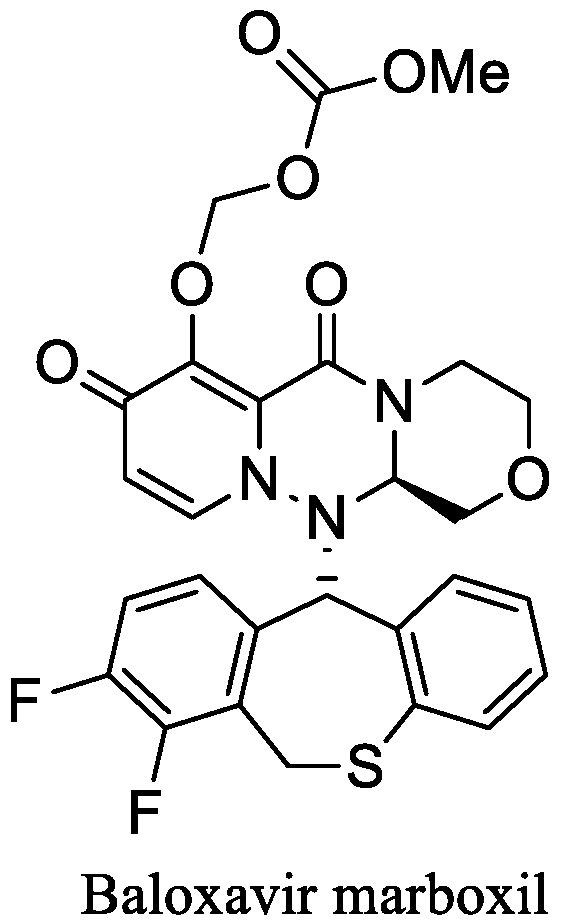
Synthesis and Cap-Dependent Endonuclease Inhibition of Baloxavir Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
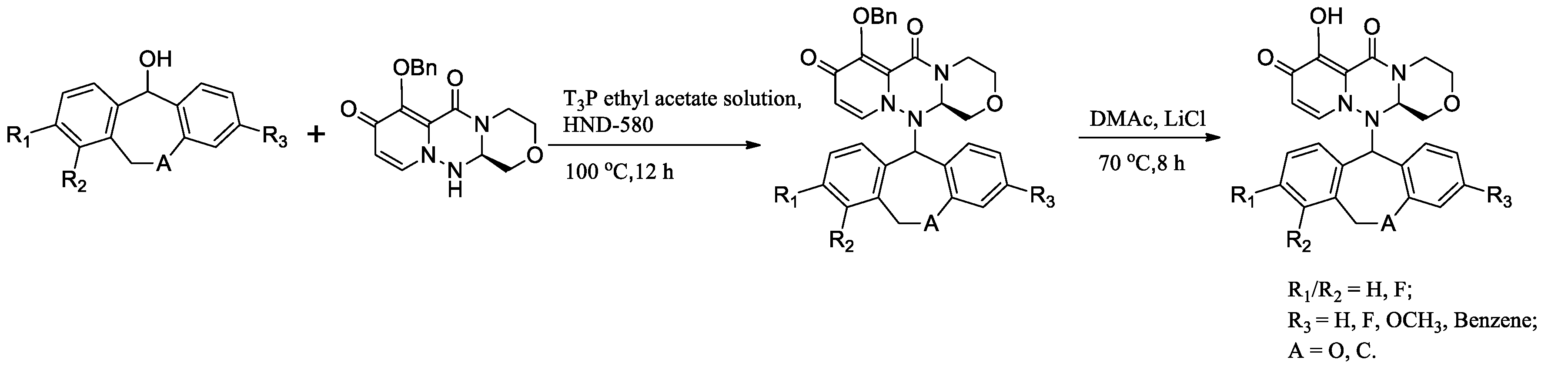
2.2. General Synthetic Method for the Compounds
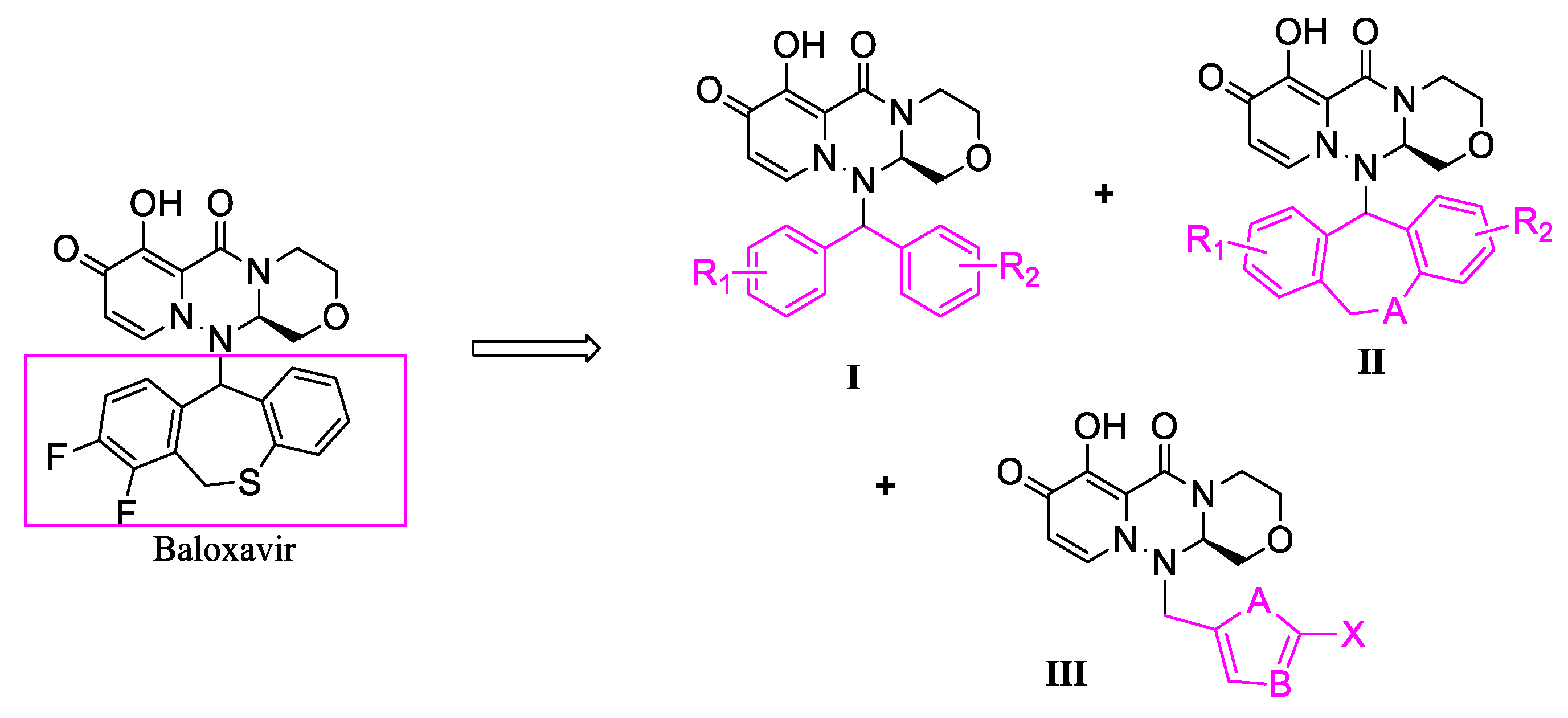
2.2.1. Synthetic Method for the Compounds I
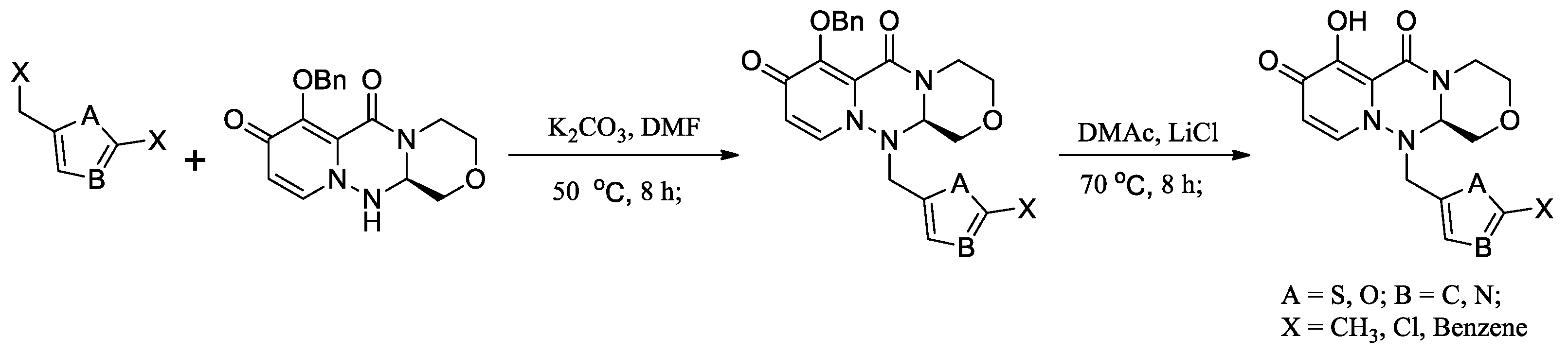
2.2.2. Synthetic Method for the Compounds II
2.2.3. Synthetic Method for the Compounds III
2.3. Drug Likeness
2.4. Biological Activity of the Synthesized Baloxavir Derivatives
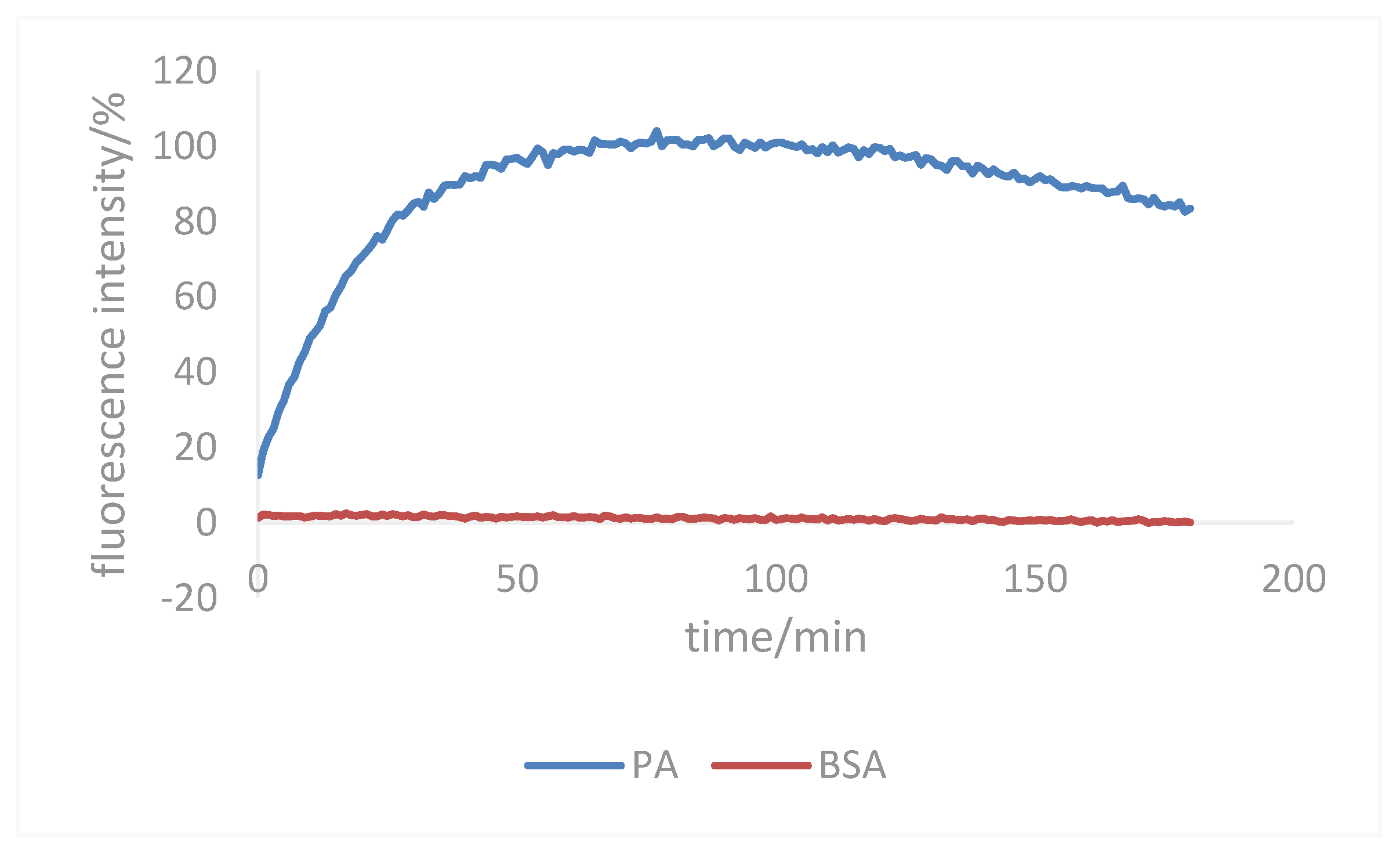
2.4.1. Establishment of a Test Method for Endonuclease Inhibitory Activity
2.4.2. Affinity Test of Compounds to Endonucleases
2.5. Molecular Docking Study
3. Results and Discussion
3.1. Design and Synthesis
3.2. Drug Likeness
3.3. Biological Activity of the Synthesized Baloxavir Derivatives
3.3.1. The Affinity of the Target Compound to Endonuclease
3.3.2. Inhibition of Target Compounds on Endonuclease
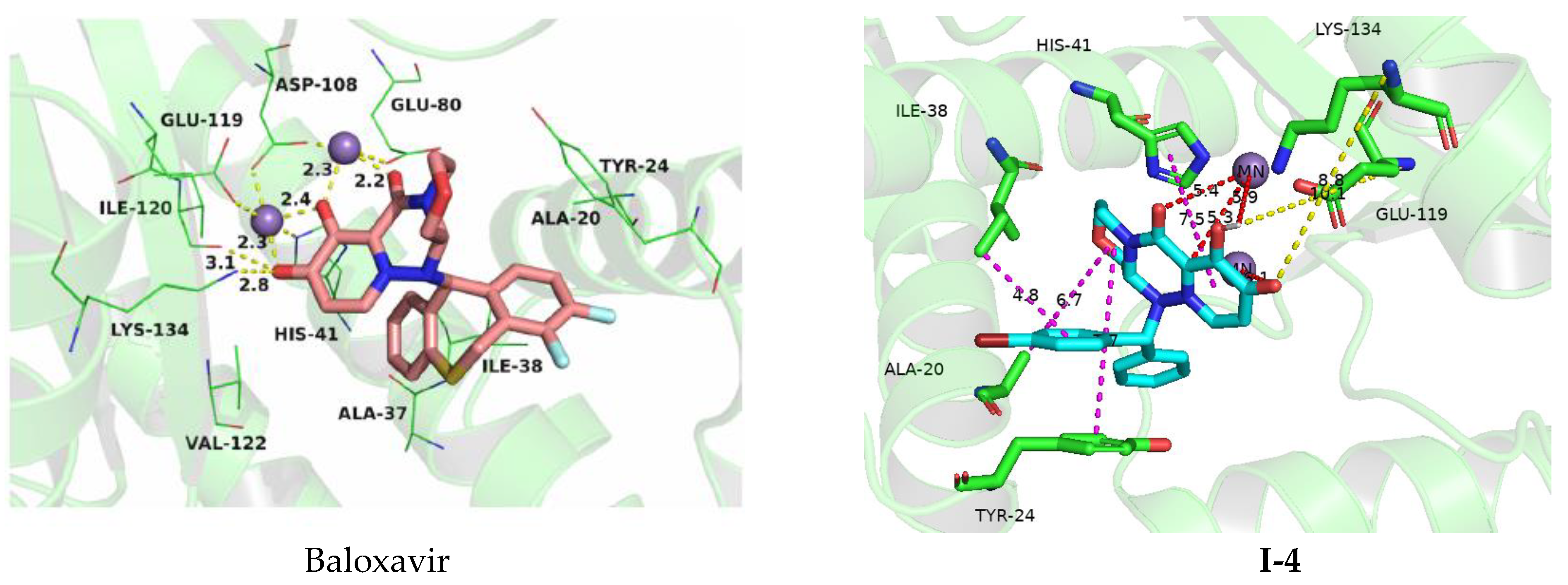
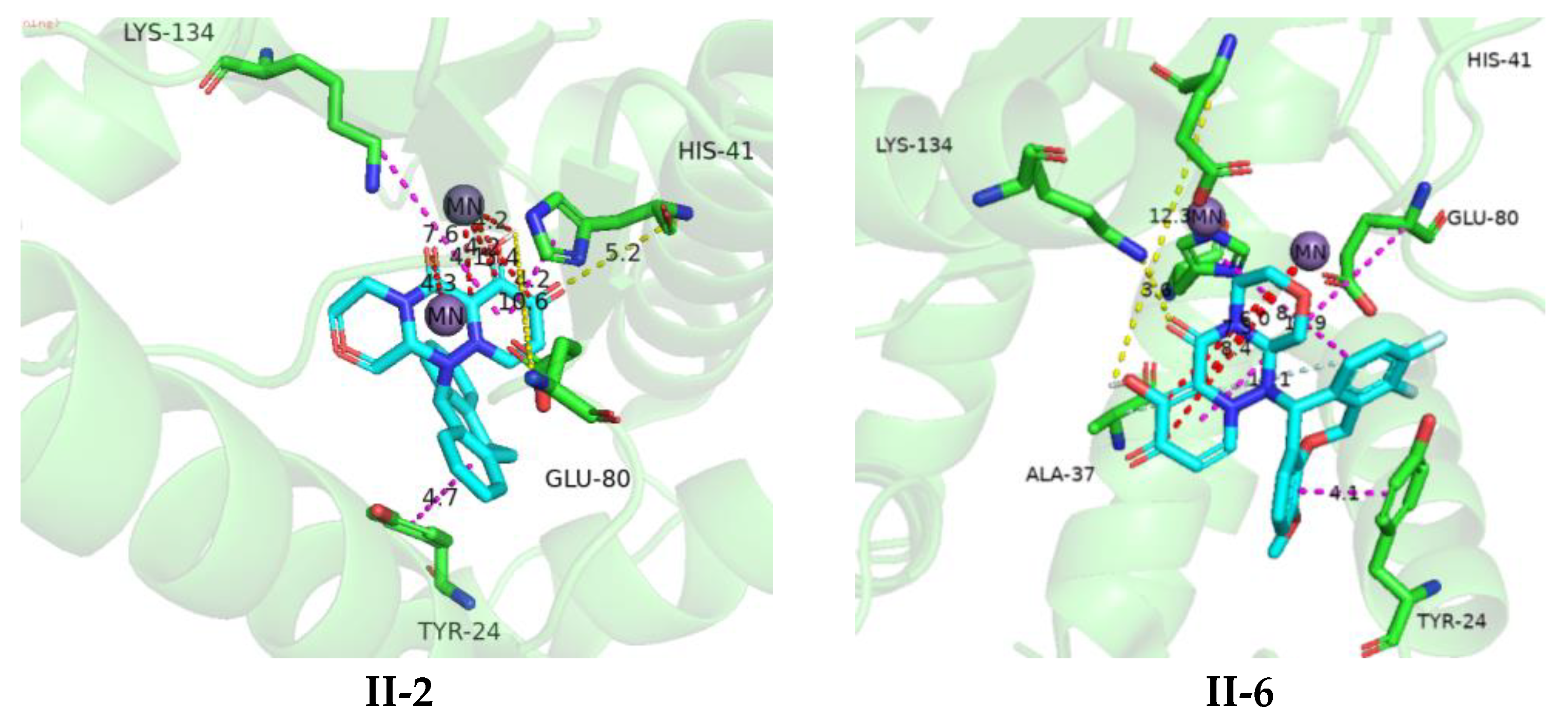
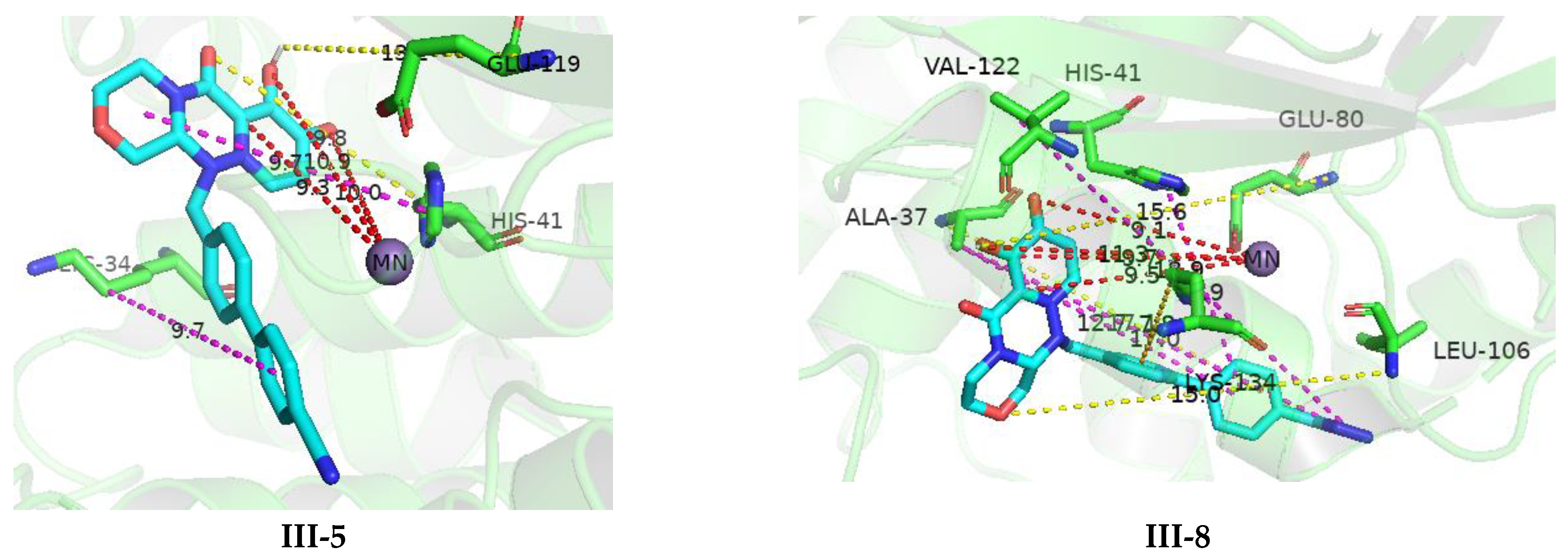
3.4. Molecular Docking
3.5. Structure–Activity Relationship
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Liu, Z.; Li, Z. Research Progress in Anti Influenza Virus Drugs. China Med. Biotechnol. 2015, 10, 533–539. [Google Scholar]
- Thompson, W.W.; Weintraub, E.; Dhankhar, P.; Cheng, P.-Y.; Brammer, L.; Meltzer, M.I.; Bresee, J.S.; Shay, D.K. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir. Viruses 2009, 3, 37–49. [Google Scholar] [CrossRef]
- Pleschka, S. Overview of influenza viruses. Curr. Top. Microbiol. Immunol. 2013, 370, 1–20. [Google Scholar]
- Xu, S.; He, X.; Chen, C.; Tian, H. Research Progress in Anti Influenza Virus Drugs. Mod. Drugs Clin. 2009, 24, 65–70. [Google Scholar]
- Cheng, N.; Chen, B.; Wang, Y. A new oral anti influenza drug-Oseltamivir. Chin. J. Clin. Pharm. 2002, 11, 55–59. [Google Scholar]
- Gu, J. Research progress of Peramivir, a novel potent neuraminidase inhibitor against influenza virus. Chin. J. New Drugs 2013, 22, 989–997. [Google Scholar]
- Zhang, Q.; Zhao, Q.; Xiong, R.; Li, J.-F.; Shen, J.-S. Research progress of anti-influenza virus agents. Acta Pharm. Sin. 2010, 45, 289–299. [Google Scholar]
- von Itzstein, M. The war against influenza: Discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 2007, 6, 967–974. [Google Scholar] [CrossRef]
- Hu, Y.; Hau, R.K.; Wang, Y.; Tuohy, P.; Zhang, Y.; Xu, S.; Ma, C.; Wang, J. Structure-property relationship studies of Influenza A virus AM2-S31N proton channel blockers. ACS Med. Chem. Lett. 2018, 9, 1111–1116. [Google Scholar] [CrossRef]
- Das, K. Antivirals targeting influenza A virus. J. Med. Chem. 2012, 55, 6263–6277. [Google Scholar] [CrossRef]
- Guti Rrze, R.A.; Naughtin, M.; Horm, S.V.; San, S.; Buchy, P. A (H5N1) virus evolution in South East Asia. Viruses 2009, 1, 335–361. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, X.; Zhang, Y. Clinical research progress of anti influenza virus drugs with different mechanisms of action. Shanghai Pharm. 2014, 35, 58–60. [Google Scholar]
- Pizzorno, A.; Abed, Y.; Boivin, G. Influenza drug resistance. Semin. Respir. Crit. Care Med. 2011, 32, 409–422. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Haynes, B.F. Influenza vaccines: mTOR inhibition surprisingly leads to protection. Nat. Immunol. 2013, 14, 1205–1207. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, W. A novel neuraminidase inhibitor—Anti influenza drug AV5080. J. Clin. Drug Ther. 2018, 16, 19–21. [Google Scholar]
- Herlocher, M.L.; Truscon, R.; Elias, S.; Yen, H.-L.; Roberts, N.A.; Ohmit, S.E.; Monto, A.S. Influenza viruses resistant to the antiviral drug oseltamivir: Transmission studies in ferrets. J. Infect. Dis. 2004, 190, 1627–1630. [Google Scholar] [CrossRef] [PubMed]
- Ivachtchenko, A.V.; Ivanenkov, Y.A.; Mitkin, O.D.; Yamanushkin, P.M.; Bichko, V.V.; Shevkun, N.A.; Karapetian, R.N.; Leneva, I.A.; Borisova, O.V.; Veselov, M.S. Novel oral anti-influenza drug candidate AV5080. J. Antimicrob. Chemother. 2014, 69, 1892–1902. [Google Scholar] [CrossRef]
- Heo, Y.A. Baloxavir: First global approval. Drugs 2018, 78, 693–697. [Google Scholar] [CrossRef]
- Sun, C.; Qiao, H. A new oral anti-influenza drug: Baloxavir marboxil. Chin. J. New Drugs Clin. Remed. 2019, 38, 262–267. [Google Scholar]
- Li, C.; Chen, H. Enhancement of influenza virus transmission by gene reassortment. Curr. Top. Microbiol. Immunol. 2014, 385, 185–204. [Google Scholar]
- Tao, J.; Lv, Q.; Li, X.; Dai, P.; Ye, Y.; Shen, Z. Research on new anti-influenza drug baloxavir marboxil. Chin. J. Clin. Med. 2019, 26, 308–311. [Google Scholar]
- Baker, D.E. Baloxavir marboxil. Hosp. Pharm. 2019, 54, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, N.; Koshimichi, H.; Ishibashi, T.; Wajima, T. Evaluation of drug-drug interaction potential between baloxavir marboxil and oseltamivir in healthy subjects. Clin. Drug Investig. 2018, 38, 1053–1060. [Google Scholar] [CrossRef]
- Hayden, F.G.; Sugayan, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir marboxil for uncomplicated in fluenza in adults andadolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Omoto, S.; Speranzini, V.; Hashimoto, T.; Noshi, T.; Yamaguchi, H.; Kawai, M.; Kawaguchi, K.; Uehara, T.; Shishido, T.; Naito, A.; et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018, 8, 9633. [Google Scholar] [CrossRef]
- Tang, C.; Ren, Q.; Luo, H.; Yin, J.; Yi, K.; Lei, Y.; Wang, Y.; Zhang, Y. Influenza Virus Replication Inhibitors and Their Uses. International Patent (PCT/CN2018/106092) WO2019/052565A1, 21 March 2019. [Google Scholar]
- Tang, L.; Yan, H.; Wu, W.; Chen, D.; Gao, Z.; Hou, J.; Zhang, C.; Jiang, Y. Synthesis and anti-influenza virus effects of novel substituted polycyclic pyridone derivatives modified from baloxavir. J. Med. Chem. 2021, 64, 14465–14476. [Google Scholar] [CrossRef]
- Miyagawa, M.; Akiyama, T.; Taoda, Y.; Takaya, K.; Takahashi Kageyama, C.; Tomita, K.; Yasuo, K.; Hattori, K.; Shano, S.; Yoshida, R.; et al. Synthesis and SAR study of carbamoyl pyridone bicycle derivatives as potentinhibitors of influenza cap-dependent endonuclease. J. Med. Chem. 2019, 62, 8101–8114. [Google Scholar] [CrossRef] [PubMed]

| Number | Structure | Number | Structure |
|---|---|---|---|
| I-1 |  C23H21N3O4; Mw: 403.43 | I-3 |  C23H19F2N3O4; Mw: 439.41 |
| I-2 |  C25H25N3O6; Mw: 463.48 | I-4 | 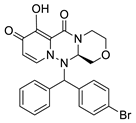 C23H20BrN3O4; Mw: 482.32 |
| Number | Structure | Number | Structure |
|---|---|---|---|
| II-1 |  C25H21N3O4; Mw: 427.45 | II-6 | 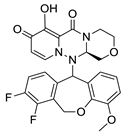 C25H21F2N3O6; Mw: 497.44 |
| II-2 |  C25H23N3O4; Mw: 429.46 | II-7 | 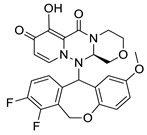 C25H21F2N3O6; Mw: 497.44 |
| II-3 | 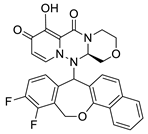 C28H21F2N3O5; Mw: 517.48 | II-8 |  C24H18F3N3O5; Mw: 485.41 |
| II-4 |  C28H21F2N3O5; Mw: 517.48 | II-9 |  C24H18F3N3O5; Mw: 485.41 |
| II-5 |  C24H19F2N3O5; Mw: 467.42 |
| Number | Structure | Number | Structure |
|---|---|---|---|
| III-1 |  C14H13ClN4O4S; Mw: 368.79 | III-5 |  C24H20N4O4; Mw: 428.44 |
| III-2 | 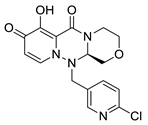 C16H15ClN4O4; Mw: 362.76 | III-6 | 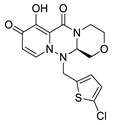 C15H14ClN3O4S; Mw: 367.80 |
| III-3 |  C21H20N4O5; Mw: 408.40 | III-7 |  C15H16N4O5; Mw: 332.31 |
| III-4 |  C16H18N4O5; Mw: 346.33 | III-8 |  C24H21N7O4; Mw: 471.47 |
| Compound | ka (1/Ms) | kd (1/s) | KD (M) | Rmax (RU) |
|---|---|---|---|---|
| Baloxavir | 689.0 | 0.04627 | 6.72 × 10−5 | 156.6 |
| I-1 | 338.0 | 0.0273 | 8.08 × 10−5 | 233.4 |
| I-2 | 248.7 | 0.008723 | 3.51 × 10−5 | 3.93 × 102 |
| I-3 | 138.7 | 0.03813 | 2.75 × 10−4 | 2.38 × 102 |
| I-4 | 194.8 | 0.05099 | 2.62 × 10−4 | 3.97 × 102 |
| II-1 | 332.3 | 0.008742 | 2.63 × 10−5 | 491.2 |
| II-2 | 275.5 | 0.01892 | 6.87 × 10−5 | 273.2 |
| II-3 | 596.3 | 0.07436 | 1.25 × 10−4 | 690.2 |
| II-4 | 2.587 | 7.15 × 10−6 | 2.76 × 10−6 | 2.18 × 104 |
| II-5 | 999.7 | 0.1065 | 1.07 × 10−4 | 144.4 |
| II-6 | 88.50 | 0.02391 | 2.70 × 10−4 | 374.5 |
| II-7 | 651.0 | 0.04824 | 7.41 × 10−5 | 127.6 |
| II-8 | 2.152 | 7.29 × 10−2 | 3.39 × 10−2 | 1573 |
| II-9 | 272.1 | 0.04264 | 1.57 × 10−4 | 3.23 × 102 |
| III-1 | 334.6 | 0.07437 | 2.22 × 10−4 | 3.77 × 102 |
| III-2 | 6.248 × 104 | 0.01521 | 2.43 × 10−7 | 5.19 × 10−2 |
| III-3 | 3103 | 0.08764 | 2.83 × 10−5 | 38.71 |
| III-4 | 4.936 × 105 | 0.08729 | 1.77 × 10−7 | 3.689 |
| III-5 | 848.4 | 0.03766 | 4.44 × 10−5 | 217.9 |
| III-6 | 843.4 | 0.07487 | 8.88 × 10−5 | 53.76 |
| III-7 | 6.220 × 104 | 0.001021 | 1.64 × 10−8 | 0.08219 |
| III-8 | 6568 | 0.0698 | 1.06 × 10−5 | 155.2 |
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| Baloxavir | 7.45 | II-7 | 5.46 |
| I-1 | 18.74 | II-8 | 6.83 |
| I-2 | 26.78 | II-9 | 8.54 |
| I-3 | 30.45 | III-1 | 0 |
| I-4 | 3.29 | III-2 | 0 |
| II-1 | 8.07 | III-3 | 13.7 |
| II-2 | 1.46 | III-4 | 0 |
| II-3 | 5.46 | III-5 | 7.37 |
| II-4 | 8.46 | III-6 | 50.55 |
| II-5 | 6.62 | III-7 | 0 |
| II-6 | 5.23 | III-8 | 6.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Wu, H.; Cui, L.; Meng, Z.; Xu, Z.; Zheng, Z.; Li, J. Synthesis and Cap-Dependent Endonuclease Inhibition of Baloxavir Derivatives. Crystals 2023, 13, 988. https://doi.org/10.3390/cryst13070988
Wang Y, Wang J, Wu H, Cui L, Meng Z, Xu Z, Zheng Z, Li J. Synthesis and Cap-Dependent Endonuclease Inhibition of Baloxavir Derivatives. Crystals. 2023; 13(7):988. https://doi.org/10.3390/cryst13070988
Chicago/Turabian StyleWang, Yiyun, Jiaru Wang, Hui Wu, Longyao Cui, Zihui Meng, Zhibin Xu, Zhonghui Zheng, and Jiarong Li. 2023. "Synthesis and Cap-Dependent Endonuclease Inhibition of Baloxavir Derivatives" Crystals 13, no. 7: 988. https://doi.org/10.3390/cryst13070988
APA StyleWang, Y., Wang, J., Wu, H., Cui, L., Meng, Z., Xu, Z., Zheng, Z., & Li, J. (2023). Synthesis and Cap-Dependent Endonuclease Inhibition of Baloxavir Derivatives. Crystals, 13(7), 988. https://doi.org/10.3390/cryst13070988






