Combustion Synthesis of MAX Phases: Microstructure and Properties Inherited from the Processing Pathway
Abstract
1. Introduction
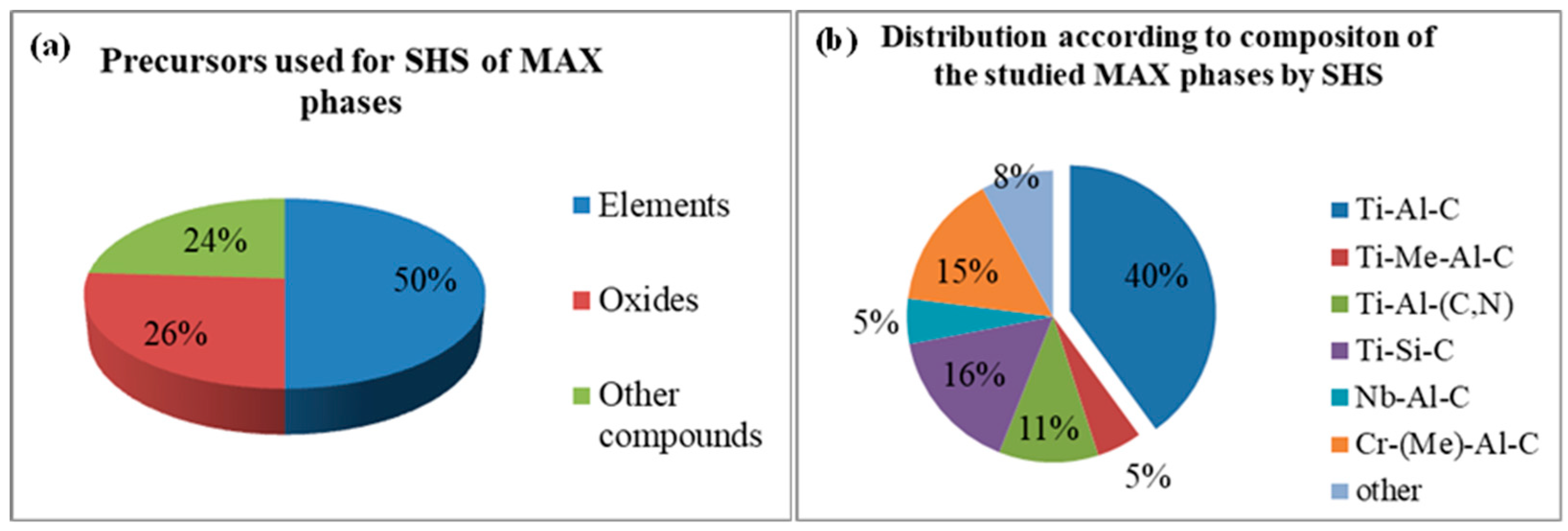
2. Precursors, Preparation of Green Bodies, Combustion Parameters, and Phase Composition
2.1. SHS from Elements
2.2. SHS from Elements and Compounds
2.3. SHS Metallothermy
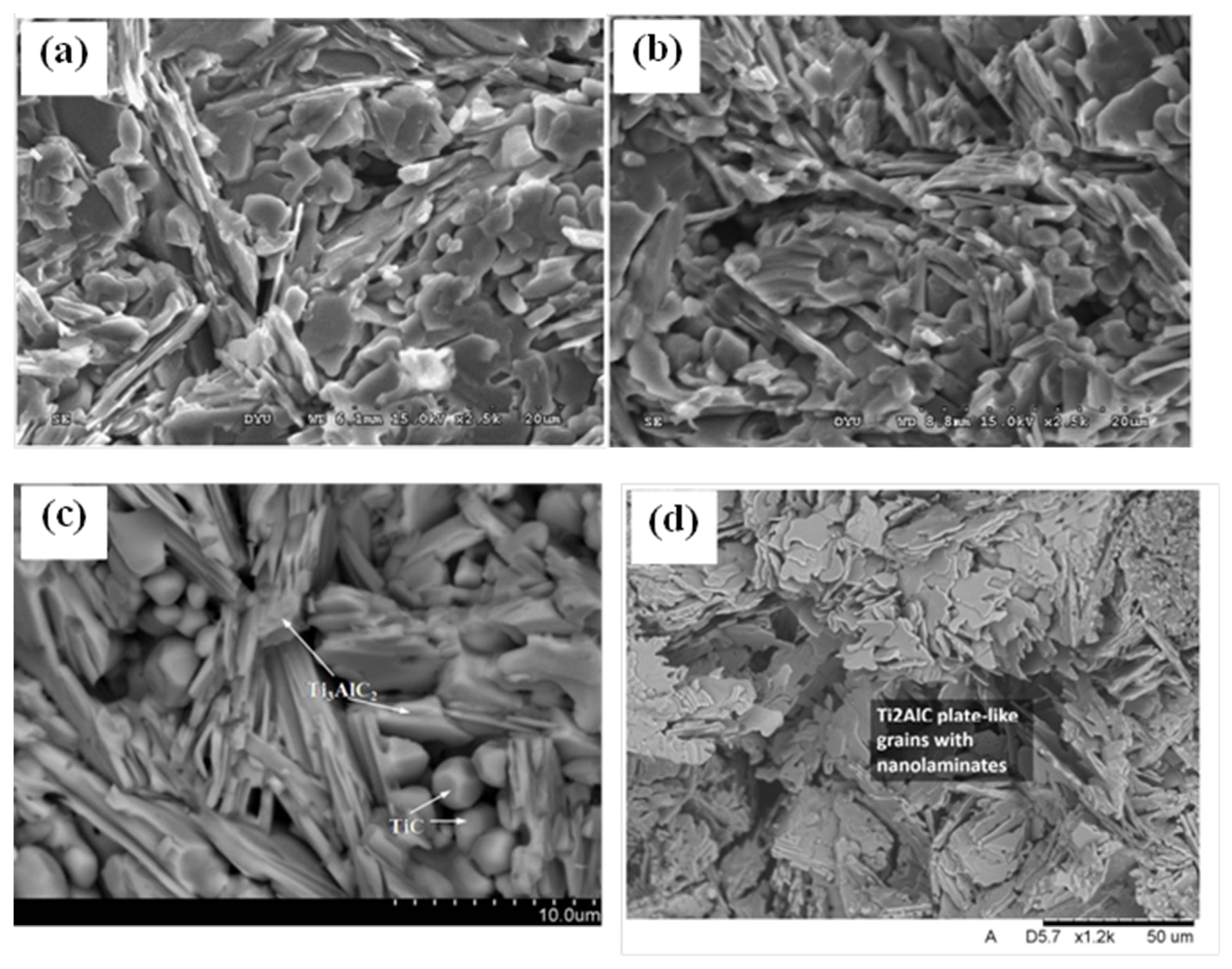
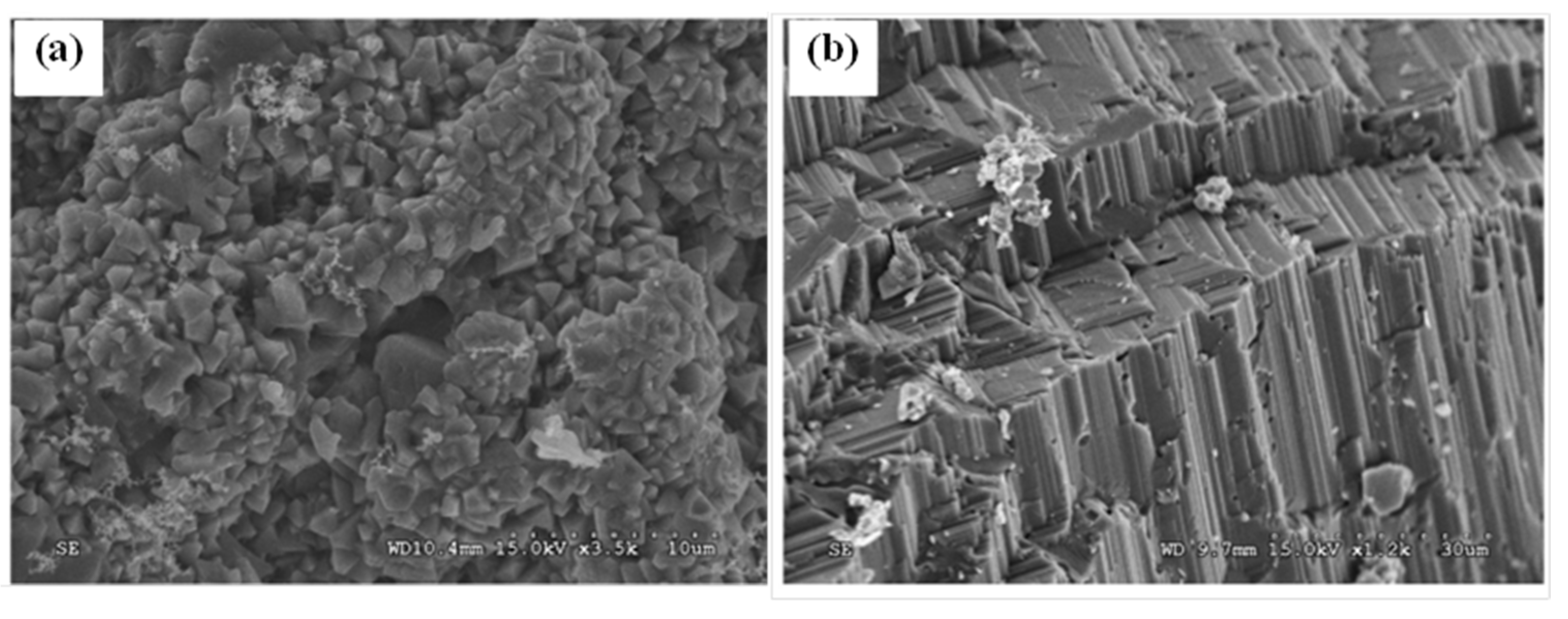
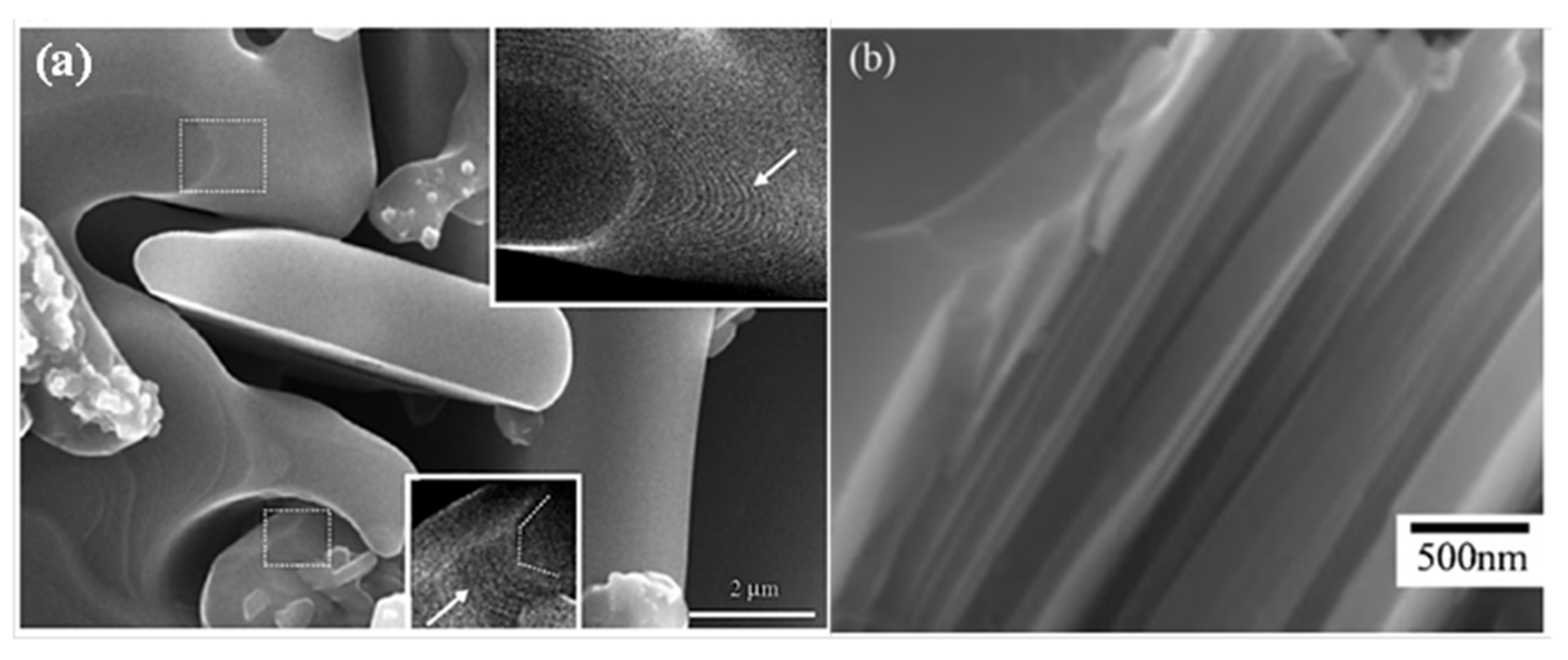
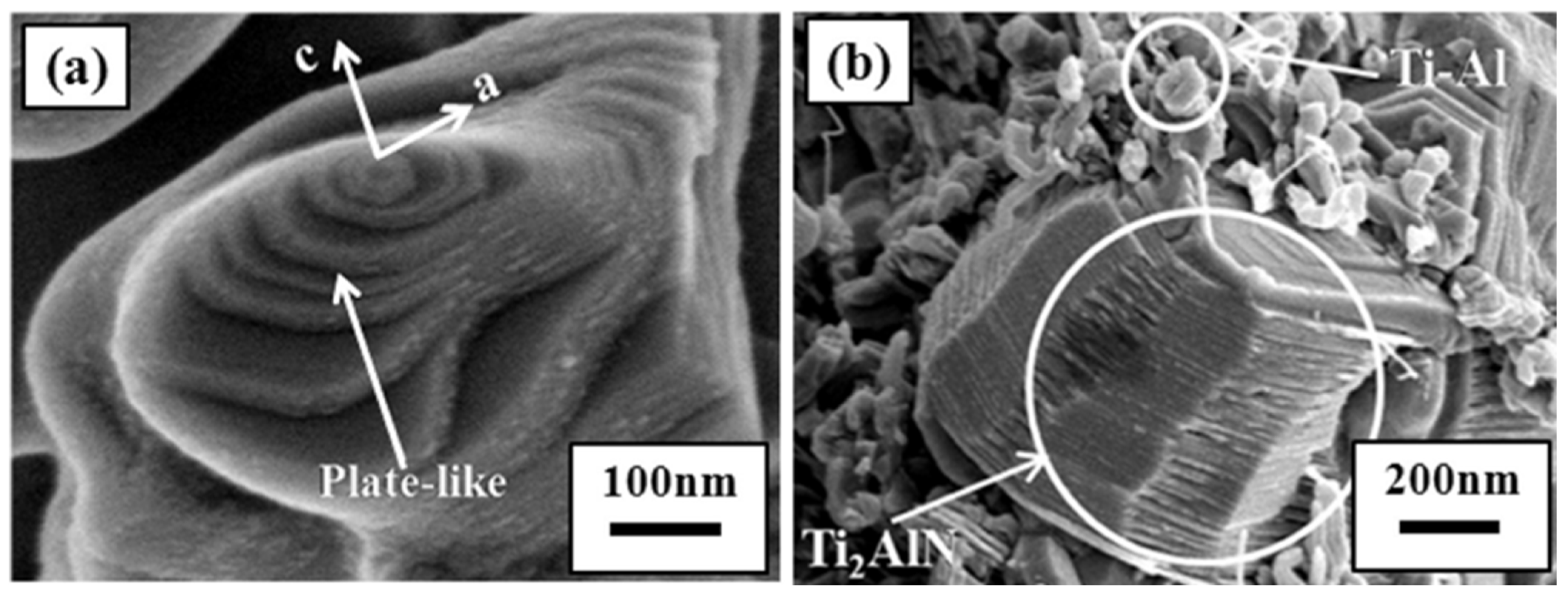
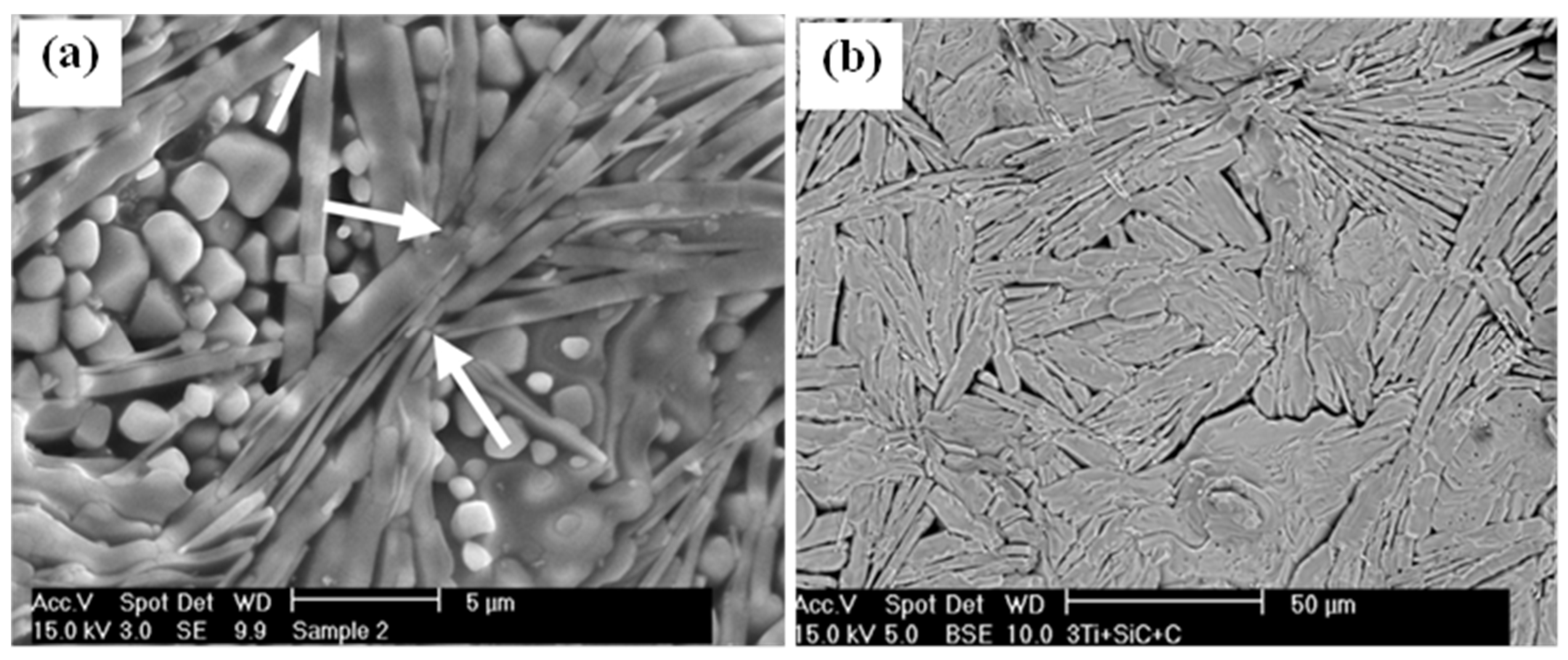
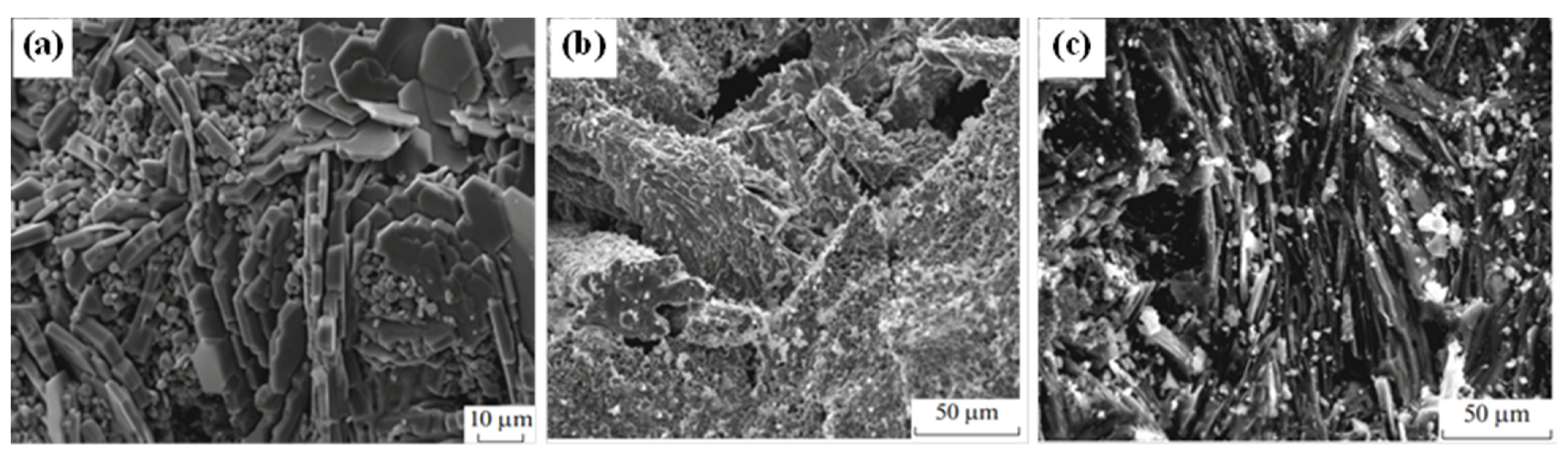
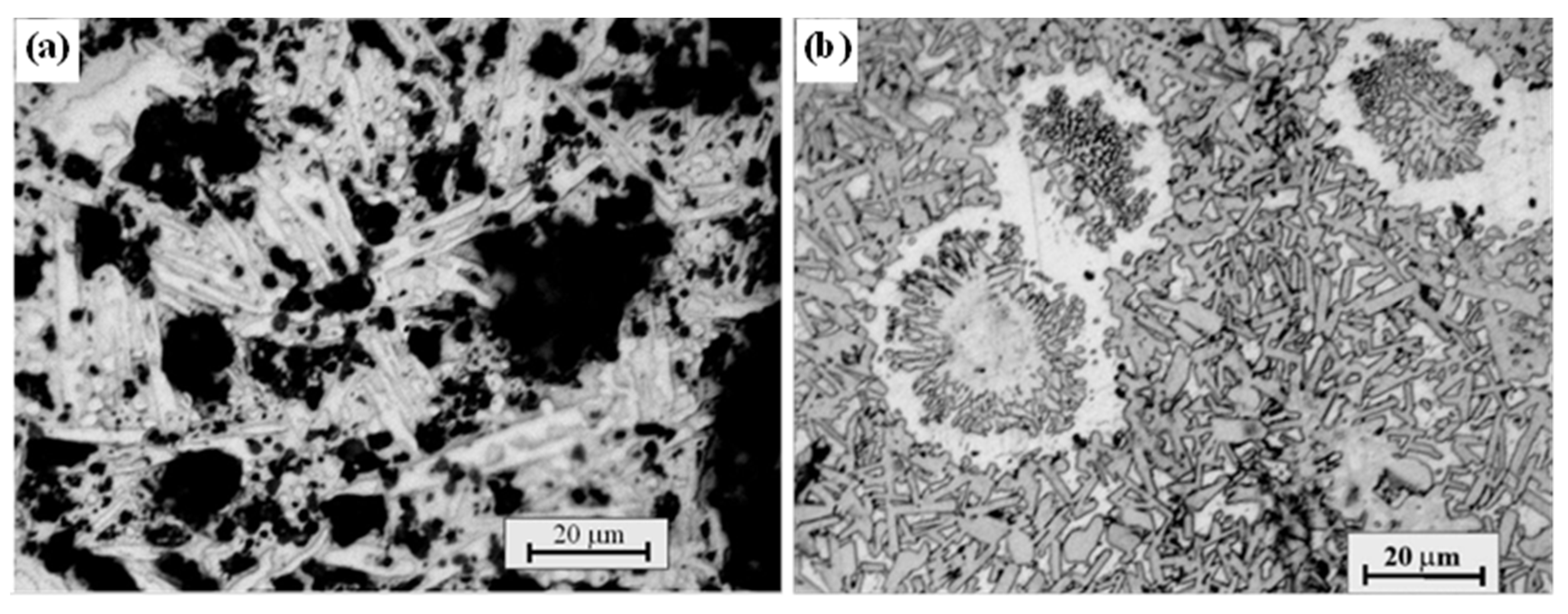
3. Crystal Structure and Microstructure Evolution
| Sample | a | c | Ref | Comment |
|---|---|---|---|---|
| (Ti0.6V0.4)2AlC | 2.998 | 13.476 | [66] | Diffraction lines of (Ti,V)2AlC shift to higher angles with increasing V content |
| (Ti0.5V0.5)2AlC | 2.981 | 13.432 | ||
| (Ti0.4V0.6)2AlC | 2.958 | 13.389 | ||
| (Ti0.3V0.7)2AlC | 2.946 | 13.348 | ||
| Ti2AlCx, | 3.040 | 13.632 | [12] | Carbon deficiency caused changes in the lattice parameters |
| Ti3AlC2x, | 3.066 | 18.536 | ||
| Cr2AlCx | 2.859 | 12.768 | ||
| Ti3AlC2 | 3.077 | 18.593 | [15] | Mechanical activation causes minor changes in the lattice spacing values |
| Ti3AlC2 − MA | 3.072 | 18.567 | ||
| Ti2AlC | 3.050 | 13.647 | [85] | Considering the high vapor pressure of Al in the raw material at high temperature, it is concluded that the derived material synthesized by SHS-PHIP is non-stoichiometric Ti2AlCx (x = 0.61445 |
| Ti3AlC2 | 3.071 | 18.536 | [87] | Due to the mutual partial displacement of the Ti and Cr atoms in the MAX phases, lattice spacing differs from the tabular value. |
| Cr2AlC | 2.856 | 12.858 | ||
| Ti3AlC2 | 3.075 | 18.567 | [88] | The relative contraction of the c parameter is 20 times larger than the contraction of the a parameter due to Al substitution by smaller Si atoms in the layered structure |
| Ti3SiC2 | 3.067 | 17.672 | ||
| Ti3Al0.5Si0.5C2 | 3.072 | 17.951 | ||
| Ti3AlC2 | 3.072 | 18.552 | [28] | Refined unit cell parameters of the SHS-grinding obtained sample match the literature data for Ti3AlC2 prepared by other methods |
| Ti3AlC2 | 3.066 | 18.525 | [89] | |
| Ti3AlC2 | 3.071 | 18.359 | [90] | |
| Ti2AlN0.25 | 2.989 | 13.654 | [56] | Both MAX phases have identical parameters regardless of the nitrogen content |
| Ti2AlN0.63 | 2.989 | 13.654 | ||
| Cr2AlC | 2.86 | 12.83 | [76] | The identical data is indicated in the common databases |
| Cr2AlC | 2.861 | 12.831 | [71] | Manganese content in the MAX phase was not estimated because the dependence of the unit cell parameters of this SS on the Mn content is obscure. |
| (CrxMn1−x)2AlC | 2.855 | 12.832 | ||
| V2AlC | 2.915 | 13.159 | [79] | V2AlC had narrow diffraction lines indicating a high degree of perfection of the crystal structure |
| Cr2GaC | 2.892 | 12.611 | [62] | The product crystallizes in the form of agglomerated anisotropic particles and their morphology differs from the conventionally prepared MAX phases |
4. Mechanical Properties
5. Summary and Outlook
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeitschko, W.; Nowotny, H. Die kristallstruktur von Ti3SiC2—Ein neuer komplexcarbid-typ. Monatshefte Chem.-Chem. Mon. 1967, 98, 329–337. [Google Scholar] [CrossRef]
- Pampuch, R.; Lis, J.; Stobierski, L.; Tymkiewicz, M. Solid combustion synthesis of Ti3SiC2. J. Eur. Ceram. Soc. 1989, 5, 283–287. [Google Scholar] [CrossRef]
- Barsoum, M.W.; El-Raghy, T. Synthesis and characterization of a remarkable ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. On the chemical diversity of the MAX phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, X.; Jia, D.; Zhou, Y.; van der Zwaag, S. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc. 2021, 41, 3851–3878. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W. MAX phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Merzhanov, A.G. History and recent developments in SHS. Ceram. Int. 1995, 21, 371–379. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Borovinskaya, I.P. Historical retrospective of SHS: An autoreview. Int. J. Self-Propagating High-Temp. Synth. 2008, 17, 242–265. [Google Scholar] [CrossRef]
- Borovinskaya, I.P.; Gromov, A.A.; Levashov, E.A.; Maksimov, Y.M.; Mukasyan, A.S.; Rogachev, A.S. (Eds.) Concise Encyclopedia of Self-Propagating High-Temperature Synthesis: History, Theory, Technology, and Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Thomas, T.; Bowen, C.R. Thermodynamic predictions for the manufacture of Ti2AlC MAX-phase ceramic by combustion synthesis. J. Alloys Compd. 2014, 602, 72–77. [Google Scholar] [CrossRef]
- Hendaoui, A.; Andasmas, M.; Amara, A.; Benaldjia, A.; Langlois, P.; Vrel, D. SHS of high-purity MAX compounds in the Ti-Al-C system. Int. J. Self-Propagating High-Temp. Synth. 2008, 17, 129–135. [Google Scholar] [CrossRef]
- Ying, G.-B.; He, X.-D.; Du, S.-Y.; Zhu, C.-C.; Zheng, Y.-T.; Wu, Y.-P.; Wang, C. Formation of Mn+1AX n phases in Ti–Cr–Al–C systems by self-propagating high-temperature synthesis. Rare Met. 2014, 33, 419–426. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, C.-A.; Ge, Z.; Wu, L. Preparation of Ti3AlC2 and Ti2AlC by self-propagating high-temperature synthesis. J. Mater. Sci. Lett. 2001, 20, 1971–1973. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y. Solid–liquid reaction synthesis of layered machinable Ti3AlC2 ceramic. J. Mater. Chem. 2002, 12, 455–460. [Google Scholar] [CrossRef]
- Potanin, A.; Loginov, P.; Levashov, E.; Pogozhev, Y.; Patsera, E.; Kochetov, N. Effect of mechanical activation on Ti3AlC2 MAX phase formation under self-propagating high-temperature synthesis. Eurasian Chem.-Technol. J. 2015, 17, 233–242. [Google Scholar] [CrossRef]
- Hendaoui, A.; Vrel, D.; Amara, A.; Langlois, P.; Andasmas, M.; Guerioune, M. Synthesis of high-purity polycrystalline MAX phases in Ti–Al–C system through mechanically activated self-propagating high-temperature synthesis. J. Eur. Ceram. Soc. 2010, 30, 1049–1057. [Google Scholar] [CrossRef]
- Shahin, N.; Kazemi, S.; Heidarpour, A. Mechanochemical synthesis mechanism of Ti3AlC2 MAX phase from elemental powders of Ti, Al and C. Adv. Powder Technol. 2016, 27, 1775–1780. [Google Scholar] [CrossRef]
- Liang, B.; Wang, M.; Li, X.; Sun, S.; Zou, Q.; Mu, Y.; Li, X. Synthesis of Ti2AlC by laser-induced self-propagating high-temperature sintering. J. Alloys Compds. 2010, 501.1, L1–L3. [Google Scholar] [CrossRef]
- Goc, K.; Prendota, W.; Chlubny, L.; Strączek, T.; Tokarz, W.; Borowiak, P.; Witulska, K.; Bućko, M.; Przewoźnik, J.; Lis, J. Structure, morphology and electrical transport properties of the Ti3AlC2 materials. Ceram. Int. 2018, 44, 18322–18328. [Google Scholar] [CrossRef]
- Sun, H.; Kong, X.; Yi, Z.; Wang, Q.; Liu, G. The difference of synthesis mechanism between Ti3SiC2 and Ti3AlC2 prepared from Ti/M/C (M=Al or Si) elemental powders by SHS technique. Ceram. Int. 2014, 40, 12977–12981. [Google Scholar] [CrossRef]
- Chumakov, Y.A.; Knyazeva, A.G. Combustion synthesis of composite in the Ti-Al-C powders mixture: Model and numerical simulation. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2021, 25, 17–35. [Google Scholar] [CrossRef]
- Khoptiar, Y.; Gotman, I. Ti2AlC ternary carbide synthesized by thermal explosion. Mater. Lett. 2002, 57, 72–76. [Google Scholar] [CrossRef]
- Ge, Z.; Chen, K.; Guo, J.; Zhou, H.; Ferreira, J.M.F. Combustion synthesis of ternary carbide Ti3AlC2 in Ti–Al–C system. J. Eur. Ceram. Soc. 2003, 23, 567–574. [Google Scholar] [CrossRef]
- Liu, G.; Chen, K.; Zhou, H.; Guo, J.; Ren, K.; Ferreira, J. Layered growth of Ti2AlC and Ti3AlC2 in combustion synthesis. Mater. Lett. 2007, 61, 779–784. [Google Scholar] [CrossRef]
- Chen, K.; Guo, J.; Fu, R.; Ferreira, J.M.F. Combustion synthesis ternary carbide Ti2AlC1−x powders. Mater. Sci. Forum 2004, 455, 191–195. [Google Scholar] [CrossRef]
- Amosov, A.P.; Latukhin, E.I.; Ryabov, A.M. Applying SHS for the fabrication of the Ti3SiC2–Ni composite. Russ. J. Non-Ferr. Met. 2019, 60, 555–565. [Google Scholar] [CrossRef]
- Koniuszewska, A.; Naplocha, K. Microwave assisted self-propagating high-temperature synthesis of Ti2AlC max phase. Compos. Theory Pract. 2016, 16, 109–112. [Google Scholar]
- Pazniak, A.; Bazhin, P.; Shplis, N.; Kolesnikov, E.; Shchetinin, I.; Komissarov, A.; Polčák, J.; Stolin, A.; Kuznetsov, D. Ti3C2Tx MXene characterization produced from SHS-ground Ti3AlC2. Mater. Des. 2019, 183, 108143. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tayebifard, S.A.; Salahi, E.; Asl, M.S.; Schmidt, G. Self-propagating high-temperature synthesis of Ti3AlC2 MAX phase from mechanically-activated Ti/Al/graphite powder mixture. Ceram. Int. 2018, 44, 9671–9678. [Google Scholar] [CrossRef]
- Averichev, O.A.; Prokopets, A.D.; Stolin, P.A. Structure formation in Ti/Ti–Al–C layered ceramic materials obtained by the method of unconfined SHS compaction. Refract. Ind. Ceram. 2019, 60, 219–222. [Google Scholar] [CrossRef]
- Dmitruk, A.; Naplocha, K. Development of Al-Ti-C porous structures for reinforcing aluminum matrix composites. Compos. Theory Pract. 2022, 22, 172–177. [Google Scholar]
- Amosov, A.P.; Latukhin, E.I.; Petrov, P.A.; Amosov, E.A.; Novikov, V.A.; Yu, A. Illarionov Self-propagating high-temperature synthesis of boron-containing MAX-phase. Key Eng. Mater. 2017, 746, 207–213. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, J.H. Combustion synthesis of (Ti1−xNbx2AlC solid solutions from elemental and Nb2O5/Al4C3-containing powder compacts. Ceram. Int. 2011, 37, 3089–3094. [Google Scholar] [CrossRef]
- Lepakova, O.K.; Karakchieva, N.I.; Golobokov, N.N.; Gal’chenko, N.K.; Afanas’ev, N.I. High-temperature synthesis of Ti–Si–B and Ti–Al–B composites and coatings. Int. J. Self-Propagating High-Temp. Synth. 2020, 29, 150–156. [Google Scholar] [CrossRef]
- Khoptiar, Y.; Gotman, I. Synthesis of dense Ti3SiC2-based ceramics by thermal explosion under pressure. J. Eur. Ceram. Soc. 2003, 23, 47–53. [Google Scholar] [CrossRef]
- Feng, A.; Orling, T.; Munir, Z.A. Field-activated pressure-assisted combustion synthesis of polycrystalline Ti3SiC2. J. Mater. Res. 1999, 14, 925–939. [Google Scholar] [CrossRef]
- Amosov, A.P.; Latukhin, E.I.; Ryabov, A.M.; Umerov, E.R.; Novikov, V.A. Application of SHS process for fabrication of copper-titanium silicon carbide composite (Cu-Ti3SiC2). J. Phys. Conf. Ser. 2018, 1115, 042003. [Google Scholar] [CrossRef]
- Lis, J.; Chlubny, L.; Witulska, K.; Borowiak, P.; Kozak, K.; Misztal, A. SHS of Ti 3 SiC 2-Based Materials in the Ti–Si–C System: Impact of Silicon Excess. Int. J. Self-Propagating High-Temp. Synth. 2019, 28, 262–265. [Google Scholar] [CrossRef]
- Afanasyev, N.I.; Lepakova, O.K.; Kitler, V.D. Non-isothermal synthesis of materials based on the MAX phases in the Ti-Si-C and Nb-Al-C systems. J. Phys. Conf. Ser. 2020, 1459, 012008. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Effects of Al content on formation of Ta2AlC by self-propagating high-temperature synthesis. J. Alloys Compd. 2009, 482, 219–223. [Google Scholar] [CrossRef]
- Inokawa, H.; Ishida, K.; Tomoshige, R.; Hokamoto, K.; Tanaka, S. Effect of Added Molybdenum on Material Properties of Zr2SC MAX Phase Produced by Self-Propagating High Temperature Synthesis. Mater. Res. Proc. 2019, 13, 79–84. [Google Scholar]
- Kovalev, D.Y.; Luginina, M.A.; Vadchenko, S.G. X-ray diffraction study of self-propagating high-temperature synthesis in the Zr–Al–C system. Russ. J. Inorg. Chem. 2017, 62, 1638–1644. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Effects of using Al4C3 as a reactant on formation of Ti3AlC2 by combustion synthesis in SHS mode. J. Alloys Compd. 2009, 473, 408–413. [Google Scholar] [CrossRef]
- Łopaciński, M.; Puszynski, J.; Lis, J. Synthesis of ternary titanium aluminum carbides using self-propagating high-temperature synthesis technique. J. Am. Ceram. Soc. 2001, 84, 3051–3053. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Effects of TiC and Al4C3 addition on combustion synthesis of Ti2AlC. J. Alloys Compd. 2009, 470, 424–428. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Chiang, C.H. Combustion synthesis of MAX phase solid solution Ti3(Al, Sn)C2. Nano Hybrids Compos. 2017, 16, 73–76. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Chabior, K.; Chachlowska, P.; Kapusta, C. Processing and properties of MAX phases–based materials using SHS technique. Arch. Metall. Mater. 2015, 60, 859–863. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, Y.C. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: A review. J. Mater. Sci. Technol. 2010, 26, 385–416. [Google Scholar]
- Chlubny, L.; Lis, J.; Bućko, M.M. SHS Synthesis of the Materials in the Ti-Al-CN System Using Intermetallics. Adv. Sci. Technol. 2006, 45, 1047–1051. [Google Scholar]
- Yeh, C.L.; Kuo, C.W.; Wu, F.S. Formation of Ti2AlC0.5N0.5 solid solutions by combustion synthesis of Al4C3-containing samples in nitrogen. J. Alloys Compd. 2010, 508, 324–328. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, F.; Cui, H.; Zhang, L.; Guo, S. Synthesis and microstructure of Ti2AlN ceramic by thermal explosion. Ceram. Int. 2017, 43, 13618–13621. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Xiao, W.; Zhang, L.; Pu, Y.; Guo, S. Rapid synthesis of Ti2AlN ceramic via thermal explosion. Mater. Lett. 2015, 149, 5–7. [Google Scholar] [CrossRef]
- Chlubny, L.; Lis, J.; Borowiak, P.; Chabior, K.; Zieleńska, K. Densification and Phase Evolution of SHS Derived Ti2AlNActive Precursor Powders During Hot Pressing Processes. Dev. Strateg. Ceram. Mater. II Ceram. Eng. Sci. Proc. 2017, 37, 211–221. [Google Scholar]
- Tian, J.; Zhai, F.; Zhang, L.; Liu, G.; Ding, Z. Effect of N2 pressure on the phase composition and morphology of Ti2AlN prepared by self-propagating combustion method. Adv. Mater. Res. 2013, 710, 199–202. [Google Scholar] [CrossRef]
- Yeh, C.-L.; Kuo, C.-W.; Wu, F.-S. Formation of Ti2AlN by Solid–Gas Combustion Synthesis with AlN-and TiN-Diluted Samples in Nitrogen. Int. J. Appl. Ceram. Technol. 2010, 7, 730–737. [Google Scholar] [CrossRef]
- Aleksanyan, A.G.; Dolukhanyan, S.K.; Mayilyan, D.G.; Muradyan, G.N.; Ter-Galstyan, O.P.; Mnatsakanyan, N.L. Formation of Ti2AlNx MAX phase by “Hydride Cycle” and SHS methods. Ceram. Int. 2022, 49, 24229–24234. [Google Scholar] [CrossRef]
- Bolotskaia, A.V.; Mikheev, M.V.; Bazhin, P.M.; Stolin, A.M. The influence of aluminum nitride nanoparticles on the structure, phase composition, and properties of TiB/Ti-based materials obtained by SHS extrusion. Inorg. Mater. Appl. Res. 2019, 10, 1191–1195. [Google Scholar] [CrossRef]
- Lis, J.; Chlubny, L.; Łopaciński, M.; Stobierski, L.; Bućko, M.M. Ceramic nanolaminates—Processing and application. J. Eur. Ceram. Soc. 2008, 28, 1009–1014. [Google Scholar] [CrossRef]
- Riley, D.P.; Kisi, E.H.; Wu, E.; McCallum, A. Self-propagating high-temperature synthesis of Ti3SiC2 from 3Ti+ SiC+ C reactants. J. Mater. Sci. Lett. 2003, 22, 1101–1104. [Google Scholar] [CrossRef]
- Riley, D.P.; Kisi, E.H.; Hansen, T.C.; Hewat, A.W. Self-propagating high-temperature synthesis of Ti3SiC2: I, Ultra-High-speed neutron diffraction study of the reaction mechanism. J. Am. Ceram. Soc. 2002, 85, 2417–2424. [Google Scholar] [CrossRef]
- Dmitruk, A.; Naplocha, K.; Lagos, M.; Egizabal, P.; Grzęda, J. Microwave assisted self-propagating high-temperature synthesis of Ti3SiC2 MAX phase. Compos. Theory Pract. 2018, 18, 241–244. [Google Scholar]
- Siebert, J.P.; Bischoff, L.; Lepple, M.; Zintler, A.; Molina-Luna, L.; Wiedwald, U.; Birkel, C.S. Sol–gel based synthesis and enhanced processability of MAX phase Cr2GaC. J. Mater. Chem. C 2019, 7, 6034–6040. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, C.W.; Chu, Y.C. Formation of Ti3AlC2/Al2O3 and Ti2AlC/Al2O3 composites by combustion synthesis in Ti–Al–C–TiO2 systems. J. Alloys Compd. 2010, 494, 132–136. [Google Scholar] [CrossRef]
- Vershinnikov, V.I.; Kovalev, D.Y. Preparation of Ti2AlC and Ti3AlC2 MAX Phases by Self-Propagating High-Temperature Synthesis with the Reduction Stage. Russ. J. Non-Ferr. Met. 2020, 61, 554–558. [Google Scholar] [CrossRef]
- Vershinnikov, V.I.; Kovalev, D.Y. Synthesis of the Ti2AlC MAX phase with a reduction step via combustion of a TiO2+ Mg+ Al+ C mixture. Inorg. Mater. 2018, 54, 949–952. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yang, W.J. Formation of MAX solid solutions (Ti,V)2AlC and (Cr,V)2AlC with Al2O3 addition by SHS involving aluminothermic reduction. Ceram. Int. 2013, 39, 7537–7544. [Google Scholar] [CrossRef]
- Kovalev, I.D.; Miloserdov, P.A.; Gorshkov, V.A.; Kovalev, D.Y. Synthesis of Nb2AlC MAX phase by SHS metallurgy. Russ. J. Non-Ferr. Met. 2020, 61, 126–131. [Google Scholar] [CrossRef]
- Miloserdov, P.A.; Gorshkov, V.A.; Kovalev, I.D.; Kovalev, D.Y. High-temperature synthesis of cast materials based on Nb2AlC MAX phase. Ceram. Int. 2019, 45, 2689–2691. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, C.W. An investigation on formation of Nb2AlC by combustion synthesis of Nb2O5–Al–Al4C3 powder compacts. J. Alloys Compd. 2010, 496, 566–571. [Google Scholar] [CrossRef]
- Yeh, C.L.; Kuo, C.W. Effects of Al and Al4C3 contents on combustion synthesis of Cr2AlC from Cr2O3–Al–Al4C3 powder compacts. J. Alloys Compd. 2011, 509, 651–655. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Khomenko, N.Y.; Miloserdova, O.M. High-temperature synthesis of composite materials based on (Cr, Mn, V)–Al–C MAX phases. Ceram. Int. 2021, 47, 25821–25825. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Karpov, A.V.; Shchukin, A.S.; Sytschev, A.E. Investigation of the composition and properties of a Cr2AlC MAX phase-based material prepared by metallothermic SHS. Phys. Met. Metallogr. 2019, 120, 471–475. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Khomenko, N.Y.; Kovalev, D.Y. The Synthesis of Cast Materials Based on the MAX Phases in a Cr–Ti–Al–C System. Russ. J. Non-Ferr. Met. 2021, 62, 732–739. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Karpov, A.V.; Kovalev, D.Y.; Sychev, A.E. Synthesis, Structure and Properties of Material Based on V2AlC MAX Phase. Phys. Met. Metallogr. 2020, 121, 765–771. [Google Scholar] [CrossRef]
- Miloserdov, P.A.; Yukhvid, V.I.; Gorshkov, V.A.; Kovalev, I.D. Aluminothermic SHS in CaCrO4–Al–C Mixtures under Nitrogen Pressure. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 123–126. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Luginina, M.A.; Sachkova, N.V.; Belikova, A.F. High-temperature synthesis of a cast material with a maximum content of the MAX phase Cr2AlC. Inorg. Mater. 2017, 53, 271–277. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yang, W.J. Effects of co-reduction of Cr2O3 and V2O5 on combustion synthesis of (Cr1−xVx)2AlC/Al2O3 solid solution composites. J. Alloys Compd. 2014, 608, 292–296. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, Y.S. Effects of Al content on formation of TaC, Ta2C, and Ta2AlC by combustion synthesis with aluminothermic reactions. Ceram. Int. 2017, 43, 15659–15665. [Google Scholar] [CrossRef]
- Vershinnikov, V.I.; Kovalev, D.Y. Formation of V2AlC MAX phase by SHS involving magnesium reduction of V2O5. Ceram. Int. 2023, 49, 6063–6067. [Google Scholar] [CrossRef]
- Gorshkov, V.A.; Miloserdov, P.A.; Sachkova, N.V. High-Temperature Synthesis of Cast Materials Based on the MAX Phase Cr2AlC Using CaCrO4+ Al+ C Mixtures. Inorg. Mater. 2020, 56, 321–327. [Google Scholar] [CrossRef]
- Kovalev, D.Y.; Gorshkov, V.A.; Boyarchenko, O.D. High-Temperature Synthesis of Mo3Al2C-Based Materials via Combustion of MoO3+ Al+ C+ Al2O3 Powder Mixtures. Inorg. Mater. 2022, 58, 939–947. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Maksakova, O.V.; Buitkenov, D.B.; Kylyshkanov, M.K.; Pogrebnjak, A.D.; Antypenko, V.P.; Konoplianchenko, Y.V. Structural-phase and tribo-corrosion properties of composite Ti3SiC2/TiC MAX-phase coatings: An experimental approach to strengthening by thermal annealing. Appl. Phys. A 2022, 128, 145. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; He, X.; Zhu, C.; Wang, R.; Sun, Y.; Chen, G.; Xiao, P. Growth morphology and microstructural characterization of nonstoichiometric Ti2AlC bulk synthesized by self-propagating high temperature combustion synthesis with pseudo hot isostatic pressing. Int. J. Refract. Met. Hard Mater. 2014, 45, 58–63. [Google Scholar] [CrossRef]
- Bai, Y.; He, X.; Li, Y.; Zhu, C.; Zhang, S. Rapid synthesis of bulk Ti2AlC by self-propagating high temperature combustion synthesis with a pseudo–hot isostatic pressing process. J. Mater. Res. 2009, 24, 2528–2535. [Google Scholar] [CrossRef]
- Stolin, A.M.; Bazhin, P.M. Manufacture of multipurpose composite and ceramic materials in the combustion regime and high-temperature deformation (SHS extrusion). Theor. Found. Chem. Eng. 2014, 48, 751–763. [Google Scholar] [CrossRef]
- Ying, G.; He, X.; Li, M.; Li, Y.; Du, S. Synthesis and mechanical properties of nano-layered composite. J. Alloys Compd. 2010, 506, 734–738. [Google Scholar] [CrossRef]
- Goc, K.; Przewoźnik, J.; Witulska, K.; Chlubny, L.; Tokarz, W.; Strączek, T.; Michalik, J.M.; Jurczyk, J.; Utke, I.; Lis, J.; et al. Structure, Morphology, Heat Capacity, and Electrical Transport Properties of Ti3(Al, Si)C2 Materials. Materials 2021, 14, 3222. [Google Scholar] [CrossRef]
- Zhu, C.-C.; Zhu, J.; Wu, H.; Lin, H. Synthesis of Ti3AlC2 by SHS and thermodynamic calculation based on first principles. Rare Met. 2015, 34, 107–110. [Google Scholar] [CrossRef]
- Kong, F.; He, X.; Liu, Q.; Qi, X.; Zheng, Y.; Wang, R.; Bai, Y. Effect of Ti3AlC2 precursor on the electrochemical properties of the resulting MXene Ti3C2 for Li-ion batteries. Ceram. Int. 2018, 44, 11591–11596. [Google Scholar] [CrossRef]
- Pietzka, M.A.; Schuster, J.C. Summary of constitutional data on the aluminum-carbon-titanium system. J. Phase Equilibria 1994, 15, 392–400. [Google Scholar] [CrossRef]


| System | Compaction Method | Density (Theoretical/Relative) | Hardness | Fracture Toughness | Ref |
|---|---|---|---|---|---|
| (Ti,V)2AlC/Al2O3 | SHS metallothermy | 4.12–4.31 g/cm3 | 4.8 GPa | 7.2 MPam1/2 | [66] |
| (Cr,V)2AlC/Al2O3 | 4.53–4.78 g/cm3 | 6.3 GPa | 9.7 MPam1/2 | ||
| Ti2AlC/Ti3AlC2/TiC | SHS/PHIP | 4.08 g/cm3/97% | 4.6 GPa | 6.6 MPam1/2 | [12] |
| Cr2AlC/TiC/Al8Cr5/Cr2Al | 4.64g/cm3/93% | 10.1GPa | 5.1 MPam1/2 | ||
| Ti2AlC | SHS/HP | 2.96g/cm3/75% | 0.62 GPa | - | [47] |
| Ti3AlC2 | 4.2g/cm3/99% | 4.22 GPa | - | ||
| Ti2AlC | SHS/PHIP | 3.99g/cm3/98% | - | - | [84] |
| Ti-Al-C | SHS extrusion | - | 4–4.5 GPa | - | [86] |
| Ti2AlC0.69 | SHS/PHIP | 97% | 5.8 GPa | 6.5 MPam1/2 | [85] |
| Ti3AlC2, Cr2AlC and TiC | SHS/PHIP | 4.55 g/cm3 | 10.53 GPa | 6.23 MPam1/2 | [87] |
| (Ti1−xNbx)2AlC SS | SHS metallothermy | 3.4–4.2 g/cm3 | - | - | [33] |
| (Ti1−xNbx)2AlC SS with alumina | 4.3–5.6 g/cm3 | - | - | ||
| Ti3(Al,Si)C2 | SHS/HP | - | - | - | [88] |
| Ti2AlC | Pressure assisted TE | 98% | 6.4 GPa | - | [22] |
| Ti2AlN | SHS/HP | 4.2 g/cm3 | - | - | [53] |
| Ti4AlN3,Ti2AlN | SHS/HP | 4.6 g/cm3 | - | - | |
| Ti2AlN0.25 | SHS/HC | 3.96 g/cm3 | - | - | [56] |
| Ti2AlN0.63 | SHS/HC | 3.03 g/cm3 | - | - | |
| Ti2AlN and Ti4AlN3 | SHS extrusion | - | 1288–1682 kgf/mm2 | - | [57] |
| Ti3SiC2, TiC | SHS/TE (reactive forging) | >95% | 4.6 GPa | - | [22] |
| Ti3SiC2 | Field activated pressure assisted SHS | 4.53 g/cm3 | 6–7 GPa | - | [36] |
| Ti3SiC2 | SHS/HP | - | 5.8 GPa | 7–8.2 MPam1/2 | [58] |
| Ti3SiC2,TiC | SHS/HP | 4.5 g/cm3 | - | - | [38] |
| Cr2AlC, Cr5Al8 | SHS metallothermy | - | 412–613 kg/mm2 | - | [71] |
| (Cr,V)2AlC | SHS metallothermy | - | 6.9 GPa | - | [72] |
| Zr2SC | SHS | - | 2.5 GPa | - | [41] |
| Zr1.4Mo0.6SC | SHS | - | 4.0 GPa | - | |
| V2AlC | SHS metallothermy | 4.85 g/cm3 | - | - | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydinyan, S. Combustion Synthesis of MAX Phases: Microstructure and Properties Inherited from the Processing Pathway. Crystals 2023, 13, 1143. https://doi.org/10.3390/cryst13071143
Aydinyan S. Combustion Synthesis of MAX Phases: Microstructure and Properties Inherited from the Processing Pathway. Crystals. 2023; 13(7):1143. https://doi.org/10.3390/cryst13071143
Chicago/Turabian StyleAydinyan, Sofiya. 2023. "Combustion Synthesis of MAX Phases: Microstructure and Properties Inherited from the Processing Pathway" Crystals 13, no. 7: 1143. https://doi.org/10.3390/cryst13071143
APA StyleAydinyan, S. (2023). Combustion Synthesis of MAX Phases: Microstructure and Properties Inherited from the Processing Pathway. Crystals, 13(7), 1143. https://doi.org/10.3390/cryst13071143





