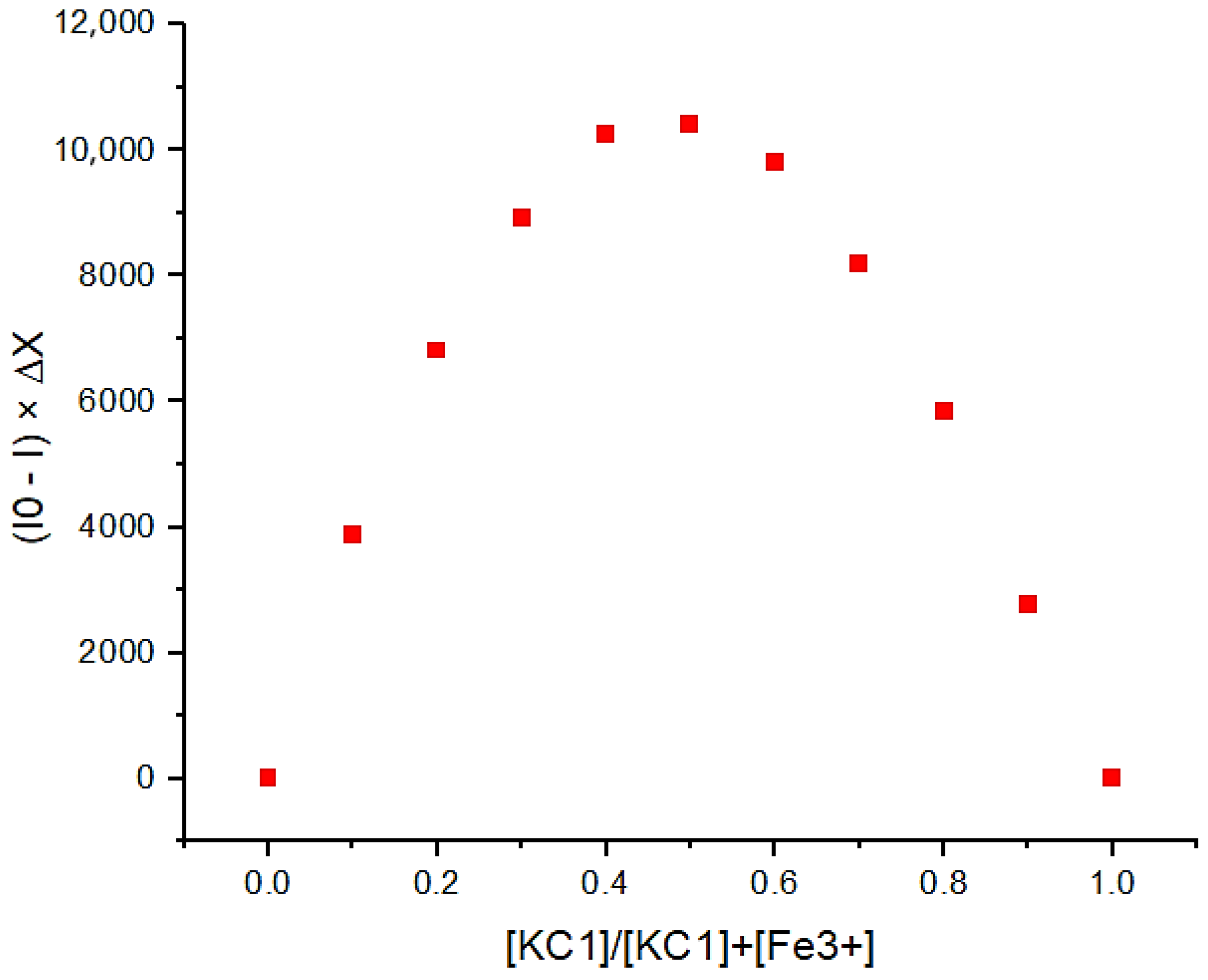
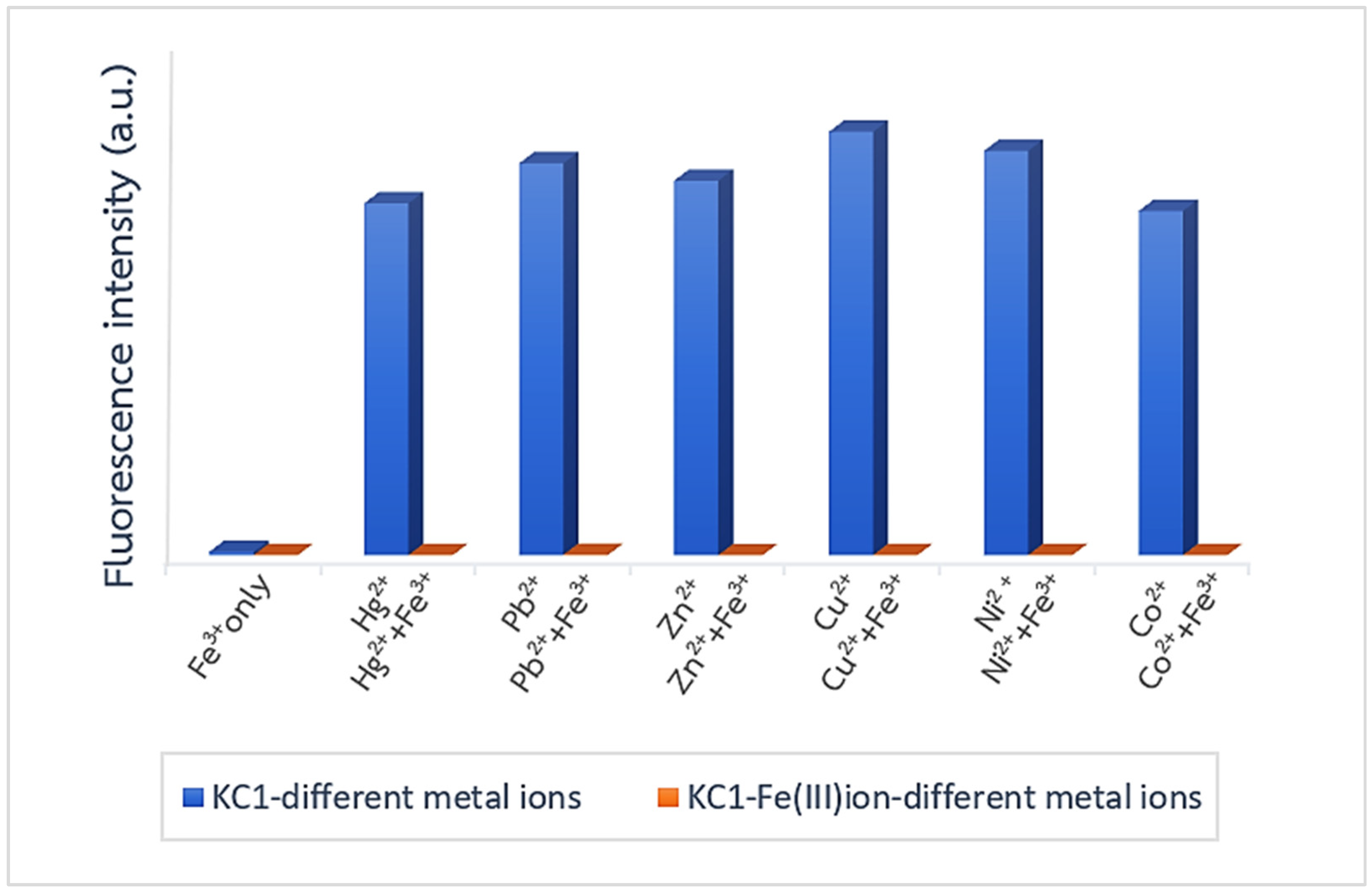
3.2. An Investigation of the Binding Behavior of KC1 and Metal Ions by Using a Fluorescence Technique
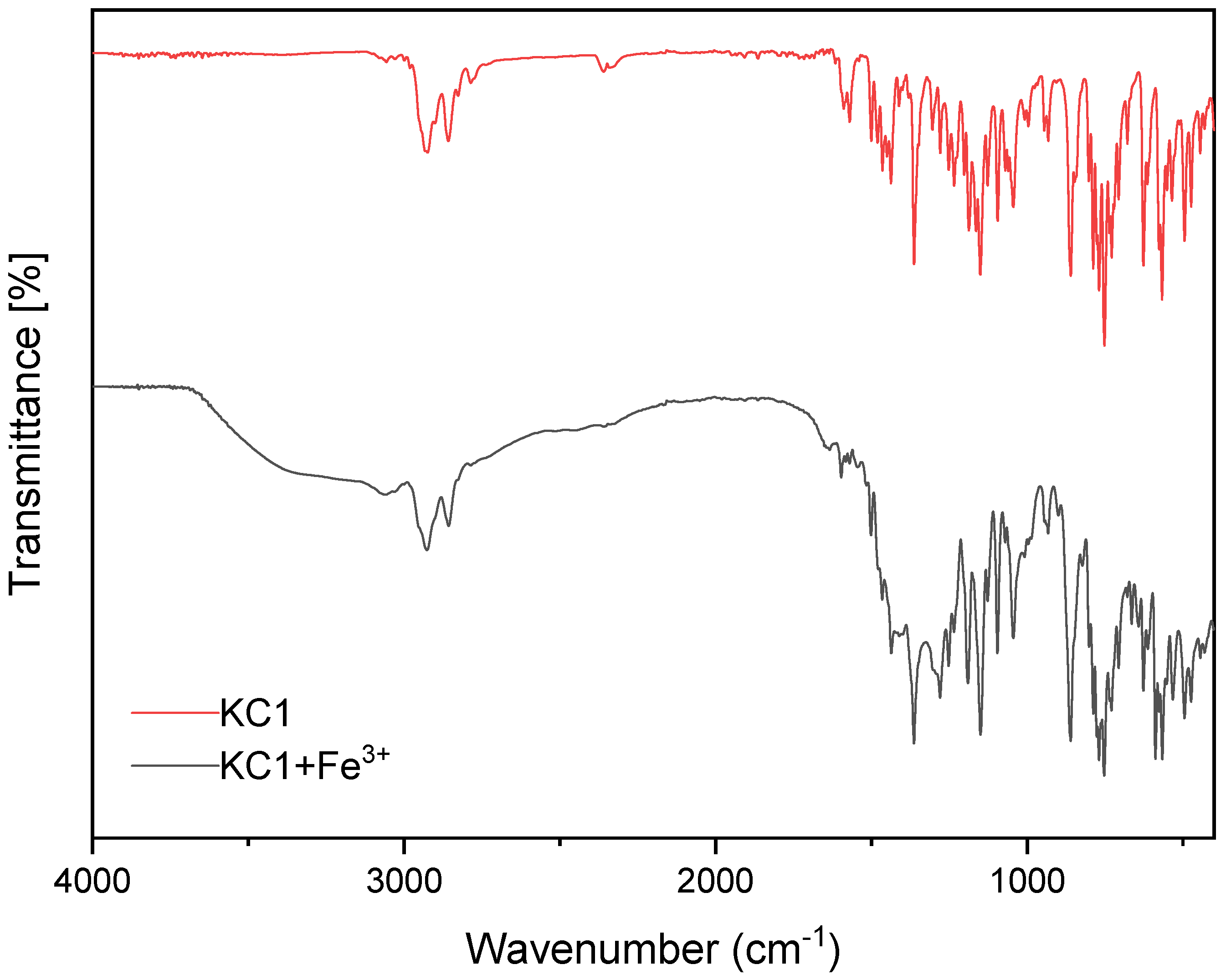
3.3. The Binding Mode Between KC1 and Fe3+ Ion via FTIR Spectroscopy
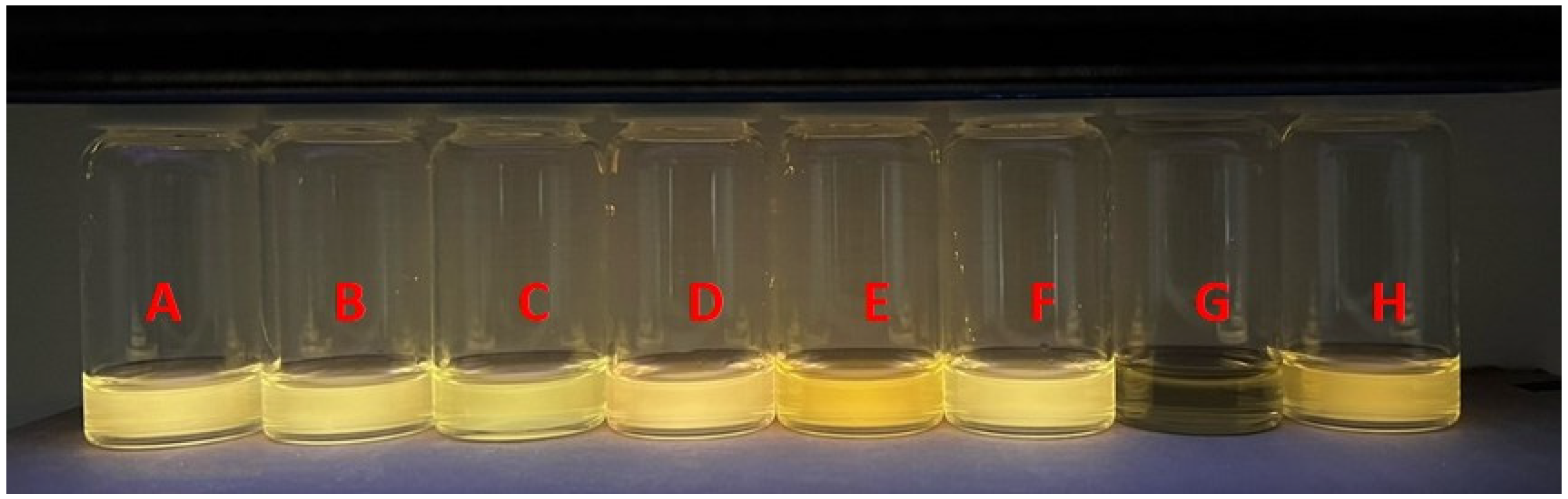
3.4. Application of KC1 and Various Concentrations of Fe3+ Ions on TLC Plates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rao, C.P.; Mandal, S. Europium-Based Metal–Organic Framework as a Dual Luminescence Sensor for the Selective Detection of the Phosphate Anion and Fe3+ Ion in Aqueous Media. Inorg. Chem. 2018, 57, 11855–11858. [Google Scholar]
- Gu, Y.-N.; Lu, J.-F.; Liu, H.; Zhao, B.; Zhou, X.-H.; Zhao, Y.-Q.; Sun, Q.-Z.; Zhang, B.-G. Two Eu3+ Based Complexes Containing Uncoordinated Lewis Basic Pyridyl Sites and Chemical Sensing of 4-Nitrophenol and Fe3+ ions. Cryst. Growth Des. 2022, 22, 4874–4884. [Google Scholar] [CrossRef]
- He, T.-S.; Lan, Y.-L.; Li, Z.-Y.; Zhu, L.-N.; Li, X.-Z. Chiral Coordination Polymers from a New 2-Deoxy-D-ribose Derivative Linker: Syntheses, Structures, and Fe3+ Fluorescent Probe Functions. Cryst. Growth Des. 2021, 21, 2233–2242. [Google Scholar] [CrossRef]
- Areti, S.; Bandaru, S.; Rao, C.P. Triazole-Linked Quinoline Conjugate of Glucopyranose: Selectivity Comparison among Zn2+, Cd2+, and Hg2+ Based on Spectroscopy, Thermodynamics, and Microscopy, and Reversible Sensing of Zn2+ and the Structure of the Complex Using DFT. ACS Omega 2016, 1, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.-L.; Tian, D.; Liu, J.; Dong, L.-Z.; Li, S.-L.; Li, D.-S.; Lan, Y.-Q. A Water-Stable Metal–Organic Framework for Highly Sensitive and Selective Sensing of Fe3+ Ion. Inorg. Chem. 2016, 55, 10580–10586. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, J.; Wan, X.-Y.; Sheng, D.-F.; Yan, H.; Zhang, S.-S.; Ma, H.-Y.; Wang, S.-N.; Li, D.-C.; Gao, Z.-Y.; et al. Nanocage-Based N-Rich Metal–Organic Framework for Luminescence Sensing toward Fe3+ and Cu2+ Ions. Inorg. Chem. 2021, 60, 671–681. [Google Scholar] [CrossRef]
- Finelli, A.; Chabert, V.; Herault, N.; Crochet, A.; Kim, C.; Fromm, K.M. Sequential Multiple-Target Sensor: In3+, Fe2+, and Fe3+ Discrimination by an Anthracene-Based Probe. Inorg. Chem. 2019, 58, 13796–13806. [Google Scholar] [CrossRef]
- Khatun, M.; Ghorai, P.; Mandal, J.; Chowdhury, S.G.; Karmakar, P.; Blasco, S.; García-Espana, E.; Saha, A. Aza-phenol Based Macrocyclic Probes Design for “CHEF-on” Multi Analytes Sensor: Crystal Structure Elucidation and Application in Biological Cell Imaging. ACS Omega 2023, 8, 7479–7491. [Google Scholar] [CrossRef]
- Tan, S.S.; Teo, Y.N.; Kool, E.T. Selective Sensor for Silver Ions Built From Polyfluorophores on a DNA Backbone. Org. Lett. 2010, 12, 4820–4823. [Google Scholar] [CrossRef]
- Bazzicalupia, C.; Bianchia, A.; García-Españab, E.; Delgado-Pinar, E. Metals in Supramolecular Chemistry. Inorg. Chim. Acta 2014, 417, 3–26. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Liu, X. Detection of Heavy Metal Ions by Ratiometric Photoelectric Sensor. J. Agric. Food Chem. 2022, 70, 11468–11480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nie, J.; Du, B. Functionalized Ionic Microgel Sensor Array for Colorimetric Detection and Discrimination of Metal Ions. ACS Appl. Mater. Interfaces 2017, 9, 20913–20921. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Rahman, M.M.; Arshad, M.N.; Asiri, A.M. Hg2+ Sensor Development Based on (E)-N’-Nitrobenzylidene Benzenesulfonohydrazide (NBBSH) Derivatives Fabricated on a Glassy Carbon Electrode with a Nafion Matrix. ACS Omega 2017, 2, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.C.; Janis, M.K.; Nguyen, H.N.; David, O.W.; Zastrow, M.L. Fluorescent Protein-Based Sensors for Detecting Essential Metal Ions across the Tree of Life. ACS Sens. 2024, 9, 1622–1643. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, H.; Sun, L.; Yan, Y.; Wang, B.; Liang, Z.; Wang, L.; Li, J. Water Stable Metal–Organic Framework Based on Phosphono containing Ligand as Highly Sensitive Luminescent Sensor toward Metal Ions. Cryst. Growth Des. 2018, 18, 7683–7689. [Google Scholar] [CrossRef]
- George, A.; Shibu, E.S.; Maliyekkal, S.M.; Bootharaju, M.S.; Pradeep, T. Luminescent, Freestanding Composite Films of Au15 for Specific Metal Ion Sensing. ACS Appl. Mater. Interfaces 2012, 4, 639–644. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, X.; Liu, Y.; Liang, L.; Peng, Y.; Wu, S.; YanZhao, Y. N-Doped Carbon Dots as Fluorescent “Turn-Off” Nanosensors for Ascorbic Acid and Fe3+ Detection ACS. Appl. Nano Mater. 2022, 5, 7268–7277. [Google Scholar] [CrossRef]
- Rajendran, S.; Ramanaiah, D.V.; Kundu, S.; Bhunia, S.K. Yellow Fluorescent Carbon Dots for Selective Recognition of As3+ and Fe3+ Ions. ACS Appl. Nano Mater. 2021, 4, 10931–10942. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, H.; Ali, R.; Umrao, S.; Srivastava, R.; Abraham, S.; Misra, A.; Singh, V.N.; Mishra, H.; Tiwari, R.S.; et al. In Situ Functionalized Fluorescent WS2-QDs as Sensitive and Selective Probe for Fe3+ and a Detailed Study of Its Fluorescence Quenching. ACS Appl. Nano Mater. 2019, 2, 566–576. [Google Scholar] [CrossRef]
- Dong, S.; Ji, W.; Ma, Z.; Zhu, Z.; Ding, N.; Nie, J.; Du, B. Thermosensitive Fluorescent Microgels for Selective and Sensitive Detection of Fe3+ and Mn2+ in Aqueous Solutions. ACS Appl. Polym. Mater. 2020, 2, 3621–3631. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, S.; Priya, A.; Datta, S.; Mukherjee, S. Luminescent Copper Nanoclusters as a Specific Cell-Imaging Probe and a Selective Metal Ion Sensor. J. Phys. Chem. C 2015, 119, 24657–24664. [Google Scholar] [CrossRef]
- Rasin, P.; Mathew, M.M.; Manakkadan, V.; Palakkeezhillam, V.N.V.; Sreekanth, A. A Highly Fluorescent Pyrene-Based Sensor for Selective Detection of Fe3+ Ion in Aqueous Medium: Computational Investigations. J. Fluoresc. 2022, 32, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. Sect. C Found. Adv. 2015, 71, 3–8. [Google Scholar]
- Bruker, S. APEX3, SAINT and SADABS, 26; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.J.; van de Streek, J.; Wood, P. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Khunarj, S.; Saijaroensakul, W.; Morom, W.; Chainok, K.; Duangthongyou, T.; Pinchaipat, B.; Wannalerse, B. Synthesis, Crystal Structure and Optical Properties of 3,5-Dihydroxyphenyl-5-(dimethylamino)naphthlene-1-sulfonate as a Fluorescence Sensor for Fluoride Ion Detection. Crystals 2022, 12, 1836. [Google Scholar] [CrossRef]
- Buasakun, J.; Srilaoong, P.; Chainok, K.; Raksakoon, C.; Rattanakram, R.; Duangthongyou, T. Dual Luminescent Coordination Polymers Based on Flexible Aliphatic Carboxylate Ligands Supplemented by Rigid Bipyridyl Ligands for 2,4-Dinitrophenol (DNP) and Iron(III) Ion Detection. Polyhedron 2021, 204, 115265. [Google Scholar] [CrossRef]


| Compound | Compound KC1 |
|---|---|
| CCDC Number | 2361572 |
| Empirical Formula | C30H33NO4S |
| Formula Weight (g/mol) | 503.67 |
| Temperature (K) | 296 |
| Wavelength (Å) | 0.71073 |
| Crystal System | Triclinic |
| Space Group | P1 |
| a (Å) | 8.7613 (2) |
| b (Å) | 9.6370 (2) |
| c (Å) | 17.5443 (5) |
| α (◦) | 79.447 (1) |
| β (◦) | 84.094 (1) |
| γ (◦) | 66.799 (1) |
| Volume/Å3 | 1337.71 (6) |
| Z | 2 |
| Density (calculated) (g/cm3) | 1.250 |
| Absorption Coefficient (mm−1) | 0.16 |
| F(000) | 536.561 |
| Crystal Size (mm3) | 0.32 × 0.32 × 0.22 |
| Theta Range for Data Collection (◦) | 5 to 57.8 |
| Index Ranges | −11 ≤ h ≤ 11, −13 ≤ k ≤ 13, −23 ≤ l ≤ 23 |
| Reflections Collected | 6982 |
| Independent Reflections | 5073 [Rint = 0.057] |
| Max. and Min. Transmission | 0.7458 and 0.6903 |
| Data/Restraints/Parameters | 5073/0/328 |
| Goodness-of-fit on F2 | 1.06 |
| Final R Indices [I > 2sigma(I)] | R1 = 0.0457, wR2 = 0.1274 |
| R Indices (all data) | R1 = 0.0706, wR2 = 0.1128 |
| Largest Diff. Peak and Hole | 0.26/−0.14 e Å−3 |
| Atom-Atom | Bond Length (Å) | Atom-Atom | Bond Length (Å) |
|---|---|---|---|
| S1-O1 | 1.4195 (14) | S1-C12 | 1.7614 (15) |
| C14-C15 | 1.382 (3) | C16-H16 | 0.93 |
| S1-O2 | 1.4235 (14) | O3-C13 | 1.4124 (18) |
| C15-H15 | 0.93 | C16-C17 | 1.380 (3) |
| S1-O3 | 1.5992 (12) | O4-C24 | 1.361 (2) |
| C15-C16 | 1.366 (3) | C17-H17 | 0.93 |
| O4-C25 | 1.422 (2) | C17-C18 | 1.394 (2) |
| N1-C1 | 1.457 (2) | C18-C19 | 1.483 (2) |
| N1-C2 | 1.449 (2) | C19-C20 | 1.392 (2) |
| O2-S1-O1 | 119.77 (8) | C24-C19-C18 | 121.14 (14) |
| C17-C16-C15 | 120.29 (18) | C3-N1-C2 | 114.14 (14) |
| O3-S1-O1 | 103.49 (8) | C24-C19-C20 | 118.34 (16) |
| C17-C16-H16 | 119.86 (12) | H1a-C1-N1 | 109.5 |
| O3-S1-O2 | 109.35 (7) | H20-C20-C19 | 119.30 (11) |
| H17-C17-C16 | 119.14 (12) | H1b-C1-N1 | 109.5 |
| C12-S1-O1 | 110.31 (8) | C21-C20-C19 | 121.4 (2) |
| C18-C17-C16 | 121.72 (18) | H1b-C1-H1a | 109.5 |
| C12-S1-O2 | 109.28 (8) | C21-C20-H20 | 119.30 (13) |
| C18-C17-H17 | 119.14 (10) | H1c-C1-N1 | 109.5 |
| C12-S1-O3 | 103.25 (6) | H21-C21-C20 | 120.32 (13) |
| C17-C18-C13 | 116.06 (15) | H1c-C1-H1a | 109.5 |
| C13-O3-S1 | 118.79 (9) | C22-C21-C20 | 119.37 (19) |
| C19-C18-C13 | 124.09 (14) | H1c-C1-H1b | 109.5 |
| C25-O4-C24 | 119.31 (14) | C22-C21-H21 | 120.32 (12) |
| C19-C18-C17 | 119.85 (15) | H2a-C2-N1 | 109.5 |
| C2-N1-C1 | 110.58 (15) | H22-C22-C21 | 119.58 (12) |
| C20-C19-C18 | 120.31 (16) | H2b-C2-N1 | 109.5 |
| C3-N1-C1 | 115.05 (15) | C23-C22-C21 | 120.84 (19) |
| Wavenumber (cm−1) | Functional Group |
|---|---|
| 2858 | -C-H |
| 1095 | -C-O |
| 1570 and 1465 | -C=C |
| 1152 | -C-N |
| 1364 and 1234 | -S=O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantaniyomporn, K.; Charoensuk, K.; Duangthongyou, T.; Chainok, K.; Wannalerse, B. A Fluorescence Sensor Based on Biphenolic Backbone for Metal Ion Detection: Synthesis and Crystal Structure. Crystals 2024, 14, 943. https://doi.org/10.3390/cryst14110943
Chantaniyomporn K, Charoensuk K, Duangthongyou T, Chainok K, Wannalerse B. A Fluorescence Sensor Based on Biphenolic Backbone for Metal Ion Detection: Synthesis and Crystal Structure. Crystals. 2024; 14(11):943. https://doi.org/10.3390/cryst14110943
Chicago/Turabian StyleChantaniyomporn, Kanokporn, Kiratikarn Charoensuk, Tanwawan Duangthongyou, Kittipong Chainok, and Boontana Wannalerse. 2024. "A Fluorescence Sensor Based on Biphenolic Backbone for Metal Ion Detection: Synthesis and Crystal Structure" Crystals 14, no. 11: 943. https://doi.org/10.3390/cryst14110943
APA StyleChantaniyomporn, K., Charoensuk, K., Duangthongyou, T., Chainok, K., & Wannalerse, B. (2024). A Fluorescence Sensor Based on Biphenolic Backbone for Metal Ion Detection: Synthesis and Crystal Structure. Crystals, 14(11), 943. https://doi.org/10.3390/cryst14110943





_CHAINOK.jpg)

