Crystallization of Intermetallic Phases Fe2Si, Fe5Si3 for High Alloyed Cast Irons
Abstract
1. Introduction
2. Materials and Methods
3. Results
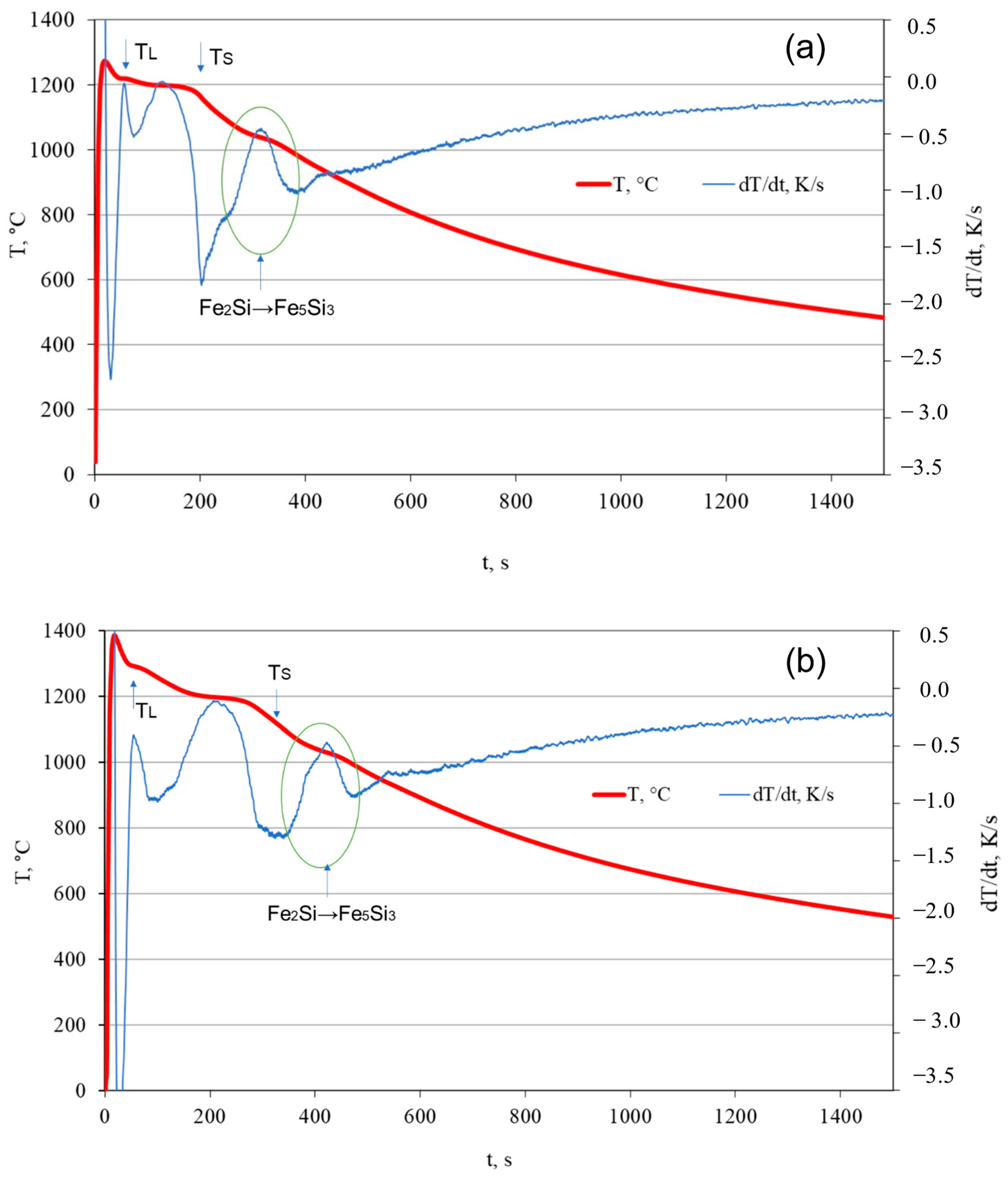
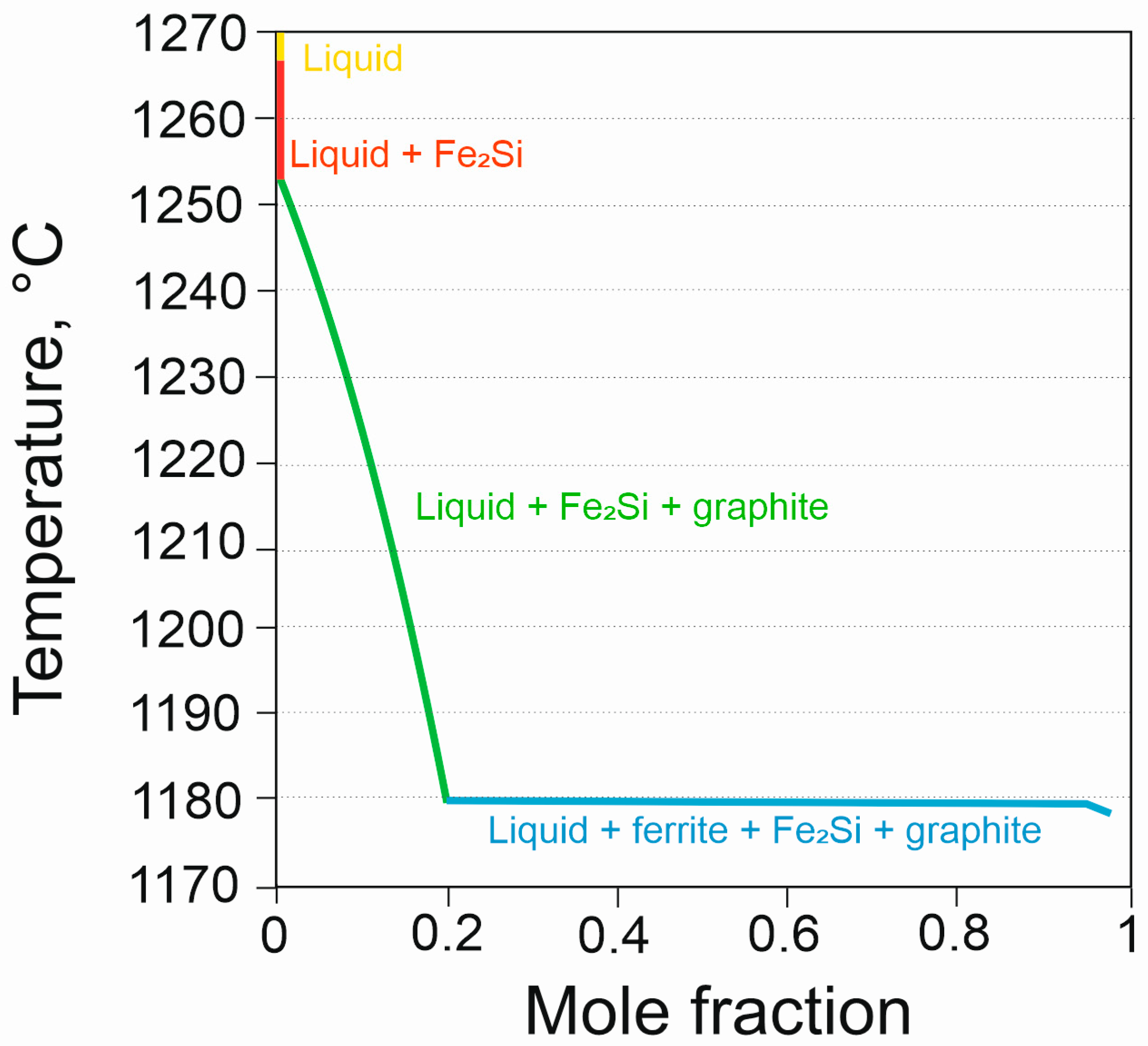
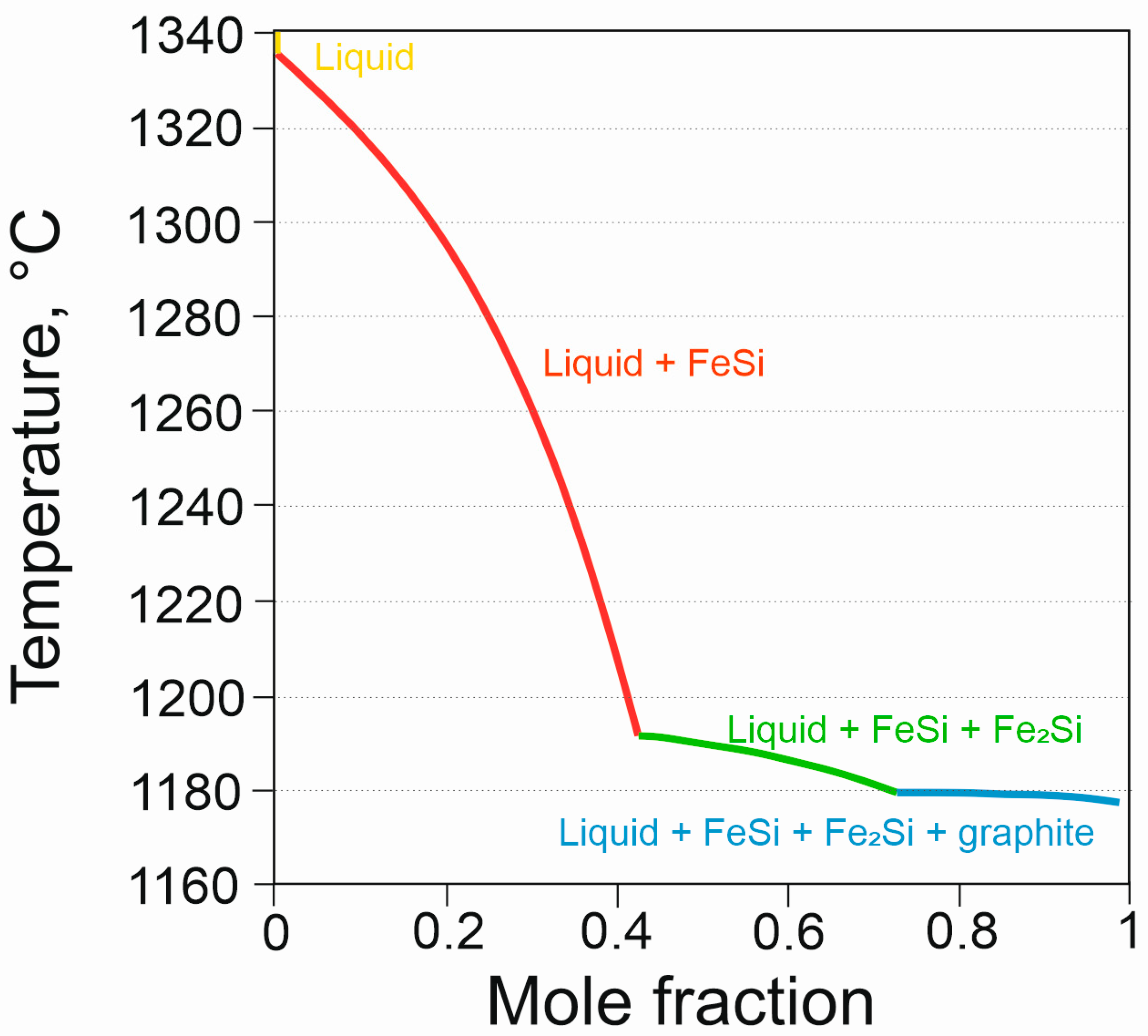
3.1. TDA Analysis
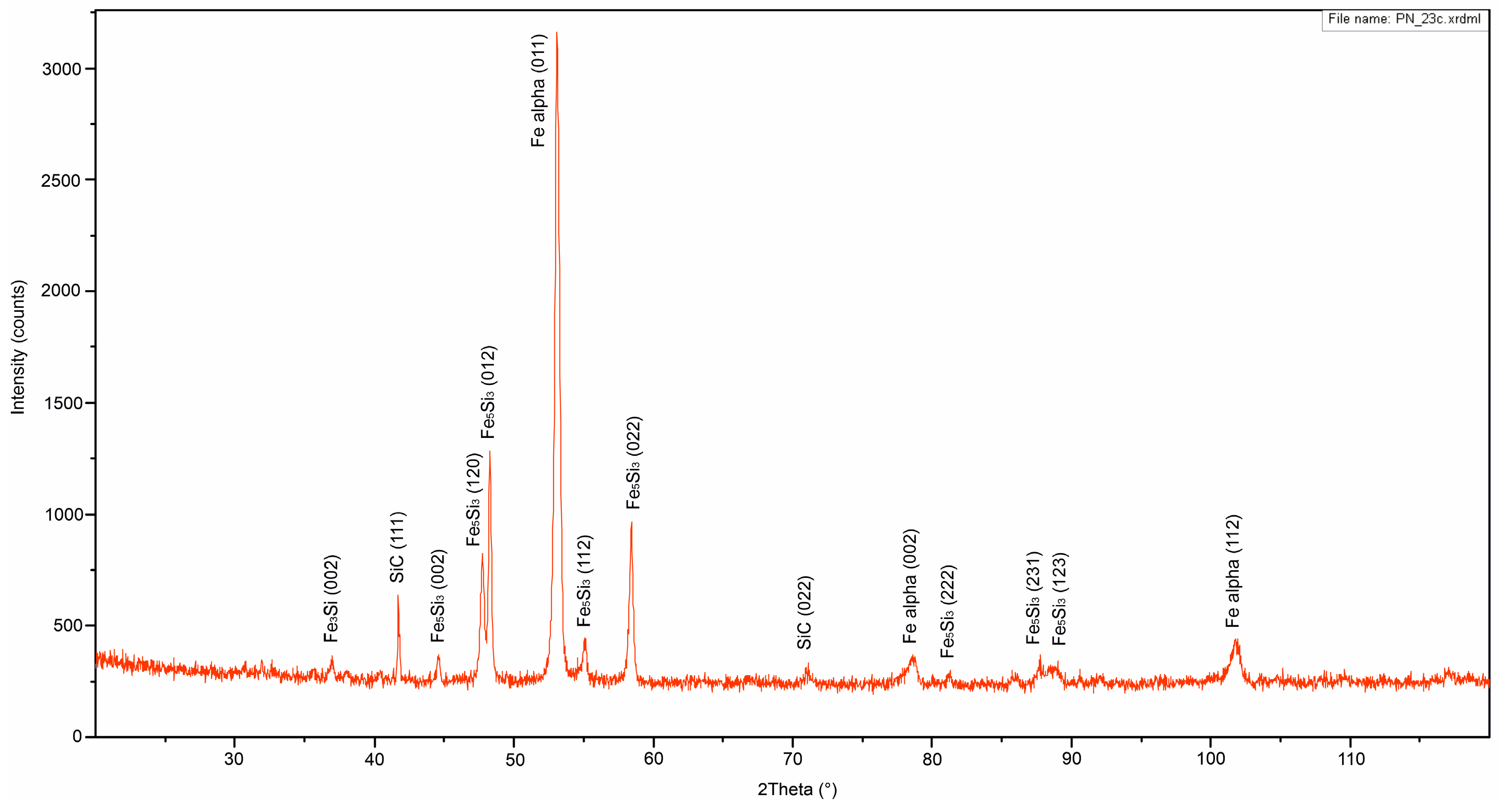
3.2. XRD Analysis
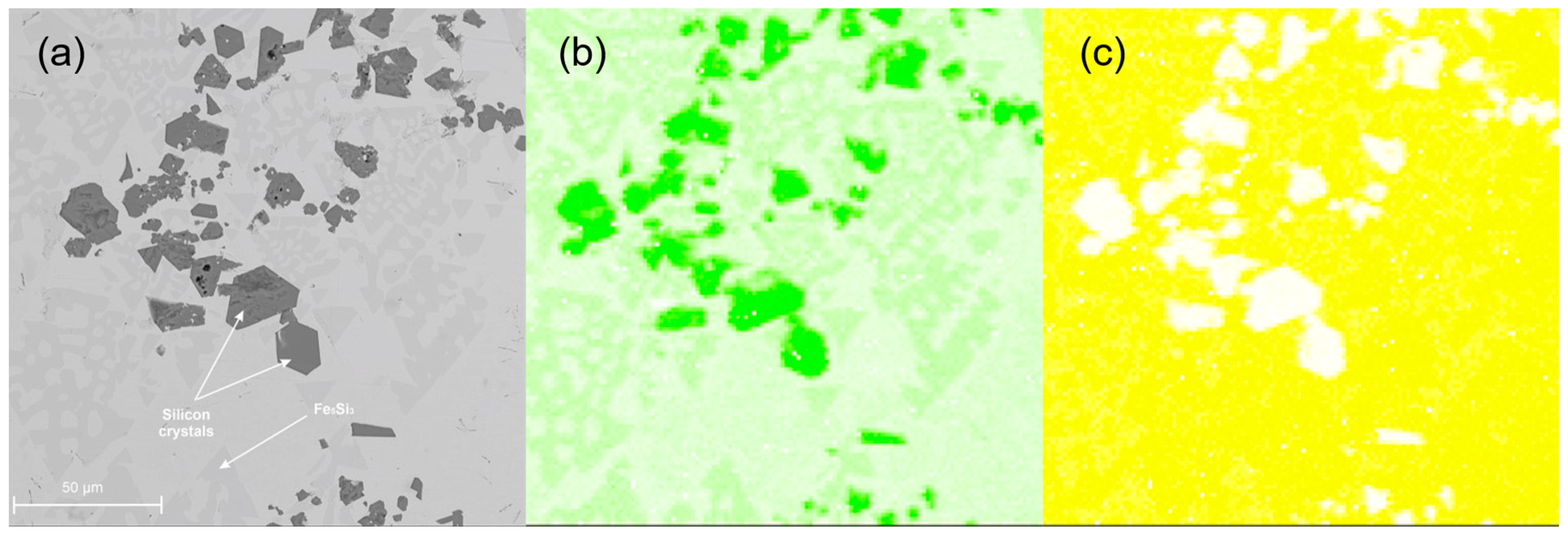
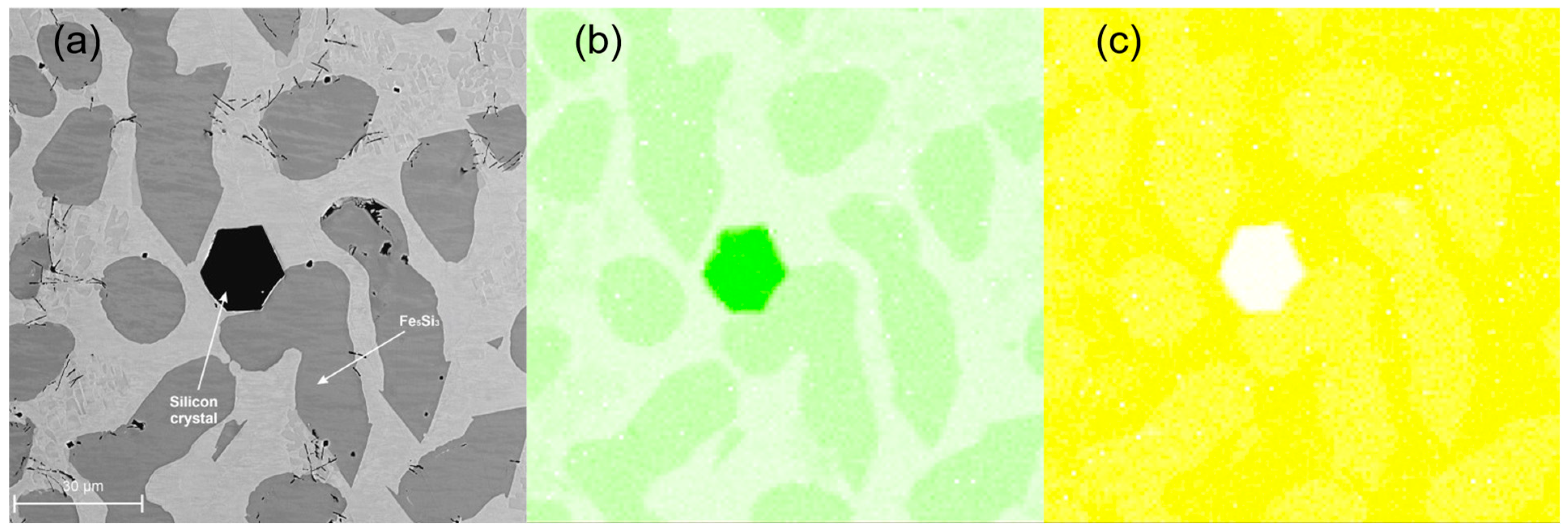
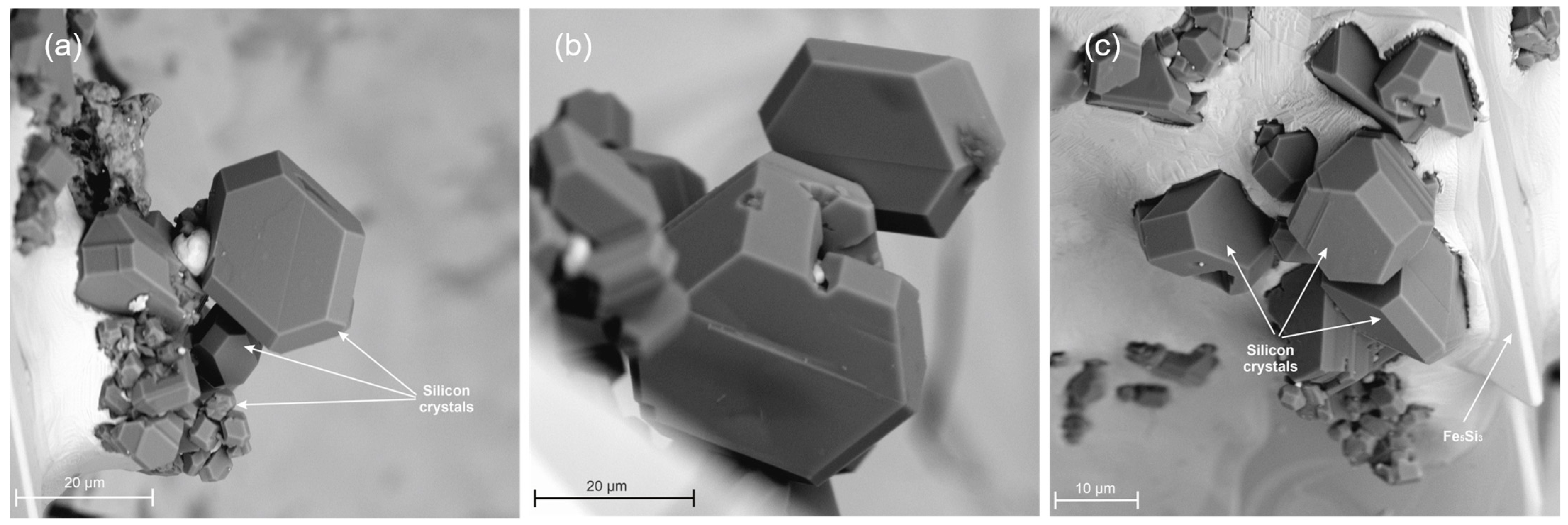
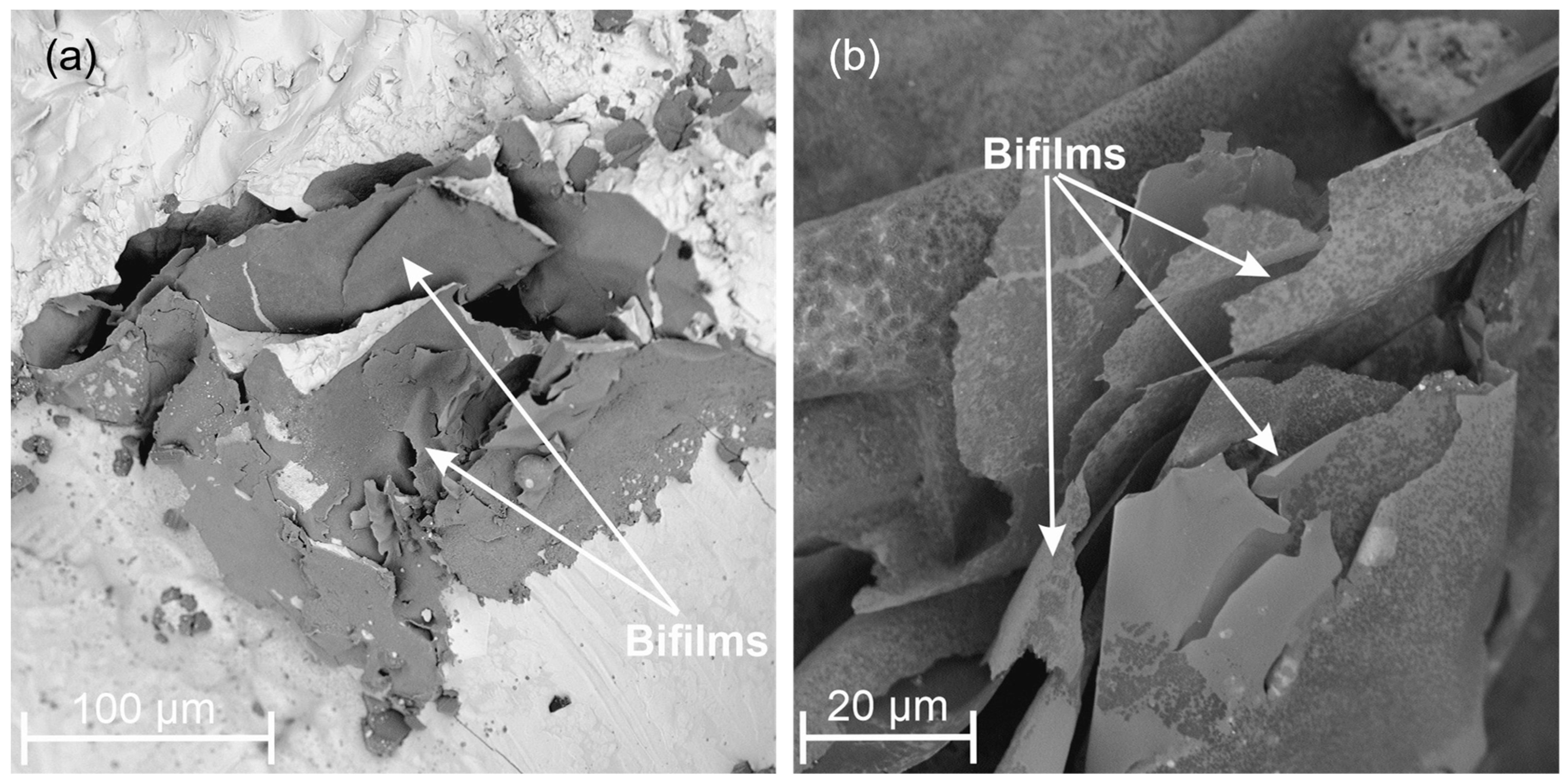
3.3. Metallographic Analysis
4. Discussion
5. Conclusions
- Solidus and liquidus temperatures were experimentally determined for the analyzed alloys: HSCI 23 (TL 1220 °C, TS 1158 °C), HSCI 25 (TL 1291 °C, TS 1126 °C).
- The phase transition temperature range, observed due to the heat effect from the transition of the Fe2Si phase into the Fe5Si3 phase, was determined. HSCI 23 (1095–978 °C), HSCI 25 (1109–987 °C).
- The spatial structure of the Fe5Si3 intermetallic phase, occurring on the surface of contraction cavities, was disclosed.
- A high level of silicon in the discussed alloys favors the emergence of oxygen inclusions of the “bifilm” type due to the increased absorption of gases by a liquid alloy.
Funding
Data Availability Statement
Conflicts of Interest
References
- Campbell, J. Complete Casting Handbook, 2nd ed.; Elsevier: Oxford, UK, 2015; p. 275. [Google Scholar]
- Pacha-Gołȩbiowska, H.; Piekarska, W. Key determinants for high-alloyed cast irons for mechanical engineering. Int. J. Appl. Mech. Eng. 2022, 27, 146–167. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Johnson, C.; Godlewski, L.; Li, M. Control of high-temperature static and transient thermomechanical behavior of SiMo ductile iron by al alloying. Int. J. Met. 2023, 17, 22–38. [Google Scholar] [CrossRef]
- Chavan, S.; Khandelwal, H. Effect of mo on micro-structural and mechanical properties of as-cast ferritic spheroidal graphite iron. Trans. Indian Inst. Met. 2021, 74, 2703–2711. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Johnson, C.; Bofah, A.; Godlewski, L.; Li, M. Improving high-temperature performance of high si-alloyed ductile iron by altering additions. Int. J. Met. 2021, 15, 874–888. [Google Scholar] [CrossRef]
- Cofiño-Villar, A.; Alvarez-Antolin, J.F.; Asensio-Lozano, J. Enhanced fracture strength in the working layer of rolls manufactured in Ni-hard cast iron alloyed with Mo, Nb and Mg. Metals 2018, 8, 725. [Google Scholar] [CrossRef]
- Vaško, A.; Uhríčik, M.; Kuchariková, L.; Tillová, E. Microstructure, mechanical and fatigue properties of SiMo- and SiCu- nodular cast irons. Procedia Struct. Integr. 2018, 13, 1527–1532. [Google Scholar] [CrossRef]
- Kotarska, A. The laser alloying process of ductile cast iron surface with titanium. Metals 2021, 11, 282. [Google Scholar] [CrossRef]
- Wieczorek, A.N. Comparative studies on the wear of ADI alloy cast irons as well as selected steels and surface-hardened alloy cast steels in the presence of abrasive. Arch. Metall. Mater. 2017, 62, 119–128. [Google Scholar] [CrossRef]
- Liu, C.; Du, Y.; Wang, X.; Zheng, Q.; Zhu, X.; Zhang, D.; Jiang, B. Comparison of the tribological behavior of quench-tempered ductile iron and austempered ductile iron with similar hardness. Wear 2023, 520, 204668. [Google Scholar] [CrossRef]
- Górny, M.; Gondek, Ł.; Angella, G.; Tyrała, E.; Kawalec, M.; Bitka, A. Structural stability of thin-walled austempered ductile iron castings. Arch. Civ. Mech. Eng. 2023, 23, 79. [Google Scholar] [CrossRef]
- Gebhardt, C.; Zhang, J.; Bezold, A.; Broeckmann, C. Microscale fatigue mechanisms in high silicon alloyed nodular cast iron. Int. J. Fatigue 2023, 168, 107402. [Google Scholar] [CrossRef]
- Baddoo, N.R. Stainless steel in construction: A review of research, applications, challenges and opportunities. J. Constr. Steel Res. 2008, 64, 1199–1206. [Google Scholar] [CrossRef]
- Nilsson, J. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Stawarz, M.; Dojka, M. Bifilm inclusions in high alloyed cast iron. Materials 2021, 14, 3067. [Google Scholar] [CrossRef]
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391–402. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pedeferri, P.; Lazzari, L.; Pastore, T. Cathodic protection and cathodic prevention in concrete: Principles and applications. J. Appl. Electrochem. 1998, 28, 1321–1331. [Google Scholar] [CrossRef]
- Stawarz, M.; Kajzer, W.; Kajzer, A.; Dojka, M. Physicochemical properties of silicon cast iron. Arch. Foundry Eng. 2017, 17, 101–106. [Google Scholar] [CrossRef]
- Campbell, J. Hoyt memorial lecture—Stop pouring, start casting. Int. J. Met. 2012, 6, 7–18. [Google Scholar] [CrossRef]
- Fox, S.; Campbell, J. Visualisation of oxide film defects during solidification of aluminium alloys. Scr. Mater. 2000, 43, 881–886. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Lordan, E.; Zhang, Y.; Karakulak, E.; Al-Helal, K.; Fan, Z. Degassing of aluminum alloy melts by high shear melt conditioning technology: An overview. Metals 2022, 12, 1772. [Google Scholar] [CrossRef]
- Dojka, M.; Stawarz, M. Bifilm defects in Ti-inoculated chromium white cast iron. Materials 2020, 13, 3124. [Google Scholar] [CrossRef] [PubMed]
- Gyarmati, G.; Fegyverneki, G.; Tokár, M.; Mende, M. Investigation on double oxide film initiated pore formation in aluminum. casting alloys. Int. J. Eng. Manag. Sci. 2020, 5, 141–153. [Google Scholar] [CrossRef]
- Rappenglück, M.A. Natural iron silicides: A systematic review. Minerals 2022, 12, 188. [Google Scholar] [CrossRef]
- Kimura, Y.; Yamada, M.; Chai, Y.W. Thermoelectric properties of nearly single-phase-FeSi2 alloys fabricated by gas-atomized powder sintering. Mater. Trans. 2019, 60, 652–661. [Google Scholar] [CrossRef]
- Sakata, T.; Sakai, Y.; Yoshino, H.; Fujii, H.; Nishida, I. Studies on the formation of FeSi2 from the FeSi Fe2Si5 eutectic. J. Less-Common Met. 1978, 61, 301–308. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Nuss, J.; Haines, J.; Jansen, M.; Vegas, A. Iron silicides and their corresponding oxides: A high-pressure study of Fe5Si3. Solid State Sci. 2004, 6, 673–678. [Google Scholar] [CrossRef]
- Yang, D.; Yu, Y.; Zhao, X.; Song, Y.; Lopez-Honorato, E.; Xiao, P.; Lai, D. Fabrication of silicon carbide (SiC) coatings from pyrolysis of Polycarbosilane/Aluminum. J. Inorg. Organomet. Polym. Mater. 2011, 21, 534–540. [Google Scholar] [CrossRef]
- Chen, H.; Barman, T. Thermo-calc and DICTRA modelling of the β-phase depletion behaviour in CoNiCrAlY coating alloys at different al contents. Comput. Mater. Sci. 2018, 147, 103–114. [Google Scholar] [CrossRef]
- Sadowski, T.; Golewski, P. Detection and numerical analysis of the most efforted places in turbine blades under real working conditions. Comput. Mater. Sci. 2012, 64, 285–288. [Google Scholar] [CrossRef]
- Chen, H.; Jackson, G.A.; Voisey, K.T.; McCartney, D.G. Modelling and experimental study on β-phase depletion behaviour of HVOF sprayed free-standing CoNiCrAlY coatings during oxidation. Surf. Coat. Technol. 2016, 291, 34–42. [Google Scholar] [CrossRef]
- Dojka, M.; Dojka, R.; Stawarz, M.; Studnicki, A. Influence of Ti and REE on primary crystallization and wear resistance of chromium cast iron. J. Mater. Eng. Perform. 2019, 28, 4002–4011. [Google Scholar] [CrossRef]
- Gyarmati, G.; Bubonyi, T.; Fegyverneki, G.; Tokár, M.; Mende, T. Interactions of primary intermetallic compound particles and double oxide films in liquid aluminum alloys. Intermetallics 2022, 149, 107681. [Google Scholar] [CrossRef]
- Gyarmati, G.; Vincze, F.; Fegyverneki, G.; Kéri, Z.; Mende, T.; Molnár, D. The effect of rotary degassing treatments with different purging gases on the double oxide- and nitride film content of liquid aluminum alloys. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2022, 53, 1244–1257. [Google Scholar] [CrossRef]





| Chemical Composition, % of Weight | ||||||
|---|---|---|---|---|---|---|
| C | Cr | Si | Mn | Ni | Mo | S |
| 0.080 | 0.037 | 0.001 | 0.594 | 0.024 | 0.01 | 0.006 |
| Co | Cu | Al | Sb | As | B | P |
| 0.010 | 0.004 | 0.046 | 0.009 | 0.108 | 0.00 | 0.016 |
| Pb | Nb | Sn | Ti | W | V | Febal |
| 0.002 | 0.031 | 0.009 | 0.001 | 0.011 | 0.006 | 98.995 |
| Chemical Composition, % of Weight | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Si | 2 C | 2 S | P | Mn | Mo | Cu | Mg | Ti | Febal | |
| HSCI 23 | 23.53 | 0.82 | 0.007 | 0.023 | 0.331 | 0.019 | 0.061 | 0.00 | 0.021 | 75.18 |
| HSCI 25 | 25.23 | 0.12 | 0.007 | 0.021 | 0.329 | 0.010 | 0.069 | 0.00 | 0.022 | 74.19 |
| Temperature, °C | ||||
|---|---|---|---|---|
| Graphite | FeSi | Fe2Si | Solidus | |
| HSCI 23 | 1267 | 1252 | 1179 | 1178 |
| HSCI 25 | 1179 | 1336 | 1192 | 1168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawarz, M. Crystallization of Intermetallic Phases Fe2Si, Fe5Si3 for High Alloyed Cast Irons. Crystals 2023, 13, 1033. https://doi.org/10.3390/cryst13071033
Stawarz M. Crystallization of Intermetallic Phases Fe2Si, Fe5Si3 for High Alloyed Cast Irons. Crystals. 2023; 13(7):1033. https://doi.org/10.3390/cryst13071033
Chicago/Turabian StyleStawarz, Marcin. 2023. "Crystallization of Intermetallic Phases Fe2Si, Fe5Si3 for High Alloyed Cast Irons" Crystals 13, no. 7: 1033. https://doi.org/10.3390/cryst13071033
APA StyleStawarz, M. (2023). Crystallization of Intermetallic Phases Fe2Si, Fe5Si3 for High Alloyed Cast Irons. Crystals, 13(7), 1033. https://doi.org/10.3390/cryst13071033






