Abstract
With Tm3F[Si3O10], a new representative of the Ln3F[Si3O10] series could be synthesized by the reaction of Tm2O3, TmF3 and SiO2 (molar ratio: 1:1:3), applying an excess of CsBr as a fluxing agent in gas-tightly sealed platinum crucibles for eight days at 750 °C, and designed to yield Tm3F3[Si3O9] or Cs2TmF[Si4O10]. Single crystals of Tm3F[Si3O10] (monoclinic, P21/n; a = 725.04(6), b = 1102.43(9), c = 1032.57(8) pm, β = 97.185(7)°; Z = 4) appear as pale celadon, transparent, air- and water-resistant rhombic plates. According to its thalenite-type structure, Tm3F[Si3O10] contains catena-trisilicate anions [Si3O10]8− and triangular [FTm3]8+ cations. The three crystallographically different Tm3+ cations are coordinated by seven plus one (Tm1) or only seven anions (Tm2 and Tm3) exhibiting a single F− anion for each polyhedron, additional to the majority of O2− anions. Furthermore, the luminescence properties of the isotypic colorless compound Y3F[Si3O10] doped with Eu3+ (red emission), Tb3+ (green emission) and Er3+ (yellow and infrared emission), respectively, are reported in presenting their different excitation and emission spectra.
1. Introduction
In the quaternary systems RE2O3–REF3–SiO2 (RE = rare-earth element), only six different types of compounds are known so far, which can be distinguished by the linkage of the involved [SiO4]4− tetrahedra via common vertices. The lanthanum fluoride oxosilicate La3F3[Si3O9] [1,2] contains cyclo-trisilicate units [Si3O9]6− and therefore shows the highest degree of silicate connectivity. By breaking one of these bonds with an extra O2− anion, the fluoride oxosilicates RE3F[Si3O10] (RE = Y [3], Dy–Er [4,5]) with a thalenite-type structure [6,7] are realized, which comprise catena-trisilicate units [Si3O10]8−. Despite the assumption that only four representatives of this formula type [3,4,5] exist, it was recently possible to obtain the isotypic Tm3F[Si3O10] by a different synthetic route. Furthermore, the compound RE4F2[Si2O7][SiO4] (≡ “RE4F2(Si3O11”), RE = Y [8], Er [9]) is built-up by isolated ortho-oxosilicate ([SiO4]4−) and oxodisilicate units ([Si2O7]6−) after another O2− anion has torn the trisilicate chain fragment apart. Three isolated [SiO4]4− tetrahedra occur in the mixed-valent fluoride ortho-oxosilicate Eu5F[SiO4]3 (≡ “EuII2EuIII3F(Si3O12)”) [10] with apatite-type structure, where all Si–O–Si bridges are lost. Moreover, discrete ortho-oxosilicate anions also dominate the structures of some oxide-fluoride derivatives, such as La7OF7[SiO4]3 [11] and Sm3OF3[SiO4] [12].
Silicate host lattices are very suitable for luminescence materials due to their generally excellent stability. Therefore, several different compounds doped with Eu3+, Tb3+ and Er3+ were described in the past [13,14,15,16,17,18,19,20]. In this work, we use Y3F[Si3O10] as the host lattice for the Ln3+ cations mentioned above and present their luminescence properties for the first time. Furthermore, the crystal structure of Tm3F[Si3O10], as a new compound of the thalenite series, is described in detail, since only lattice constants have been mentioned before [21].
2. Materials and Methods
Single crystals of Tm3F[Si3O10] have been synthesized by the reaction of thulium oxide (Tm2O3, ChemPur, Karlsruhe, Germany: 99.9%), thulium trifluoride (TmF3, ChemPur, Karlsruhe, Germany: 99.9%) and silicon dioxide (SiO2, Merck, Darmstadt, Germany: silica gel, p. a.) in a molar ratio of 1:1:3 with an excess of cesium bromide (CsBr, ChemPur, Karlsruhe, Germany: 99.9%) as the fluxing agent in gas-tightly sealed platinum ampoules (Horst zu Jeddeloh GmbH, Winsen/Luhe, Germany) for eight days at 750 °C. The original target products were Tm3F3[Si3O9] or Cs2TmF[Si4O10], if Cs+, as part of the flux, would become incorporated, as it happened in the case of Cs2YF[Si4O10] [22]. Following cooling by 10 °C/h down to room temperature and washing the crude product with water, the compound emerged as light green, transparent, air- and water-resistant rhombic plates. Crystallographic data of Tm3F[Si3O10] and details of its structural refinement, as well as atomic parameters and coefficients of the anisotropic thermal displacement factors are summarized in Table 1 and Table 2. Selected interatomic distances and angles fill Table 3 and Table 4 informs about the motifs of mutual adjunction [23,24].

Table 1.
Tm3F[Si3O10]: Crystallographic data and details of the structure refinement.

Table 2.
Atomic parameters and coefficients of the anisotropic thermal displacement factors (Uij/pm2) for Tm3F[Si3O10] (all atoms reside at the Wyckoff position 4e).

Table 3.
Selected interatomic distances (d/pm) and bond angles (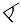 /°) for Tm3F[Si3O10].
/°) for Tm3F[Si3O10].
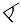 /°) for Tm3F[Si3O10].
/°) for Tm3F[Si3O10].

Table 4.
Motifs of mutual adjunction [23,24] for the Tm3F[Si3O10] structure.
The colorless samples for luminescence investigations Y3F[Si3O10]:Ln3+ (Ln = Eu, Tb, Er) could be synthesized via the classical route [3]. For this purpose, mixtures of yttrium oxide (Y2O3, ChemPur, Karlsruhe, Germany: 99.9%), yttrium trifluoride (YF3, ChemPur, Karlsruhe, Germany: 99.9%) and silicon dioxide (SiO2, Merck, Darmstadt, Germany: silica gel, p. a.) in the molar ratio 1:4:9 with a slight excess of cesium chloride (CsCl, ChemPur, Karlsruhe, Germany: 99.9%) as fluxing agent, and doped with 3% of the lanthanoid trifluorides LnF3 (Ln = Eu, Tb, Er; each ChemPur, Karlsruhe, Germany: 99.9%) replacing YF3, were heated in arc-sealed tantalum ampoules (Plansee, Reutte, Austria) for seven days at 700 °C. For example, to yield circa 500 mg of (Y2.9Er0.1)F[Si3O10], 271.36 mg of Y2O3 was mixed with 43.83 mg of YF3, 2.25 mg of ErF3 as dopant, and 168.06 mg of SiO2 and a slight excess of cesium bromide (550 mg). The lack of color for Y3F[Si3O10] results from the fact that all included ions (Y3+ and Si4+ as well as F− and O2−) exhibit electronic closed-shell configurations ([Ne] for the anions and Si4+, [Kr] for Y3+). As no visible charge-transfer processes from the anions to the cations are active, there is no way to show any color within the 380–750-nm region. So consequently, all components or combinations, such as Y2O3, SiO2, YF3, SiF4, YOF, SiOF2 and Y2Si2O7 appear colorless as well, no matter if covalent or ionic. For this reason, their huge band gaps feature the key property for energy preservation after irradiation followed by transfer processes and avoid thermal quenching. Since Y3+ as a non-lanthanoid cation exhibits the same ionic radius (ri(Y3+) = 96–102 pm for C.N. = 7–8) [25] as the mid-size Ln3+ cations, despite being so much lighter in weight, yttrium(III) compounds with “hard anions”, such as F− and O2− according to the Pearson concept [26], provide perfect host materials for doping with luminescent Ln3+ guests with similar ionic radii, but open-shell representatives (e.g., Eu3+: [Xe]4f 6, ri = 101–107 pm; Tb3+: [Xe]4f 8, ri = 98–104 pm; Er3+: [Xe]4f 11, ri = 95–100 pm) [25] harvest the visible light upon UV irradiation from electronic f–f transitions.
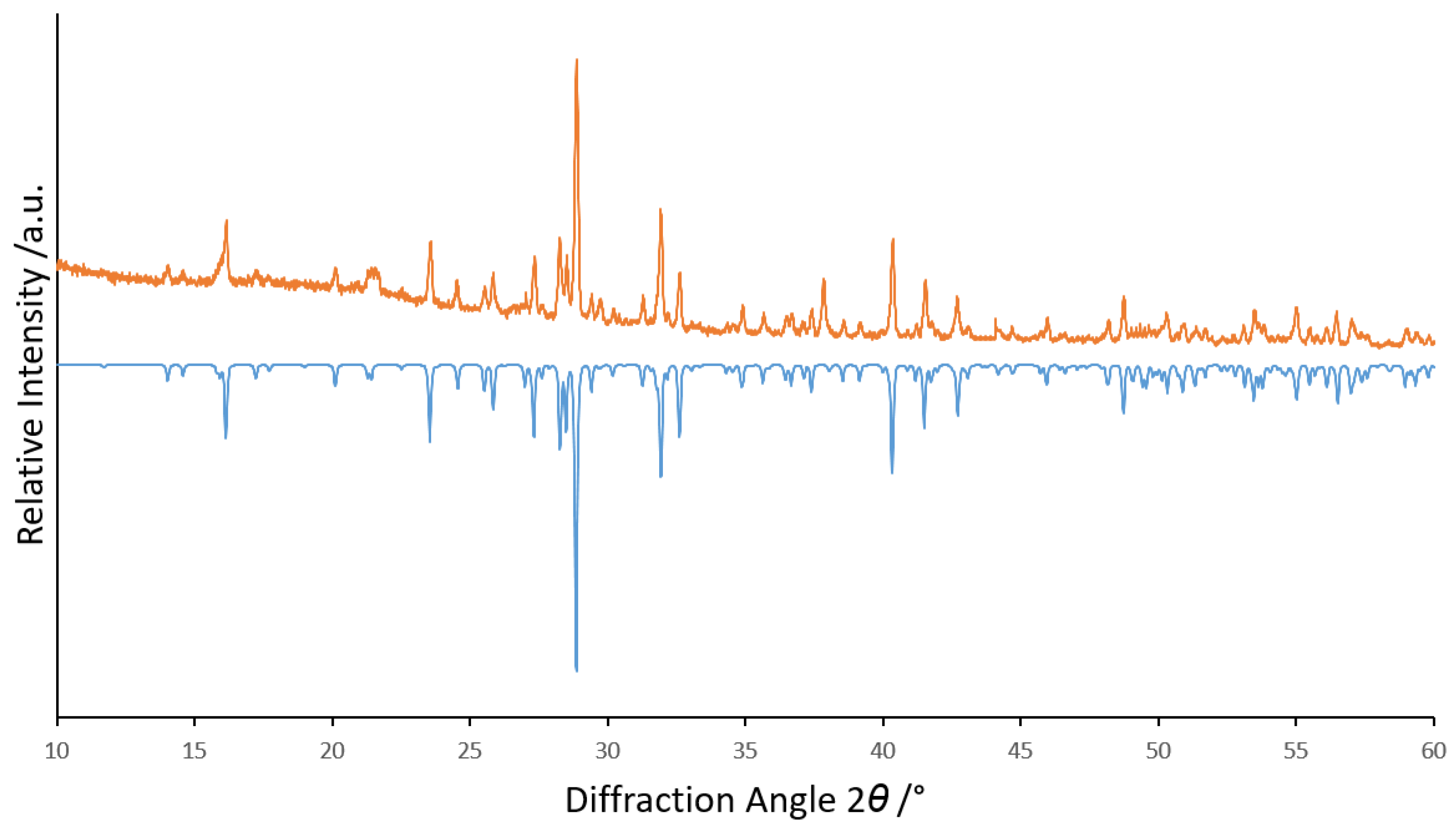
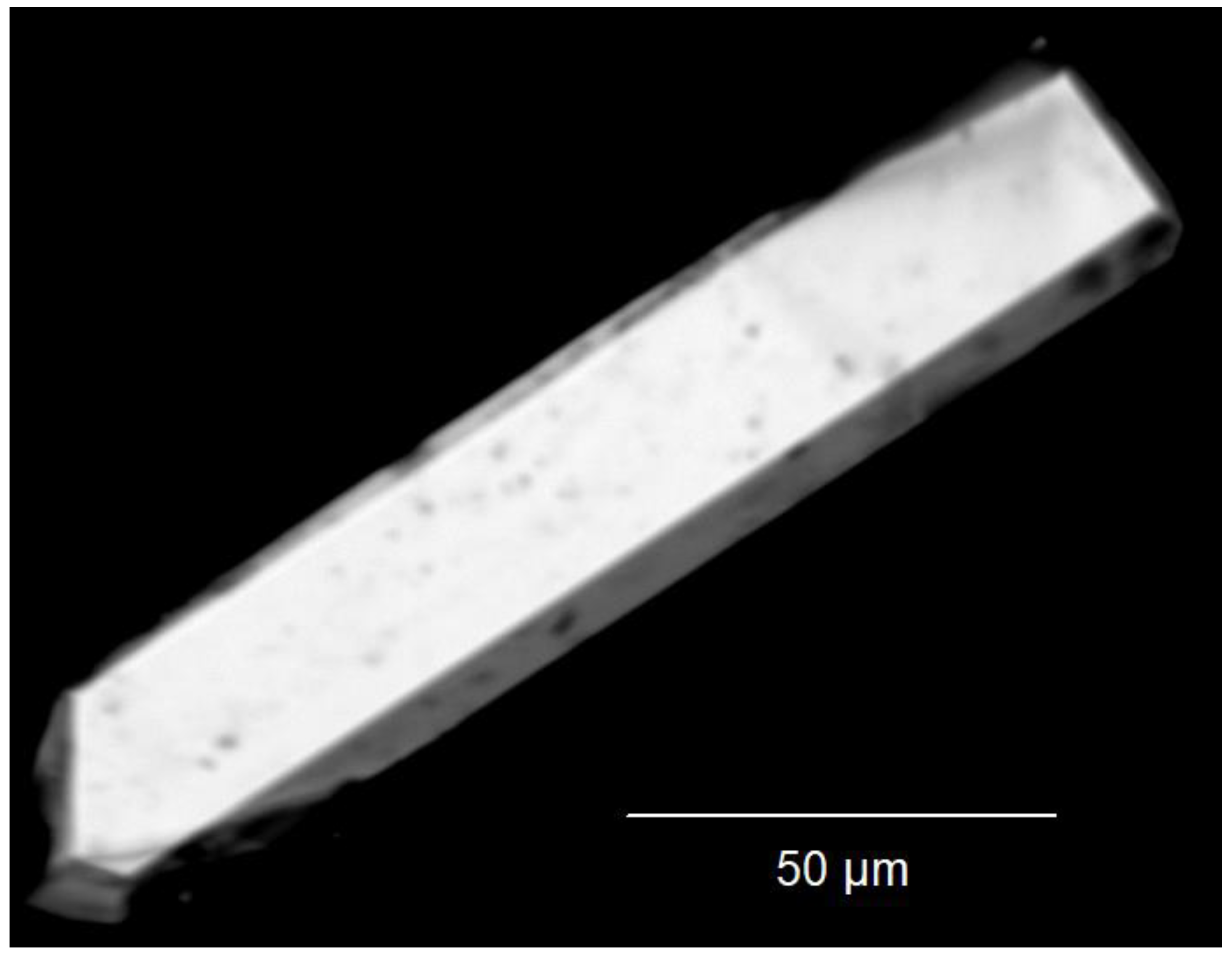
Quick satisfying phase-purity checks with PXRD of each product were made with a STADI-P powder X-ray diffractometer (Stoe & Cie, Darmstadt, Germany; Cu-Kα radiation), but no Rietveld refinement appeared to be necessary (Figure 1). Semi-quantitative EDXS checks were made with an electron-beam X-ray microprobe (SX-100, Cameca, Gennevilliers, France) and Figure 2 shows a scanning electron micrograph of Tm3F[Si3O10]. Photoluminescence emission and excitation spectra of each sample were recorded at room temperature on a Jobin Yvon fluorescence spectrometer (Fluorolog 3, Horiba, Kyoto, Japan) equipped with two 0.22 m double monochromators (SPEX, 1680, Horiba, Kyoto, Japan) and a 450 W xenon lamp. The emission spectra were corrected for photomultiplier sensitivity, the excitation spectra for lamp intensity and both for the transmission of the monochromators.
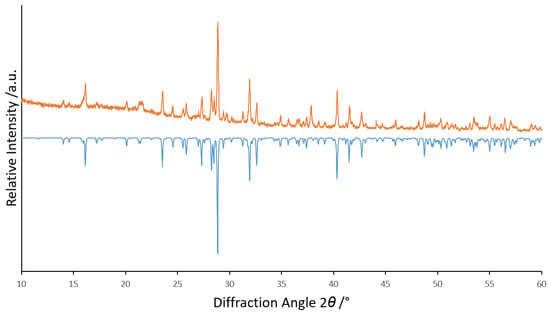
Figure 1.
Powder X-ray diffractogram of (Y2.9Er0.1)F[Si3O10] (top: measured in orange, bottom: calculated in blue with data from [3]).
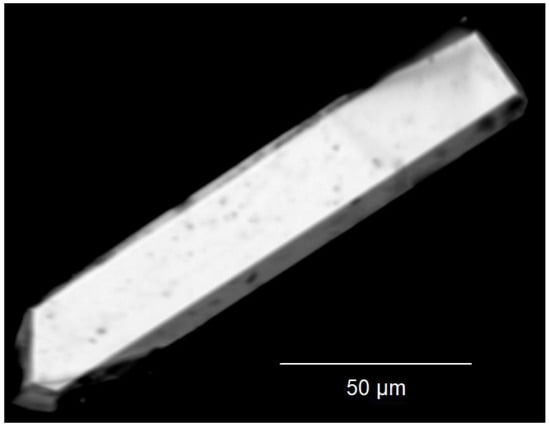
Figure 2.
Scanning electron micrograph (SEM) of the measured, in this case bar-shaped, single crystal of Tm3F[Si3O10].
3. Results and Discussion
3.1. Structure Description
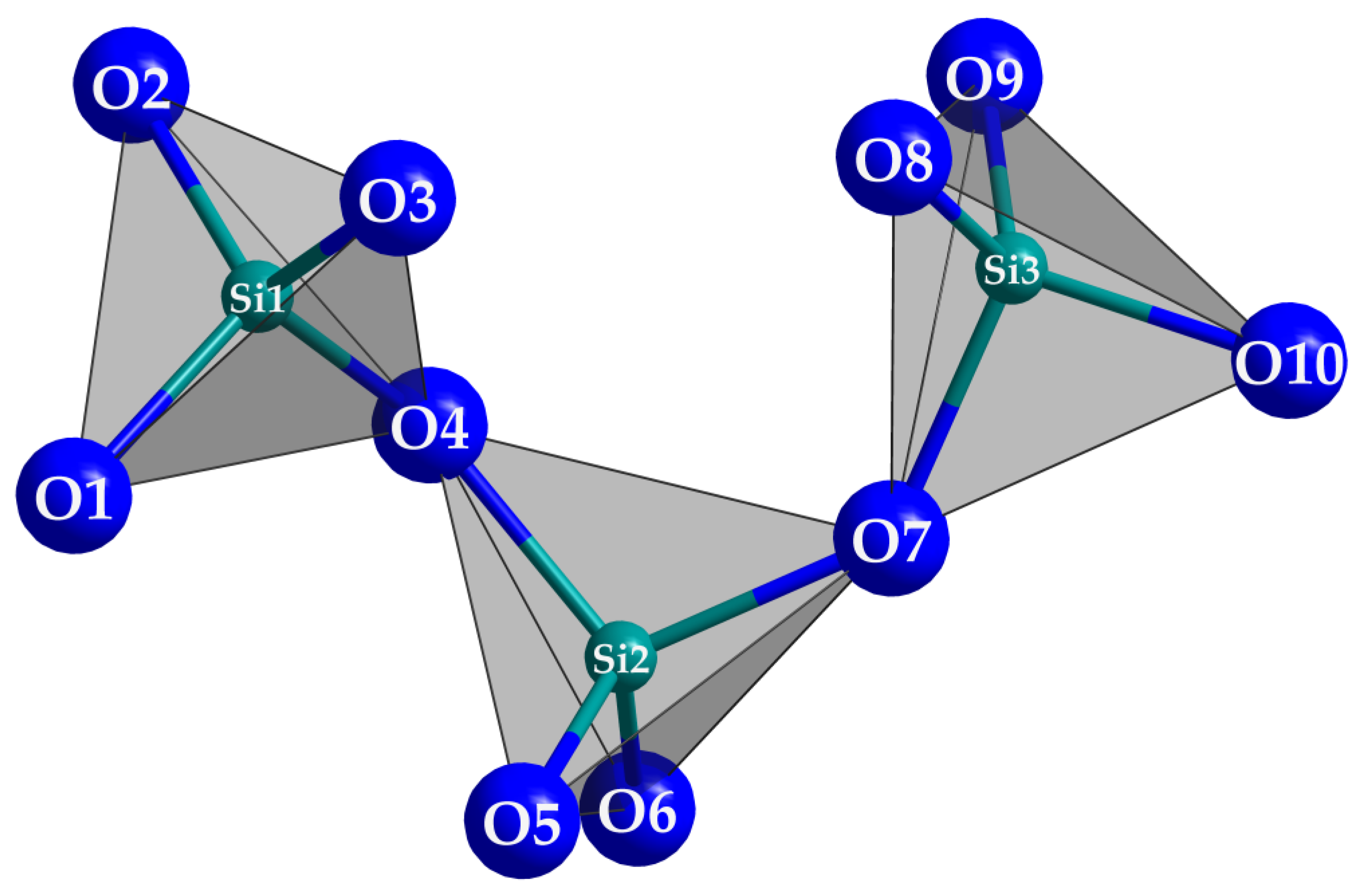
Instead of the expected hypothetical quinary thulium oxosilicate with the formula Cs2TmF[Si4O10], that was scheduled to form analogously to Cs2YF[Si4O10] [22], Tm3F[Si3O10] was surprisingly obtained in several experiments, which represents an extension of the domain of existence in the thalenite-type series (RE3F[Si3O10]; RE = Dy, Ho, Er and Y [4,5]). Tm3F[Si3O10] crystallizes isotypically to monoclinic Y3F[Si3O10] [3] in space group P21/n with a = 725.04(6), b = 1102.43(9), c = 1032.57(8) pm, β = 97.185(7)° and four formula units per unit cell. The three crystallographically independent [SiO4]4− tetrahedra are linked via common vertices to an open, horseshoe-shaped chain fragment [Si3O10]8− (Figure 3), which has to be addressed as a catena-oxotrisilicate unit. The tetrahedra are aligned and eclipsed in respect to the O4 bridge (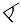 (Si1–O4–Si2) = 133°), but staggered considering the O7 connection (
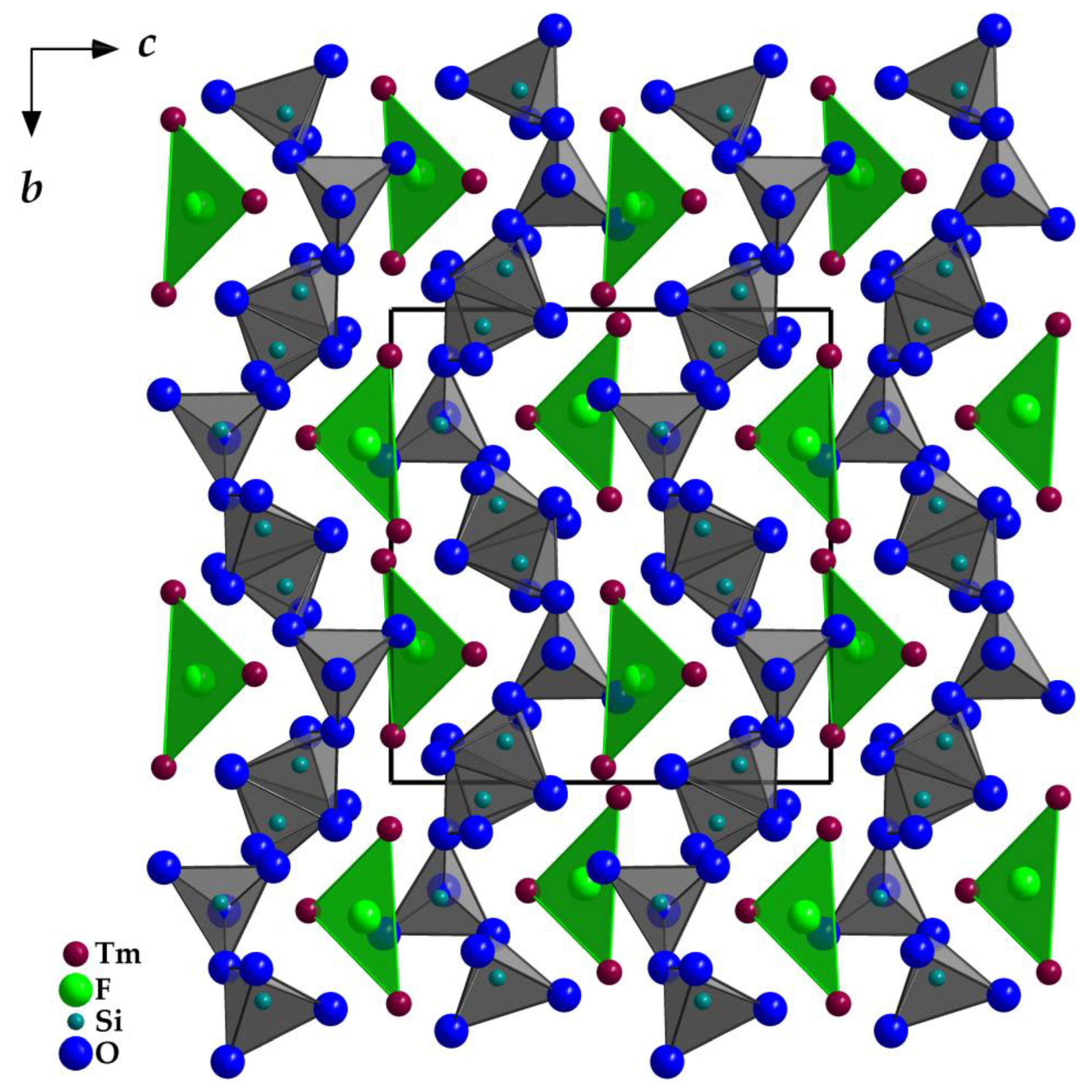
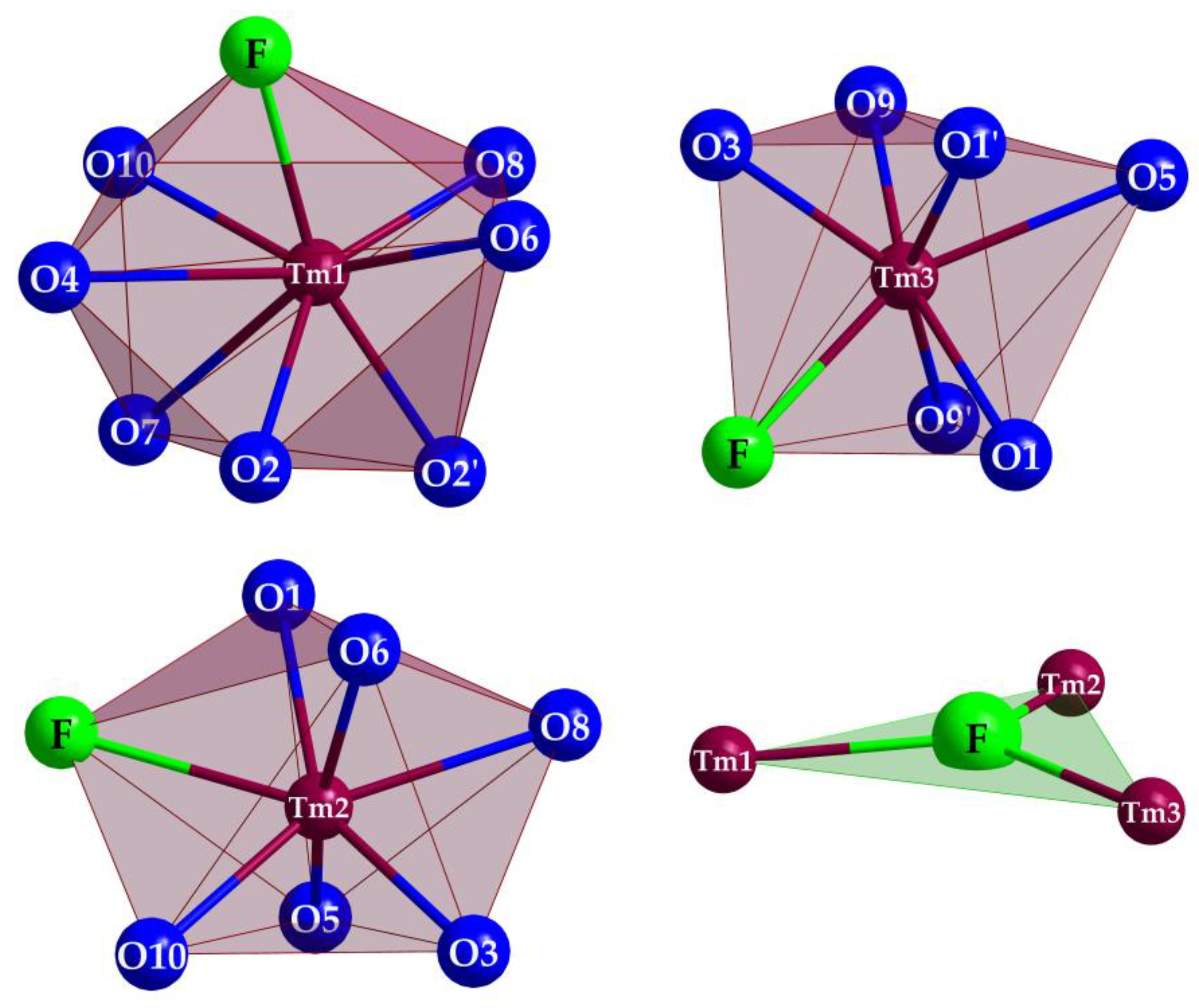
(Si1–O4–Si2) = 133°), but staggered considering the O7 connection (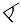 (Si2–O7–Si3) = 138°). In the unit cell, the opening of the [Si3O10]8− horseshoes is arranged alternatingly to the left and the right (Figure 4) along the [010] direction. The Si–O distances with values of 160–165 pm match very well with the distances of the two known types of thulium oxodisilicates (e.g., B-type Tm2Si2O7: d(Si–O) = 160–165 pm [30] or C-type Tm2Si2O7: d(Si–O) = 161–164 pm [31]). The triangular, almost planar, isolated [FTm3]8+ units (d(F–Tm) = 220–235 pm; Figure 5) are located between the [Si3O10]8− chain fragments (Figure 2). The deflection of the F− anion from the (Tm3+)3 triangle amounts to 20 pm and the Tm–F–Tm angles range from 110 to 134°. In the structure, three crystallographically distinguishable Tm3+ cations are found. (Tm1)3+ is surrounded by one F− and six plus one O2− anions in the shape of a distorted square antiprism. The coordination polyhedra of (Tm2)3+ can be described as a strongly distorted monocapped trigonal antiprism, consisting of one fluorine and six oxygen atoms and (Tm3)3+ shows an environment of one F− and six O2− anions arranged as a distorted monocapped trigonal prism (Figure 5). The Tm–O distances with values of 220–268 plus 292 pm correspond excellently with those in thulium oxodisilicates, oxide ortho-oxosilicate or sulfide oxodisilicate, respectively (e.g., B-type Tm2Si2O7: d(Tm–O) = 220–274 + 287 pm [30], Tm2O[SiO4]: d(Tm–O) = 218–250 + 322 pm [32], Tm4S3[Si2O7]: d(Tm–O) = 225–251 + 317 pm [33]). The distances between thulium and fluorine (d(Tm–F) = 220–235 pm) are also in good agreement with the separations in, for example, BaTm2F8 (d(Tm–F) = 222–231 pm) [34] or TmF[AuF4]2 (d(Tm–F) = 212–238 pm) [35]. For visualization of the crystal-structure features, Figure 3, Figure 4 and Figure 5 were created using the DIAMOND [36] program.
(Si2–O7–Si3) = 138°). In the unit cell, the opening of the [Si3O10]8− horseshoes is arranged alternatingly to the left and the right (Figure 4) along the [010] direction. The Si–O distances with values of 160–165 pm match very well with the distances of the two known types of thulium oxodisilicates (e.g., B-type Tm2Si2O7: d(Si–O) = 160–165 pm [30] or C-type Tm2Si2O7: d(Si–O) = 161–164 pm [31]). The triangular, almost planar, isolated [FTm3]8+ units (d(F–Tm) = 220–235 pm; Figure 5) are located between the [Si3O10]8− chain fragments (Figure 2). The deflection of the F− anion from the (Tm3+)3 triangle amounts to 20 pm and the Tm–F–Tm angles range from 110 to 134°. In the structure, three crystallographically distinguishable Tm3+ cations are found. (Tm1)3+ is surrounded by one F− and six plus one O2− anions in the shape of a distorted square antiprism. The coordination polyhedra of (Tm2)3+ can be described as a strongly distorted monocapped trigonal antiprism, consisting of one fluorine and six oxygen atoms and (Tm3)3+ shows an environment of one F− and six O2− anions arranged as a distorted monocapped trigonal prism (Figure 5). The Tm–O distances with values of 220–268 plus 292 pm correspond excellently with those in thulium oxodisilicates, oxide ortho-oxosilicate or sulfide oxodisilicate, respectively (e.g., B-type Tm2Si2O7: d(Tm–O) = 220–274 + 287 pm [30], Tm2O[SiO4]: d(Tm–O) = 218–250 + 322 pm [32], Tm4S3[Si2O7]: d(Tm–O) = 225–251 + 317 pm [33]). The distances between thulium and fluorine (d(Tm–F) = 220–235 pm) are also in good agreement with the separations in, for example, BaTm2F8 (d(Tm–F) = 222–231 pm) [34] or TmF[AuF4]2 (d(Tm–F) = 212–238 pm) [35]. For visualization of the crystal-structure features, Figure 3, Figure 4 and Figure 5 were created using the DIAMOND [36] program.
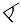 (Si1–O4–Si2) = 133°), but staggered considering the O7 connection (
(Si1–O4–Si2) = 133°), but staggered considering the O7 connection ( (Si2–O7–Si3) = 138°). In the unit cell, the opening of the [Si3O10]8− horseshoes is arranged alternatingly to the left and the right (Figure 4) along the [010] direction. The Si–O distances with values of 160–165 pm match very well with the distances of the two known types of thulium oxodisilicates (e.g., B-type Tm2Si2O7: d(Si–O) = 160–165 pm [30] or C-type Tm2Si2O7: d(Si–O) = 161–164 pm [31]). The triangular, almost planar, isolated [FTm3]8+ units (d(F–Tm) = 220–235 pm; Figure 5) are located between the [Si3O10]8− chain fragments (Figure 2). The deflection of the F− anion from the (Tm3+)3 triangle amounts to 20 pm and the Tm–F–Tm angles range from 110 to 134°. In the structure, three crystallographically distinguishable Tm3+ cations are found. (Tm1)3+ is surrounded by one F− and six plus one O2− anions in the shape of a distorted square antiprism. The coordination polyhedra of (Tm2)3+ can be described as a strongly distorted monocapped trigonal antiprism, consisting of one fluorine and six oxygen atoms and (Tm3)3+ shows an environment of one F− and six O2− anions arranged as a distorted monocapped trigonal prism (Figure 5). The Tm–O distances with values of 220–268 plus 292 pm correspond excellently with those in thulium oxodisilicates, oxide ortho-oxosilicate or sulfide oxodisilicate, respectively (e.g., B-type Tm2Si2O7: d(Tm–O) = 220–274 + 287 pm [30], Tm2O[SiO4]: d(Tm–O) = 218–250 + 322 pm [32], Tm4S3[Si2O7]: d(Tm–O) = 225–251 + 317 pm [33]). The distances between thulium and fluorine (d(Tm–F) = 220–235 pm) are also in good agreement with the separations in, for example, BaTm2F8 (d(Tm–F) = 222–231 pm) [34] or TmF[AuF4]2 (d(Tm–F) = 212–238 pm) [35]. For visualization of the crystal-structure features, Figure 3, Figure 4 and Figure 5 were created using the DIAMOND [36] program.
(Si2–O7–Si3) = 138°). In the unit cell, the opening of the [Si3O10]8− horseshoes is arranged alternatingly to the left and the right (Figure 4) along the [010] direction. The Si–O distances with values of 160–165 pm match very well with the distances of the two known types of thulium oxodisilicates (e.g., B-type Tm2Si2O7: d(Si–O) = 160–165 pm [30] or C-type Tm2Si2O7: d(Si–O) = 161–164 pm [31]). The triangular, almost planar, isolated [FTm3]8+ units (d(F–Tm) = 220–235 pm; Figure 5) are located between the [Si3O10]8− chain fragments (Figure 2). The deflection of the F− anion from the (Tm3+)3 triangle amounts to 20 pm and the Tm–F–Tm angles range from 110 to 134°. In the structure, three crystallographically distinguishable Tm3+ cations are found. (Tm1)3+ is surrounded by one F− and six plus one O2− anions in the shape of a distorted square antiprism. The coordination polyhedra of (Tm2)3+ can be described as a strongly distorted monocapped trigonal antiprism, consisting of one fluorine and six oxygen atoms and (Tm3)3+ shows an environment of one F− and six O2− anions arranged as a distorted monocapped trigonal prism (Figure 5). The Tm–O distances with values of 220–268 plus 292 pm correspond excellently with those in thulium oxodisilicates, oxide ortho-oxosilicate or sulfide oxodisilicate, respectively (e.g., B-type Tm2Si2O7: d(Tm–O) = 220–274 + 287 pm [30], Tm2O[SiO4]: d(Tm–O) = 218–250 + 322 pm [32], Tm4S3[Si2O7]: d(Tm–O) = 225–251 + 317 pm [33]). The distances between thulium and fluorine (d(Tm–F) = 220–235 pm) are also in good agreement with the separations in, for example, BaTm2F8 (d(Tm–F) = 222–231 pm) [34] or TmF[AuF4]2 (d(Tm–F) = 212–238 pm) [35]. For visualization of the crystal-structure features, Figure 3, Figure 4 and Figure 5 were created using the DIAMOND [36] program.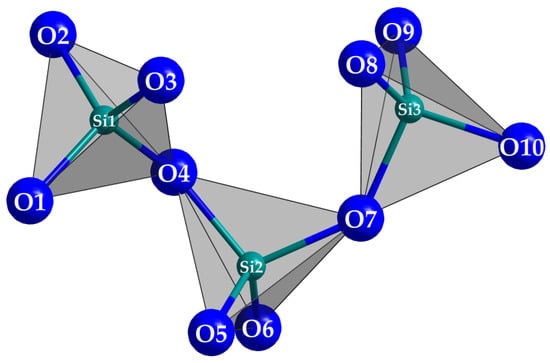
Figure 3.
The horseshoe-shaped catena-oxotrisilicate anion [Si3O10]8− in Tm3F[Si3O10].
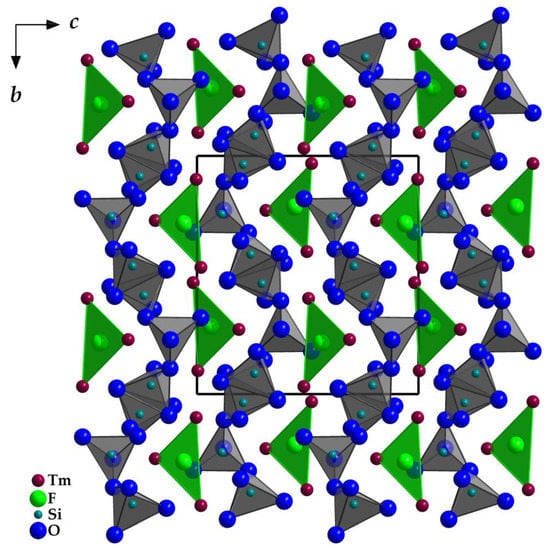
Figure 4.
View at the monoclinic crystal structure of Tm3F[Si3O10] along [100] emphasizing the discrete [FTm3]8+ and [Si3O10]8− units.
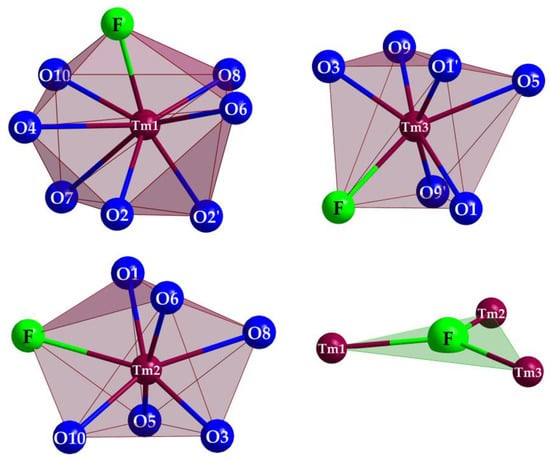
Figure 5.
Coordination of the polyhedra of the three crystallographically independent Tm3+ cations and the triangular [FTm3]8+ unit in Tm3F[Si3O10].
3.2. Spectroscopy
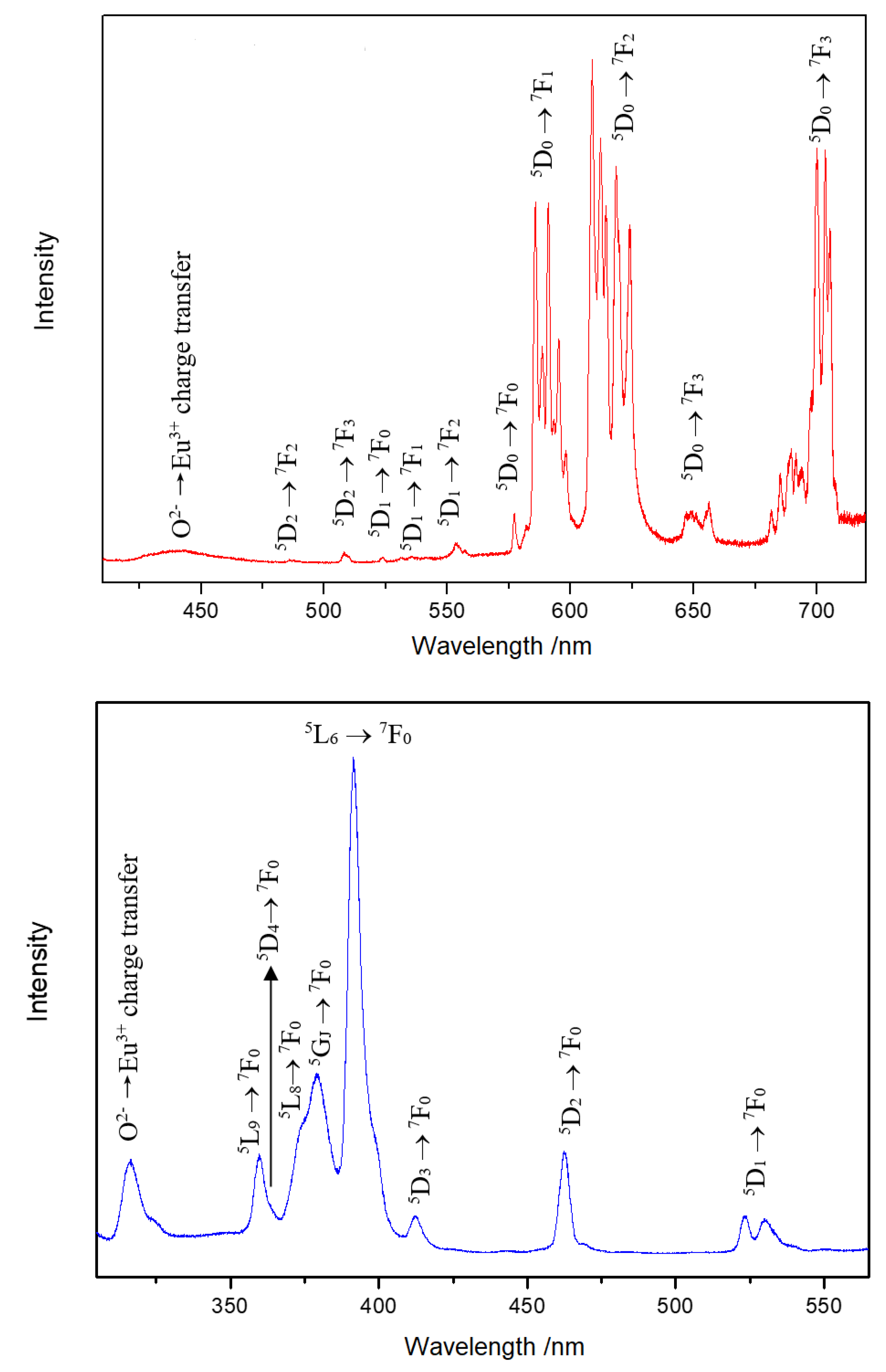
In the Y3F[Si3O10]:Ln3+ phosphors (Ln = Eu, Tb, Er), the doping Ln3+ cations are obviously able to replace all three different Y3+-cation sites. The emission spectrum of Y3F[Si3O10]:Eu3+ detected at 390 nm (Figure 6, top) presents strong peaks due to the 5D0 → 7F2 transition, as well as the peaks for the 5D0 → 7F1 transition. The 7F1 level is split into nine components due to three different sites and the low symmetry for Eu3+ in the crystal structure. The existence of a weak 5D0 → 7F0 peak indicates that only Cn, Cnv or Cs symmetries are possible. The bands for 5D1 → 7FJ transitions were also observed. A weak broad band at 442 nm was detected, which suggests the occurrence of the charge-transfer transition O2–-2p → Eu3+-5d. The excitation spectrum detected at 585 nm (Figure 6, bottom) shows f–f transitions of the Eu3+ cation together with a broad band peaking at 316 nm, which can be assigned again to the O2− → Eu3+ charge-transfer process.
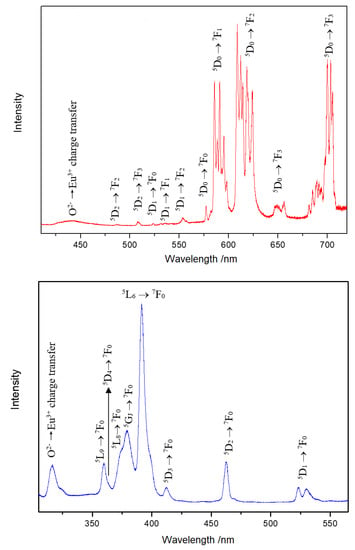
Figure 6.
Emission spectrum of Y3F[Si3O10]:Eu3+ excited at 390 nm at room temperature (top). Excitation spectrum of Y3F[Si3O10]:Eu3+ detected at the emission energy of 585 nm at room temperature (bottom).
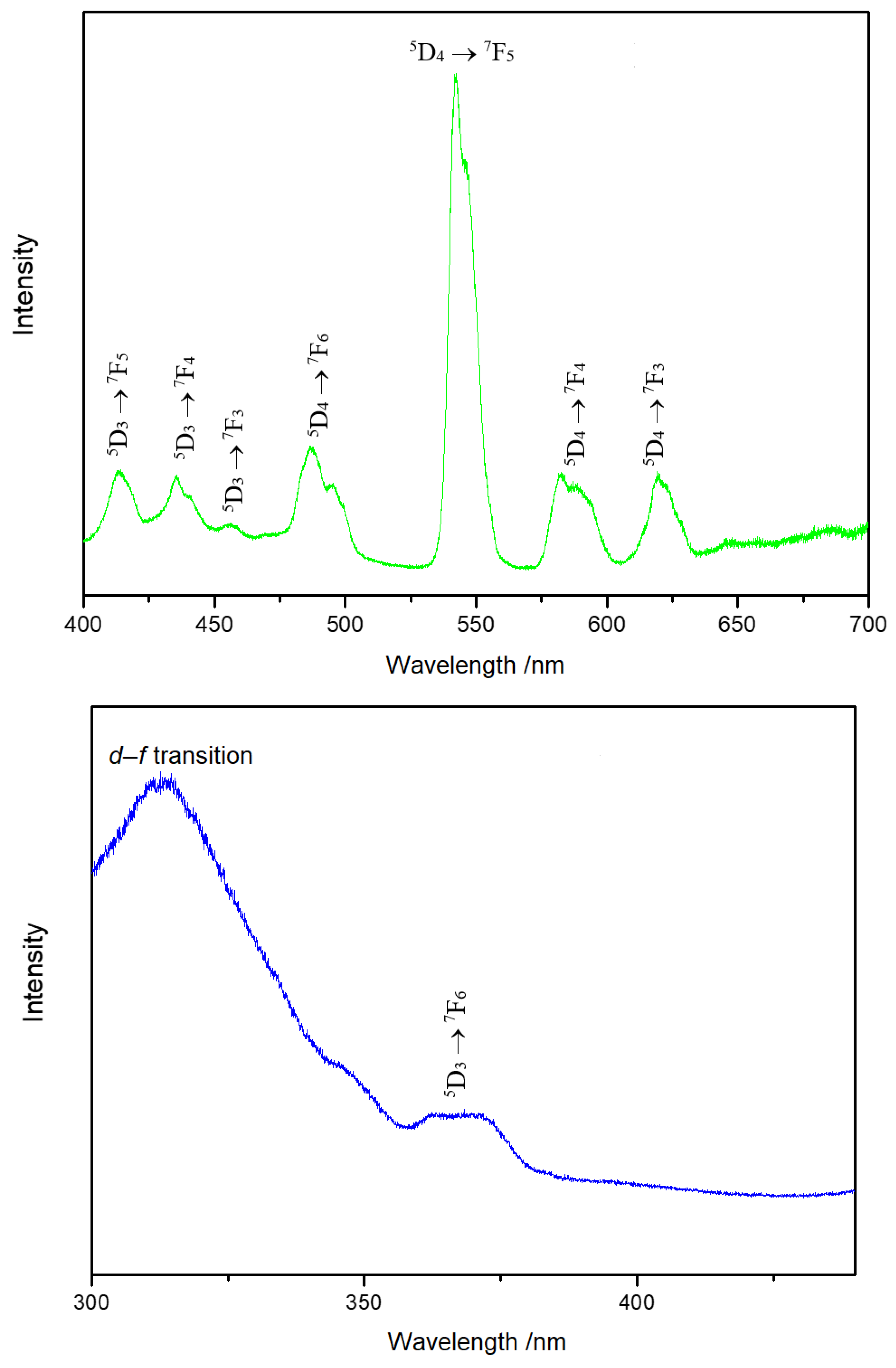
In the emission spectrum of Y3F[Si3O10]:Tb3+ at 370 nm (Figure 7, top), broad bands were detected for the 5D3 → 7FJ and 5D4 → 7FJ transitions. Due to the lot of degeneration possibilities of the f–f transitions, these bands usually appear to be broad. The excitation spectrum of Y3F[Si3O10]:Tb3+ detected at 485 nm (Figure 7, bottom) shows the 5D3 → 7F6 transition of Tb3+ together with an obvious strong broad band, which results from the allowed transition from the 4f8 to the 4f75d1 configuration.
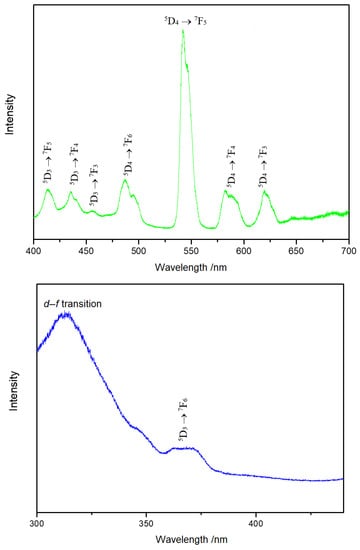
Figure 7.
Emission spectrum of Y3F[Si3O10]:Tb3+ excited at 370 nm at room temperature (top). Excitation spectrum of Y3F[Si3O10]:Tb3+ detected at the emission energy of 485 nm at room temperature (bottom).
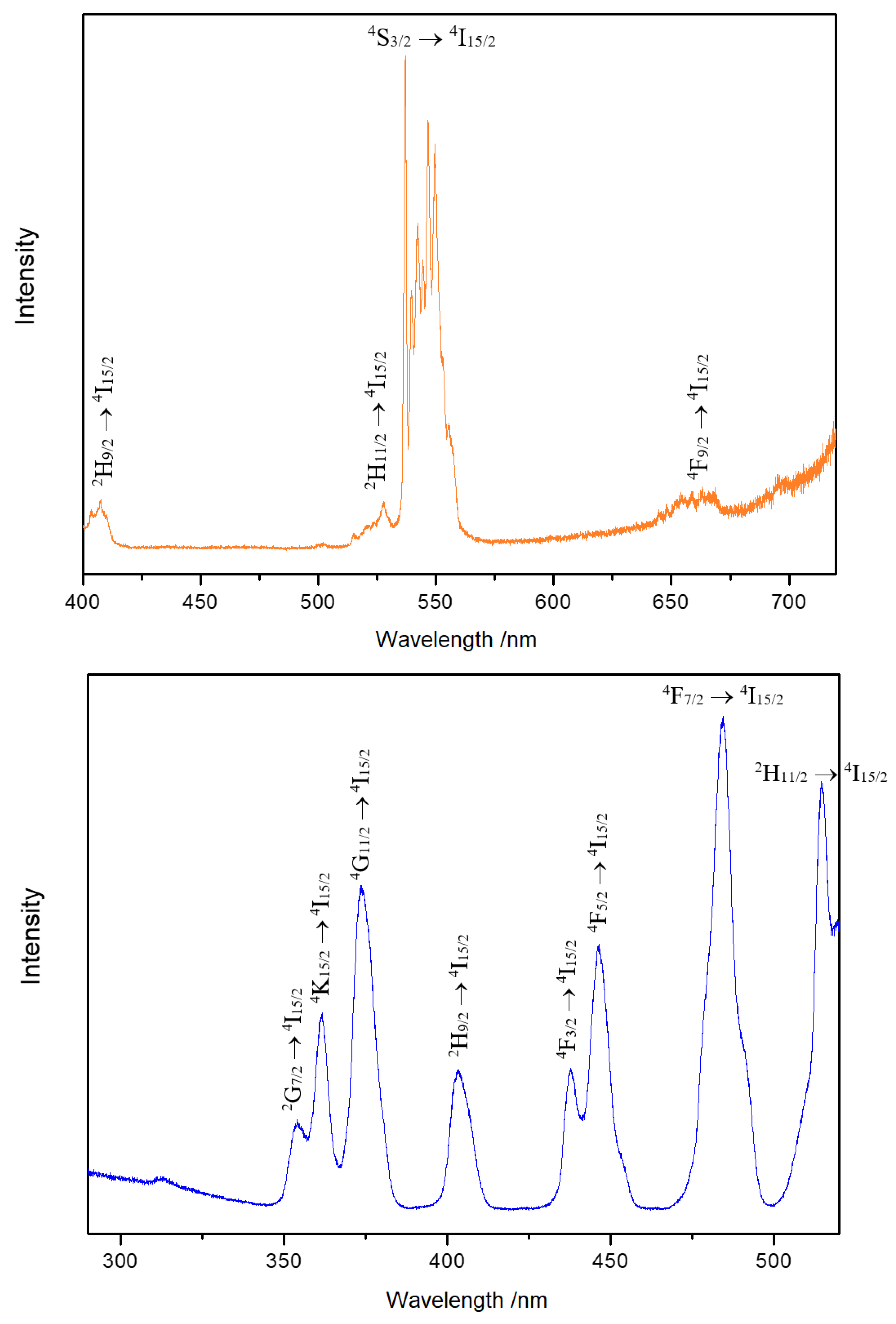
A large number of bands between 536 and 550 nm were detected for 4S3/2 → 4I15/2 in the emission spectrum of Y3F[Si3O10]:Er3+ at 375 nm (Figure 8, top). The excitation spectrum (Figure 8, bottom) recorded at 536 nm shows strong peaks due to f–f transitions. This indicates that the luminescence of Er3+ is almost exclusively owed to f–f transitions of the Er3+ cations.
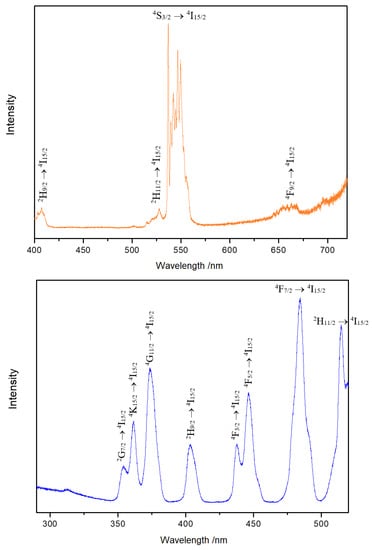
Figure 8.
Emission spectrum of Y3F[Si3O10]:Er3+ excited at 375 nm at room temperature (top). Excitation spectrum of Y3F[Si3O10]:Er3+ detected at the emission energy of 536 nm at room temperature (bottom).
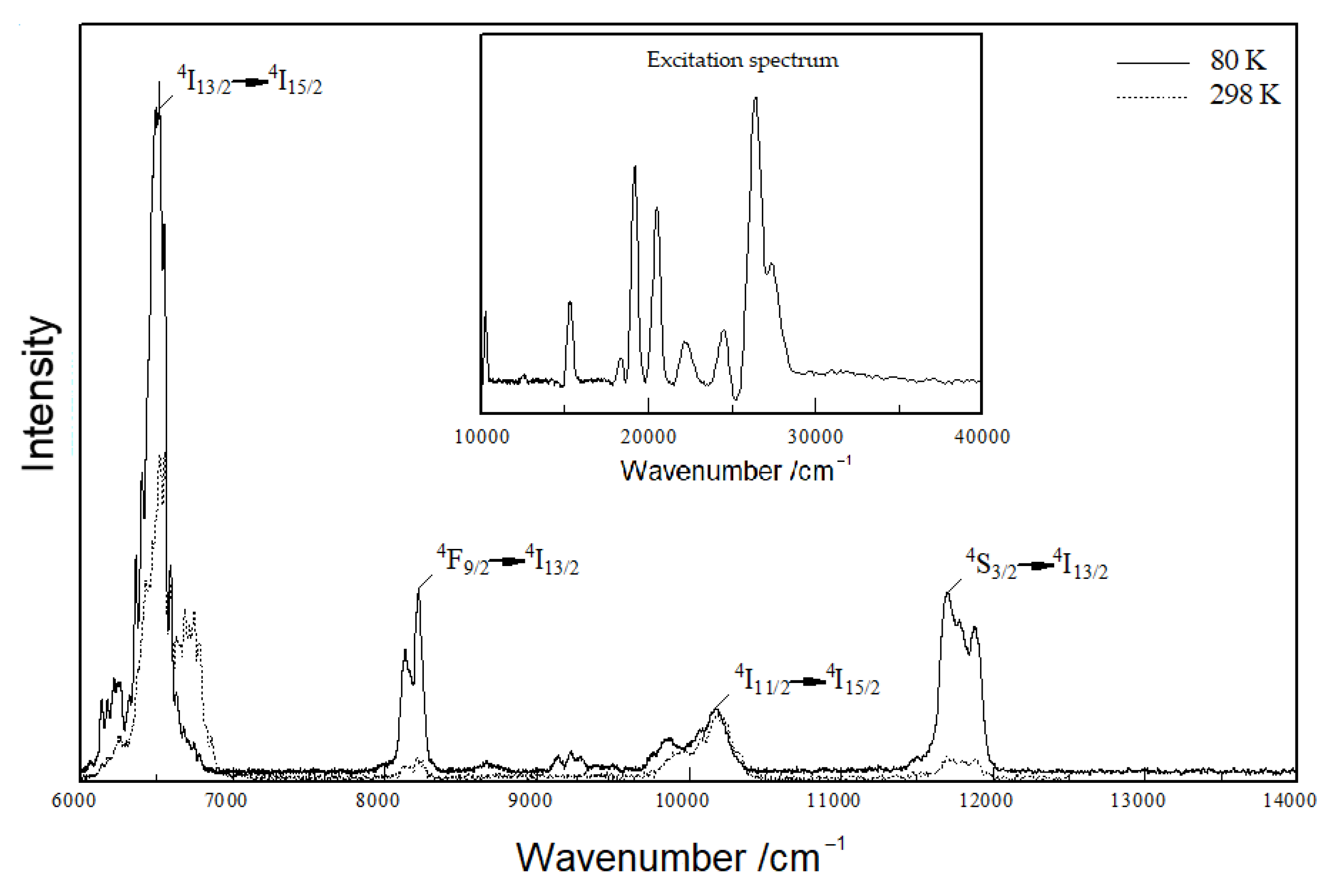
Figure 9 shows that Y3F[Si3O10]:Er3+ also offers infrared emission bands, the strongest at 850 nm (≡ 11765 cm−1), 1000 nm (≡ 10000 cm−1), 1200 nm (≡ 8333 cm−1) and 1500 nm (≡ 6667 cm−1), which can be attributed to 4S3/2 → 4I13/2, 4I11/2 → 4I15/2, 4F9/2 → 4I13/2 and 4I13/2 → 4I15/2 transitions, respectively. However, this special kind of Er3+ IR-luminescence seems more suitable for living tissue probing [37] than for lighting applications.
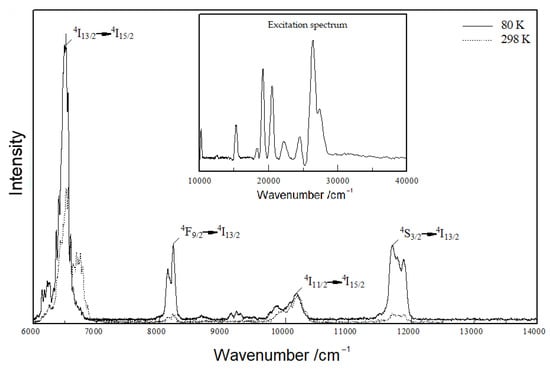
Figure 9.
Emission spectrum of Y3F[Si3O10]:Er3+ excited at 375 nm at room temperature (dotted curve) and 80 K (solid curve); excitation spectrum inserted of Y3F[Si3O10]:Er3+ detected at the emission energy of 536 nm at room temperature.
4. Conclusions
The crystal structure of Tm3F[Si3O10] is presented for the first time with the so far heaviest Ln3+ cation of the Ln3F[Si3O10] series (Ln = Dy–Tm), exhibiting the monoclinic thalenite-type arrangement of Y3F[Si3O10]. The latter itself was used for doping experiments with selected Ln3+ cations replacing 3% of its Y3+ content. With Eu3+ as dopant, the typical red luminescence resulting from 5D0 → 7F2 and 5D0 → 7F1 transitions could be detected and interpreted, while the Tb3+- and Er3+-doped YF3[Si3O10] samples shine green and yellow light, respectively, accompanied by rather strong emissions in the infrared region for the Er3+ case.
Author Contributions
Conceptualization, M.C.S. and T.S.; methodology, M.C.S., M.P. and S.Z.; software, M.C.S. and S.Z.; validation, M.C.S., T.S. and C.W.; formal analysis, M.C.S., T.S. and C.W.; investigation, M.C.S., T.S. and C.W.; resources, T.S. and C.W.; data curation, M.C.S., T.S., S.Z. and C.W.; writing—original draft preparation, M.C.S., I.H., R.J.C.L., C.W. and T.S.; writing—review and editing, M.C.S., I.H., R.J.C.L., C.W. and T.S.; visualization, M.C.S., S.Z., R.J.C.L. and I.H.; supervision, T.S.; project administration, T.S.; funding acquisition, C.W. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the State of Baden-Württemberg (Stuttgart) for financial support. Moreover, we are thankful for the funding from the Deutsche Forschungsgemeinschaft (Bonn) within the SFB 1166 priority program “lanthanoid-specific functionalities in molecule and material”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the conclusions are included within the manuscript and available upon request from the corresponding authors.
Acknowledgments
We thank Falk Lissner for the single-crystal X-ray diffraction measurement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müller-Bunz, H.; Schleid, T. La3F3[Si3O9]: Ein Fluorid-cyclo-Trisilicat des Lanthans. Z. Kristallogr. 1997, S12, 141. [Google Scholar]
- Müller-Bunz, H.; Schleid, T. La3F3[Si3O9]: Das erste Fluoridsilicat aus dem tenären System LaF3/La2O3/SiO2. Z. Anorg. Allg. Chem. 1999, 625, 1377–1383. [Google Scholar] [CrossRef]
- Schleid, T.; Müller-Bunz, H. Einkristalle von Y3F[Si3O10] im Thalenit-Typ. Z. Anorg. Allg. Chem. 1998, 624, 1082–1084. [Google Scholar] [CrossRef]
- Schleid, T.; Müller-Bunz, H. Er3F[Si3O10]: Ein Fluorid-catena-Trisilicat des Erbiums. Z. Kristallogr. 1997, S12, 134. [Google Scholar]
- Müller-Bunz, H.; Schleid, T. Darstellung und Aufbau der Lanthanoidfluorid-catena-Trisilicate M3F[Si3O10] (M = Dy, Ho, Er) im Fluorthalenit-Typ (Y3F[Si3O10]). Z. Anorg. Allg. Chem. 2000, 626, 845–852. [Google Scholar] [CrossRef]
- Kornev, A.N.; Batalieva, N.G.; Maksimov, B.A.; Ilyukhin, V.V.; Belov, N.V. Crystalline structure of thalenite, Y3[Si3O10](OH). Dokl. Akad. Nauk SSSR 1972, 202, 1324–1327. [Google Scholar]
- Yakubovich, O.V.; Voloshin, A.V.; Pakhomovskii, Y.A.; Simonov, M.A. Refined crystal structure of thalenite. Kristallografiya 1988, 33, 605–608. [Google Scholar]
- Schäfer, M.C.; Hartenbach, I.; Schleid, T. Tetrayttrium difluoride disilicate orthosilicate, Y4F2[Si2O7][SiO4]. Acta Crystallogr. 2013, E69, i71. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Schleid, T. Er4F2[Si2O7][SiO4]: Das erste Selten-Erd-Fluoridsilicat mit zwei verschiedenen Silicat-Anionen. Z. Anorg. Allg. Chem. 2001, 627, 218–223. [Google Scholar] [CrossRef]
- Wickleder, C.; Hartenbach, I.; Lauxmann, P.; Schleid, T. Eu5F[SiO4]3 und Yb5S[SiO4]3. Z. Anorg. Allg. Chem. 2002, 628, 1602–1606. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Schleid, T. La7OF7[SiO4]3: Das erste Selten-Erd-Oxidfluorid-ortho-Silicat. Z. Kristallogr. 2002, S19, 115. [Google Scholar]
- Zimmerhofer, F.; Netzer, F.; Tribus, M.; Huppertz, H. Crystal strucuture determination and characterization of Sm3SiO5F3. Z. Naturforsch. 2022, 77b, 657–665. [Google Scholar] [CrossRef]
- Cannas, C.; Mainas, M.; Musinu, A.; Piccaluga, G.; Speghini, A.; Bertinelli, M. Nanocrystalline luminescent Eu3+-doped Y2SiO5 prepared by sol-gel technique. Opt. Mater. 2005, 27, 1506–1510. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, P.; Duan, C.; Yan, K.; Yin, M.; Lou, L.; Xia, S.; Krupa, J.-C. Preparation and size effect on concentration quenching of nanocrystalline Y2SiO5:Eu. Chem. Phys. Lett. 1998, 292, 133–136. [Google Scholar] [CrossRef]
- Ananias, D.; Ferdov, S.; Paz, F.A.A.; Sa Ferreira, R.A.; Ferreira, A.; Geraldes, C.F.G.C.; Carlos, L.D.; Lin, Z.; Rocha, J. Photoluminescent Layered Lanthanide Silicate Nanoparticles. Chem. Mater. 2008, 20, 205–212. [Google Scholar] [CrossRef]
- Reichardt, J.; Stiebler, M.; Hirrle, R.; Kemmler-Sack, S. Cathodo- and Photoluminescence in Oxyorthosilicates of X1 and X2 Type: System Y2−xGdxSiO5: Tb3+. Phys. Stat. Sol. 1990, A119, 631–642. [Google Scholar] [CrossRef]
- Lammers, M.J.J.; Blasse, G. Luminescence of Tb3+-and Ce3+-Activated Rare Earth Silicates. J. Electrochem. Soc. 1987, 134, 2068–2072. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, G.; Xu, X. Crystal growth and spectroscopic properties of erbium doped Lu2SiO5. J. Cryst. Growth 2010, 312, 2103–2106. [Google Scholar] [CrossRef]
- Hayhurst, T.; Shalimoff, G.; Edelstein, N.M.; Boatner, L.A.; Abraham, M.M. Optical spectra and Zeeman effect for Er3+ in LuPO4 and HfSiO4. J. Chem. Phys. 1981, 74, 5449–5452. [Google Scholar] [CrossRef]
- Oskam, K.D.; Kaspers, K.A.; Meijerink, A.; Müller-Bunz, H.; Schleid, T. Luminescence of La3F3[Si3O9]:Ce3+. J. Lumin. 2002, 99, 101–105. [Google Scholar] [CrossRef]
- Schleid, T.; Müller-Bunz, H.; Janka, O. Geo-Inspired Phosphors Based on Rare-Earth Metal(III) Fluorides with Complex Oxoanions: I. Fluoride Oxocarbonates and Oxosilicates. In Minerals as Advanced Materials II; Krivovichev, S.V., Ed.; Springer: Berlin, Heidelberg, Germany, 2011; pp. 353–366. [Google Scholar]
- Schäfer, M.C.; Schleid, T. Synthese und Kristallstruktur des Fluorid-ino-Oxosilicats Cs2YFSi4O10. Z. Anorg. Allg. Chem. 2007, 633, 1018–1023. [Google Scholar] [CrossRef]
- Hoppe, R. The Coordination Number — An “Inorganic Chameleon”. Angew. Chem. Int. Ed. 1970, 9, 25–34. [Google Scholar] [CrossRef]
- Hoppe, R. On the Symbolic Language of the Chemist. Angew. Chem. Int. Ed. 1980, 19, 110–125. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1975, A32, 751–767. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Herrendorf, W.; Bärnighausen, H. HABITUS: Program for the Optimization of the Crystal Shape for Numerical Absorption Correction in X-SHAPE; Version 1.06; Fa. Stoe: Darmstadt, Germany, 1999. [Google Scholar]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97: Programs for Solution and Refinement of Crystal Structures from X-ray Diffraction Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Wilson, A.J.C. (Ed.) International Tables for Crystallography, 2nd edit.; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 1992; Volume C. [Google Scholar]
- Hartenbach, I.; Lissner, F.; Schleid, T. Crystal Structure of B-Type Tm2Si2O7 (≡ Tm4[Si3O10][SiO4]). Z. Naturforsch. 2003, 58b, 925–927. [Google Scholar] [CrossRef]
- Felsche, J. Polymorphism and crystal data of the rare-earth disilicates of type RE2Si2O7. J. Less-Common Met. 1970, 21, 1–14. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Schleid, T. Über die Oxidsilicate M2O[SiO4] der schweren Lanthanoide (M = Dy–Lu) im A-Typ. Z. Anorg. Allg. Chem. 1999, 625, 613–618. [Google Scholar] [CrossRef]
- Sieke, C.; Hartenbach, I.; Schleid, T. Sulfidisch derivatisierte Oxodisilicate der schweren Lanthanide vom Formeltyp M4S3[Si2O7] (M = Gd–Tm). Z. Naturforsch. 2002, 57b, 1427–1432. [Google Scholar] [CrossRef]
- Izotova, O.E.; Aleksandrov, V.B. Crystalline Structure of BaTm2F8. Dokl. Akad. Nauk SSSR 1970, 192, 1037–1039. [Google Scholar]
- Engelmann, U.; Müller, B.G. Tetrafluoroaurate(III) der Lanthaniden MF[AuF4]2 (M = Tm, Yb, Lu). Z. Anorg. Allg. Chem. 1993, 619, 1661–1668. [Google Scholar] [CrossRef]
- Crystal Impact GbR. DIAMOND: Visual Crystal Structure Information System; Crystal Impact GbR: Bonn, Germany, 1999. [Google Scholar]
- Cao, C.; Xie, Y.; Li, S.-W.; Hong, C. Er3+-Ions-Doped Multiscale Nanoprobes for Fluorescence Imaging in Cellular and Living Mice. Nanomaterials 2021, 11, 2676. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).