Comparison and Contrast of Calcite vs. Dolomite after Heat Treatment to Enhance Toluidine Blue Removal from Water
Abstract
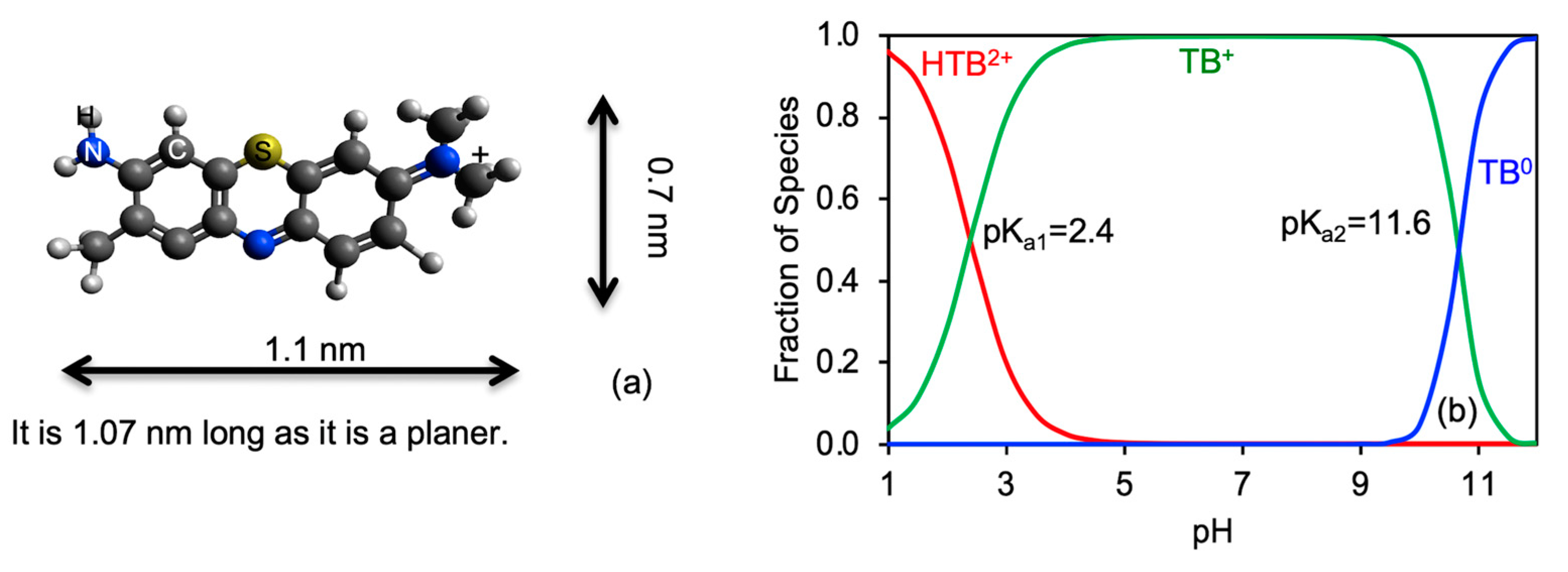
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TB Sorption Study
2.3. Instrumental Analyses
3. Results and Discussions
3.1. TB Removal in Isotherm Studies
3.2. Kinetic Study of TB Removal
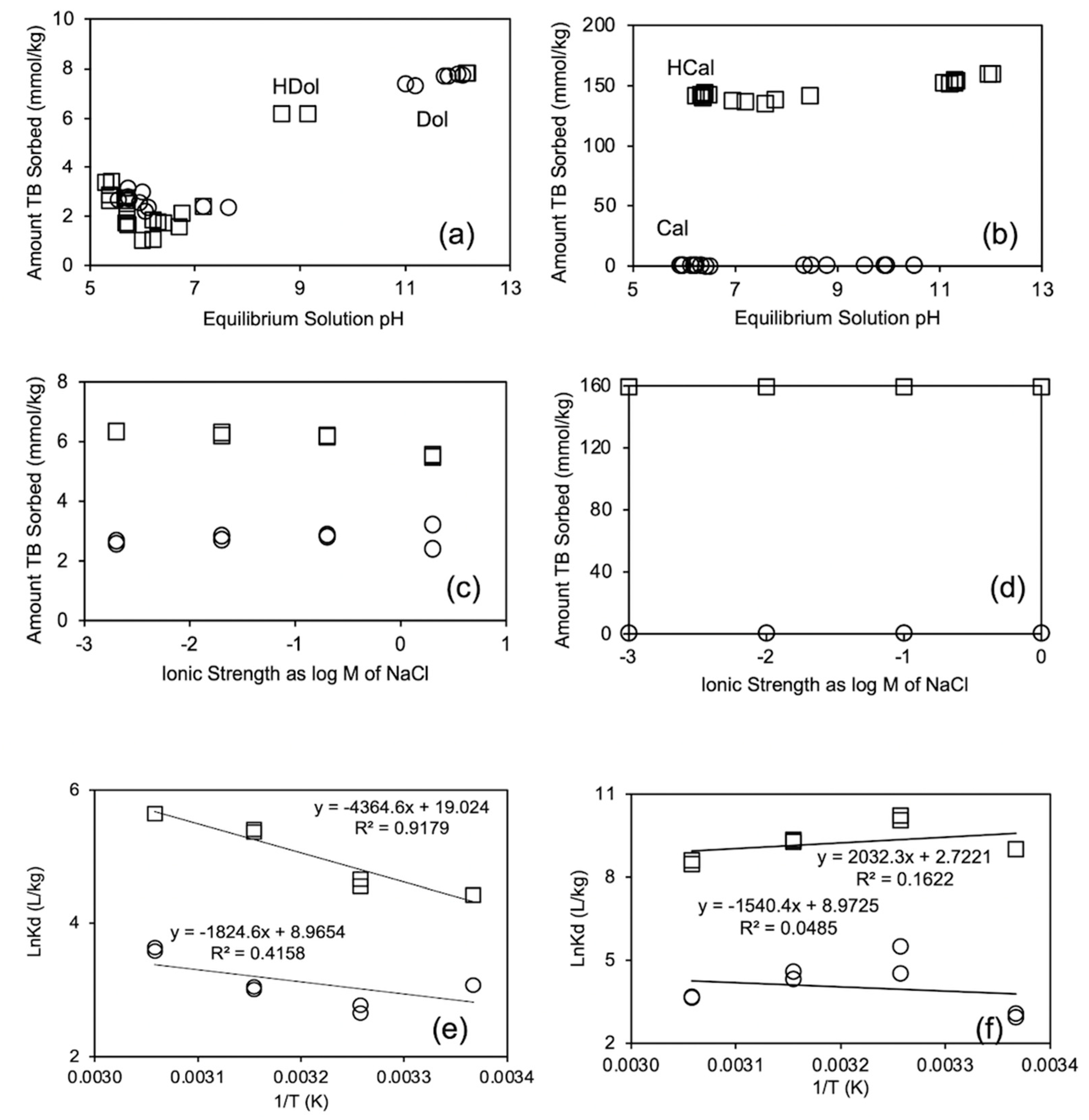
3.3. TB Removal as Influenced by Equilibrium Solution pH, Ionic Strength, and Temperature
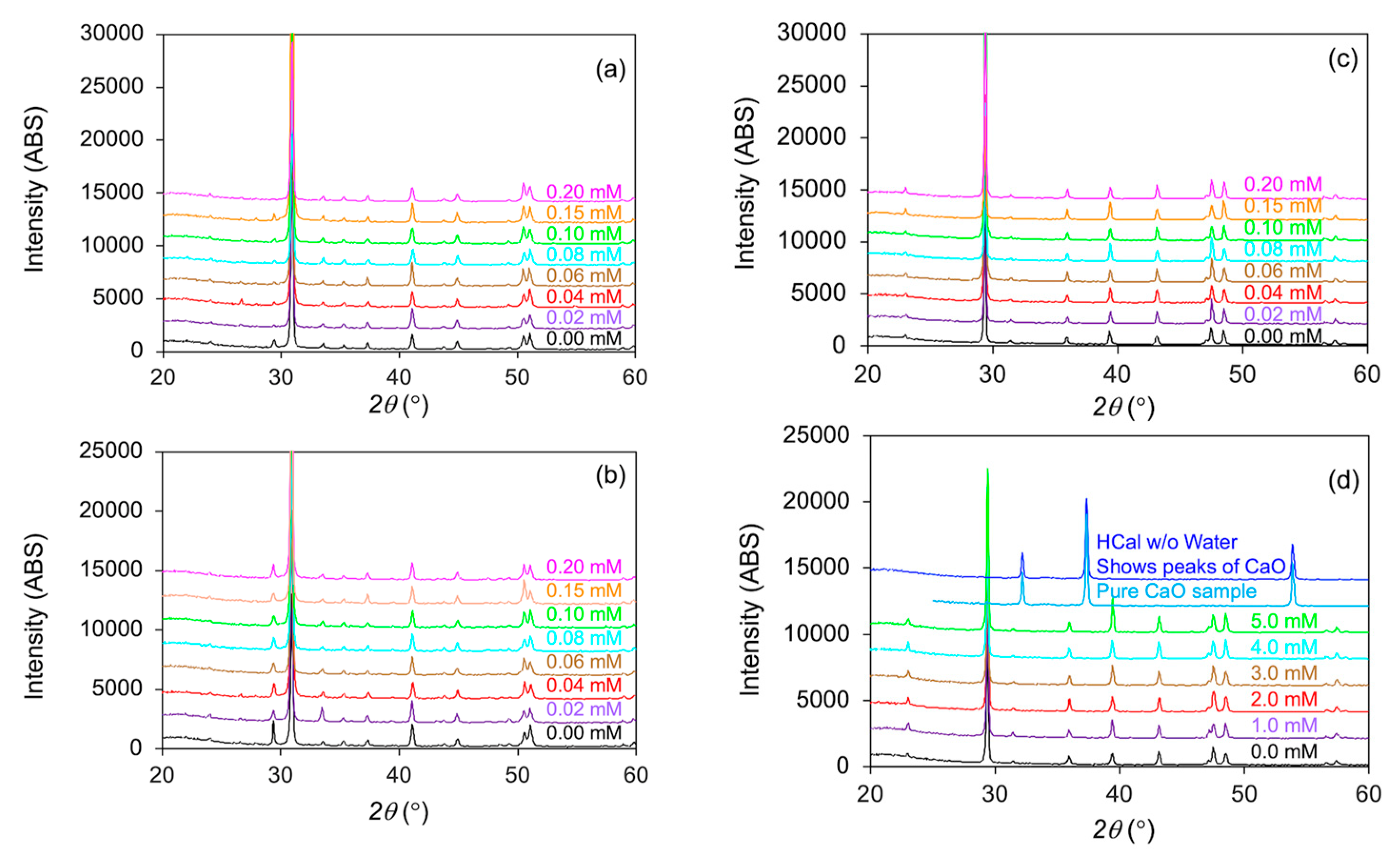
3.4. XRD Analyses
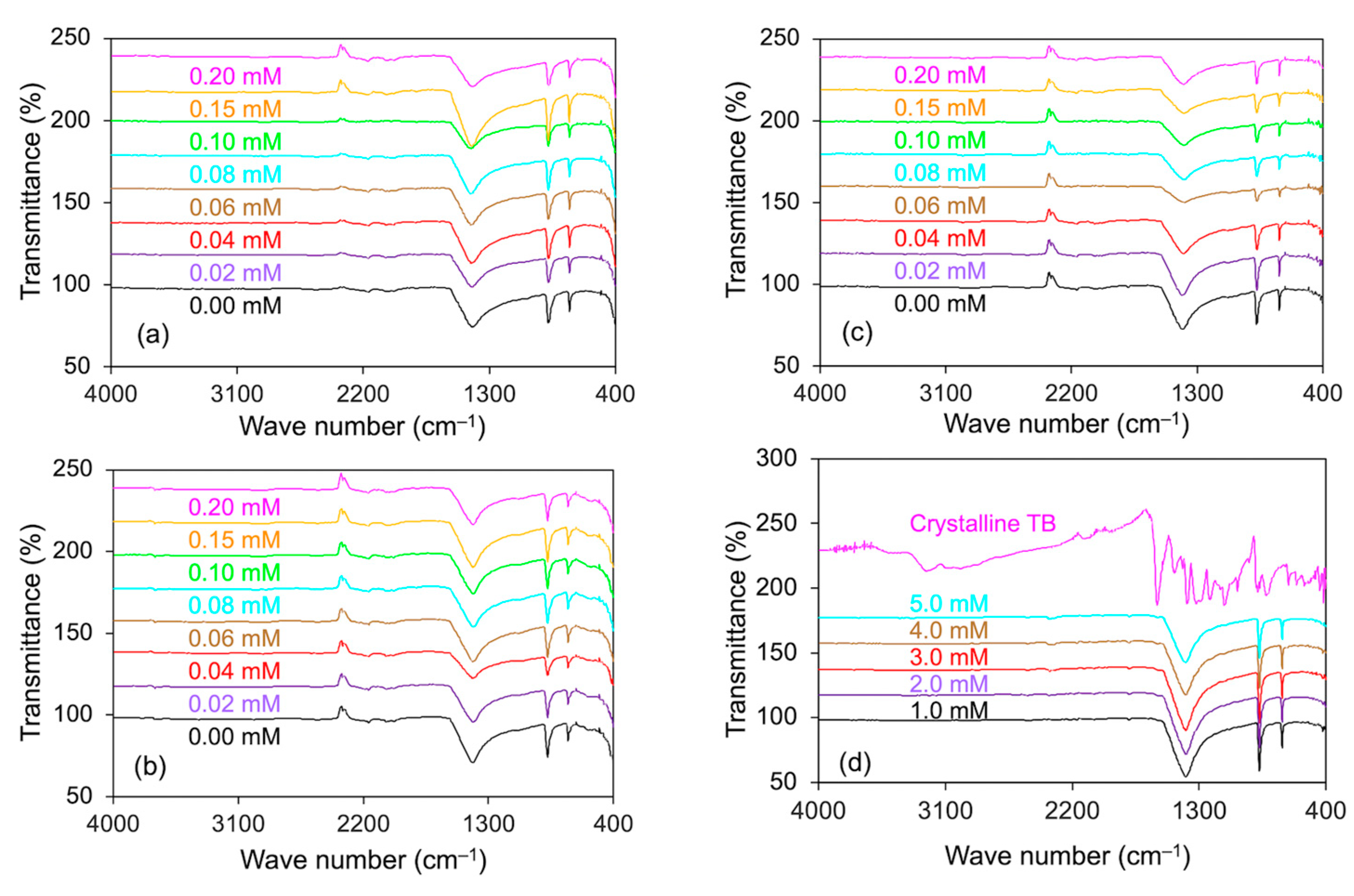
3.5. FTIR Analyses
3.6. SEM Observation and EDS Analyses
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parvin, F.; Islam, S.; Akm, S.I.; Urmy, Z.; Ahmed, S.; Islam, A.S. A study on the solutions of environment pollutions and worker’s health problems caused by textile manufacturing operations. Biomed. J. Sci. Tech. Res. 2020, 28, 21831–21844. [Google Scholar]
- Fosso-Kankeu, E.; Potgieter, J.; Waanders, F.B. Removal of malachite green and toluidine blue dyes from aqueous solution using a clay-biochar composite of bentonite and sweet sorghum bagasse. Int. J. Appl. Eng. Res. 2019, 14, 1324–1333. [Google Scholar]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The adsorption kinetics and removal of cationic dye, Toluidine Blue O, from aqueous solution with Turkish zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, D.; Lamkiewicz, J.; Shyichuk, A. Determination of sodium dodecyl sulfate by means of photometric titration with o-toluidine blue dye. J. Surfactants Deterg. 2018, 21, 751–756. [Google Scholar] [CrossRef]
- Jebaramya, J.; Ilanchelian, M.; Prabahar, S. Spectral studies of toluidine blue o in the presence of sodium dodecyl sulfate. Digest J. Nanomater. Biostruct. 2009, 4, 789–797. [Google Scholar]
- Vleugels, L.F.; Féat, A.; Voets, I.K.; Tuinier, R. Toluidine blue-sodium lauryl ether sulfate complexes: Influence of ethylene oxide length. Dye. Pigment. 2017, 141, 420–427. [Google Scholar] [CrossRef]
- Lafi, R.; Rezma, S.; Hafiane, A. Removal of toluidine blue from aqueous solution using orange peel waste (OPW). Desalination Water Treat. 2015, 56, 2754–2765. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Y.; Zhang, W.; Li, B. Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv. Powder Technol. 2019, 30, 493–501. [Google Scholar] [CrossRef]
- Salim, H.A.M.; Salih, S.A.M. Photodegradation study of Toluidine Blue dye in aqueous solution using magnesium oxide as a photocatalyst. Int. J. Chem. 2015, 7, 143. [Google Scholar] [CrossRef]
- Salim, H.A.M.; Idrees, S.A.; Rashid, R.A.; Mohammed, A.A.; Simo, S.M.; Khalo, I.S. Photo-catalytic degradation of toluidine blue dye in aqueous medium under fluorescent light. In Proceedings of the 2018 International Conference on Advanced Science and Engineering (ICOASE), Duhok, Iraq, 9–11 October 2018; pp. 384–388. [Google Scholar]
- Sree, G.V.; Nagaraaj, P.; Kalanidhi, K.; Aswathy, C.A.; Rajasekaran, P. Calcium oxide a sustainable photocatalyst derived from eggshell for efficient photodegradation of organic pollutants. J. Clean. Prod. 2020, 270, 122294. [Google Scholar] [CrossRef]
- Borhade, A.V.; Kale, A.S. Calcined eggshell as a cost effective material for removal of dyes from aqueous solution. Appl. Water Sci. 2017, 7, 4255–4268. [Google Scholar] [CrossRef]
- Rápó, E.; Szép, R.; Keresztesi, Á.; Suciu, M.; Tonk, S. Adsorptive removal of cationic and anionic dyes from aqueous solutions by using eggshell household waste as biosorbent. Acta Chim. Slov. 2018, 65, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Bessashia, W.; Hattab, Z.; Berredjem, Y.; Djellabi, R.; Zerdoum, R.; Allaoui, A.; Gheid, A.; Guerfi, K. Utilization of powdered eggshell waste for rhodamine B removal: Evaluation of adsorptive efficiencies and modeling studies. Sens. Lett. 2018, 16, 128–136. [Google Scholar] [CrossRef]
- Amarasinghe, A.; Wanniarachchi, D. Eco-Friendly photocatalyst derived from egg shell waste for dye degradation. J. Chem. 2019, 2019, 8184732. [Google Scholar] [CrossRef]
- Qu, T.; Yao, X.; Owens, G.; Gao, L.; Zhang, H. A sustainable natural clam shell derived photocatalyst for the effective adsorption and photodegradation of organic dyes. Sci. Rep. 2022, 12, 2988. [Google Scholar] [CrossRef]
- Ikram, M.; Khalid, A.; Shahzadi, A.; Haider, A.; Naz, S.; Naz, M.; Shahzadi, I.; Ul-Hamid, A.; Haider, J.; Nabgan, W.; et al. Enhanced photocatalytic degradation with sustainable CaO nanorods doped with Ce and cellulose nanocrystals: In silico molecular docking studies. ACS Omega 2022, 7, 27503–27515. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Venkatraman, S.K.; Imthiaz, S.; Drweesh, E.A.; Elnagar, M.M.; Koppala, S.; Swamiappan, S. Synthesis and characterization of calcium and magnesium based oxides and titanates for photocatalytic degradation of rhodamine B: A comparative study. Sci. Rep. 2023, 13, 3615. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008, 223, 152–161. [Google Scholar] [CrossRef]
- Rauf, M.A.; Qadri, S.M.; Ashraf, S.; Al-Mansoori, K.M. Adsorption studies of Toluidine Blue from aqueous solutions onto gypsum. Chem. Eng. J. 2009, 150, 90–95. [Google Scholar] [CrossRef]
- El Haouti, R.; Ouachtak, H.; El Guerdaoui, A.; Amedlous, A.; Amaterz, E.; Haounati, R.; Addi, A.A.; Akbal, F.; El Alem, N.; Taha, M.L. Cationic dyes adsorption by Na-Montmorillonite Nano Clay: Experimental study combined with a theoretical investigation using DFT-based descriptors and molecular dynamics simulations. J. Mol. Liquids 2019, 290, 111139. [Google Scholar] [CrossRef]
- Shi, Y.; Baker, J.; Feng, C.; Wang, X.; Li, Z. Removal of toluidine blue from water using 1:1 layered clay minerals. Adv. Powder Technol. 2022, 33, 103608. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, A.; Rayniak, A.; Strommen, J.; Allen, L.; Xu, S. Heat treatment of calcite to enhance its removal of color dye. Crystals 2024, 14, 450. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, A.; Rayniak, A.; Xu, S. Anionic dye alizarin red S removal using heat treated dolomite. Crystals 2024, 14, 187. [Google Scholar] [CrossRef]
- Ilanchelian, M.; Raj, C.R.; Ramaraj, R. Spectral studies on the cyclodextrin inclusion complexes of toluidine blue O and Meldola’s blue in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2000, 36, 9–20. [Google Scholar] [CrossRef]
- Karadağ, E.; Topaç, F.; Kundakci, S.; Üzüm, Ö.B. Novel composite sorbent AAm/MA hydrogels containing starch and kaolin for water sorption and dye uptake. Bull. Mater. Sci. 2014, 37, 1637–1646. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Wang, X.; Carlson, K.; Li, Z. Removal of toluidine blue and safranin O from single and binary solutions using zeolite. Crystals 2021, 11, 1181. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Mahapatra, S. Aragonite crystals with unconventional morphologies. J. Mater. Chem. 1999, 9, 2953–2957. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. Int. J. Orig. Work All Asp. Raman Spectrosc. Incl. High. Order Process. Also Brillouin Rayleigh Scatt. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Weir, C.E.; Lippincott, E.R. Infrared studies of aragonite, calcite, and vaterite type structures in the borates, carbonates, and nitrates. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65, 173–180. [Google Scholar] [CrossRef]
- Ji, J.; Ge, Y.; Balsam, W.; Damuth, J.E.; Chen, J. Rapid identification of dolomite using a Fourier Transform Infrared Spectrophotometer (FTIR): A fast method for identifying Heinrich events in IODP Site U1308. Mar. Geol. 2009, 258, 60–68. [Google Scholar] [CrossRef]
- Liu, M.; Shi, G.; Zhang, L.; Zhao, G.; Jin, L. Electrode modified with toluidine blue-doped silica nanoparticles, and its use for enhanced amperometric sensing of hemoglobin. Anal. Bioanal. Chem. 2008, 391, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J. Photochem. Photobiol. B Biol. 2003, 71, 87–98. [Google Scholar] [CrossRef]
- D’Ilario, L.; Martinelli, A. Toluidine blue: Aggregation properties and structural aspects. Model. Simul. Mater. Sci. Eng. 2006, 14, 581–595. [Google Scholar] [CrossRef]
- Gilani, A.G.; Dezhampanah, H.; Poormohammadi-Ahandani, Z. A comparative spectroscopic study of thiourea effect on the photophysical and molecular association behavior of various phenothiazine dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ziane, S.; Marouf-Khelifa, K.; Benmekki, H.; Schott, J.; Khelifa, A. Removal of a reactive textile azo dye by dolomitic solids: Kinetic, equilibrium, thermodynamic, and FTIR studies. Desalination Water Treat. 2015, 56, 695–708. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, L.; Xing, G.; Bai, Z.; Huang, J.; Zhang, Z. Microscale flower-like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv. 2019, 9, 7338–7348. [Google Scholar] [CrossRef]
- Yadav, P.; Saini, R.; Bhaduri, A. Facile synthesis of MgO nanoparticles for effective degradation of organic dyes. Environ. Sci. Pollut. Res. 2023, 30, 71439–71453. [Google Scholar] [CrossRef]
- Park, H.K.B.; Kumar, P.; Kebaili, I.; Boukhris, I.; Joo, Y.H.; Sung, T.H.; Kumar, A. Optimization and modelling of magnesium oxide (MgO) photocatalytic degradation of binary dyes using response surface methodology. Sci. Rep. 2024, 14, 9412. [Google Scholar]
- Ameta, R.; Kumar, D.; Jhalora, P. Photocatalytic degradation of methylene blue using calcium oxide. Acta Chim. Pharm. Indica 2014, 4, 20–28. [Google Scholar]
- Veeranna, K.D.; Lakshamaiah, M.T.; Narayan, R.T. Photocatalytic degradation of indigo carmine dye using calcium oxide. Int. J. Photochem. 2014, 2014, 530570. [Google Scholar] [CrossRef]
- Mohamed, F.; Shaban, M.; Aljohani, G.; Ahmed, A.M. Synthesis of novel eco-friendly CaO/C photocatalyst from coffee and eggshell wastes for dye degradation. J. Mater. Res. Technol. 2021, 14, 3140–3149. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Maharani, B.S.; Ragaditha, R. Calcium oxide nanoparticle production and its application as photocatalyst. J. Adv. Res. Appl. Sci. Eng. Technol. 2023, 30, 168–181. [Google Scholar] [CrossRef]
- Janbooranapinij, K.; Yimponpipatpol, A.; Ngamthanacom, N.; Panomsuwan, G. Conversion of industrial carpet waste into adsorbent materials for organic dye removal from water. Clean. Eng. Technol. 2021, 4, 100150. [Google Scholar] [CrossRef]


| Sorption Parameters | Cal | HCal | Dol | HDol |
|---|---|---|---|---|
| Sm (mmol/kg) | 2 | 220 | 3.5 | 10.5 |
| KL (L/mmol) | 74 | 113 | 329 | 23 |
| qe (mmol/kg) | 2 | 160 | 3 | 6 |
| Kqe2 (mmol/kg-h) | 0.3 | 10,000 | 4 | 2 |
| k (kg/mmol-h) | 0.06 | 0.38 | 0.54 | 0.06 |
| r2 for pseudo-second-order fitting | 0.995 | 0.98 | 0.99 | 0.97 |
| Minerals | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (kJ/mol-K) | |||
|---|---|---|---|---|---|---|
| 296 K | 306 K | 316 K | 326 K | |||
| Dol | −6.98 | −7.73 | −8.47 | −9.22 | 15.17 | 0.075 |
| HDol | −10.47 | −12.05 | −13.63 | −15.21 | 36.29 | 0.158 |
| Cal | −9.35 | −10.10 | −10.84 | −11.59 | 12.80 | 0.075 |
| HCal | 10.25 | 10.03 | 9.80 | 9.58 | 16.89 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Strommen, J.; Garza, A.; Bowman, A.; Rayniak, A.; Schulz, L.; Allen, L.; Xu, S. Comparison and Contrast of Calcite vs. Dolomite after Heat Treatment to Enhance Toluidine Blue Removal from Water. Crystals 2024, 14, 874. https://doi.org/10.3390/cryst14100874
Li Z, Strommen J, Garza A, Bowman A, Rayniak A, Schulz L, Allen L, Xu S. Comparison and Contrast of Calcite vs. Dolomite after Heat Treatment to Enhance Toluidine Blue Removal from Water. Crystals. 2024; 14(10):874. https://doi.org/10.3390/cryst14100874
Chicago/Turabian StyleLi, Zhaohui, Jadyn Strommen, Aaron Garza, Anna Bowman, Angie Rayniak, Laura Schulz, Lori Allen, and Shangping Xu. 2024. "Comparison and Contrast of Calcite vs. Dolomite after Heat Treatment to Enhance Toluidine Blue Removal from Water" Crystals 14, no. 10: 874. https://doi.org/10.3390/cryst14100874
APA StyleLi, Z., Strommen, J., Garza, A., Bowman, A., Rayniak, A., Schulz, L., Allen, L., & Xu, S. (2024). Comparison and Contrast of Calcite vs. Dolomite after Heat Treatment to Enhance Toluidine Blue Removal from Water. Crystals, 14(10), 874. https://doi.org/10.3390/cryst14100874







