Abstract
Titanium alloys, particularly Ti6Al4V, are commonly used in biomedical applications. However, the inclusion of aluminum (Al) and vanadium (V) in this alloy can cause cytotoxic effects in the human body, resulting in Alzheimer’s disease and cancer. This study compares the performance of biocompatible alloys containing non-toxic elements, such as tin (Sn) and niobium (Nb), which are considered safe for implantation. Two sets of alloys were selected, Ti5Sn and Ti5Sn5Nb, and their properties were compared to Ti6Al4V. First, the alloys were prepared using a power metallurgical technique. Then, their phase analysis, hardness, wear resistance, strength, and corrosion performance in simulated body fluid (SBF) solution were characterized. Optical microscopy was used to study the microstructure, XRD was used to identify phases, and electrochemical testing was conducted to assess the alloys’ anodic and cathodic characteristics. Nanoindentation techniques were used to analyze surface characteristics, such as elastic modulus, nano hardness, and wear resistance. The results showed the alloys containing Nb and Sn had lower corrosion rates in SBF solution compared to Al-containing alloys. Moreover, Nb-containing alloys exhibited the highest hardness, 72% higher than Al-containing alloys. The corrosion-resistant properties of the alloys containing Nb and Sn were higher than those without Nb or Sn, suggesting they may be ideal for orthopedic implants in humans.
1. Introduction
Biocompatibility and corrosion resistance are the two most significant attributes of biomaterials. The most commonly used metallic biomaterials include stainless steels, cobalt alloys, titanium, and titanium alloys [1,2]. Compared to competitive alloys, such as SS 316L and CoCr alloys, titanium alloys have superior mechanical properties, improved biocompatibility, low densities (ranging from 4430 kg/m3 to 4850 g/cm3 depending on the alloying elements), and non-toxicity. In a study conducted by FA Anene et al. [3] the mechanical characteristics of several Ti and Ti-based alloys were demonstrated [4,5]. When titanium and its alloys come into contact with bone, no fibrous tissue barrier is formed. For applications that require high mechanical strength, such as hip joint implants, titanium alloys should be used because they have excellent mechanical qualities compared to pure titanium [6,7]. Among all titanium alloys, Ti6Al4V is the most commonly used implanted material due to its strength of 1000 MN/m2 (19%) and increased corrosion resistance of 40% compared to pure titanium [8]. However, the material ions, such as Al and V [9], can create toxicity in the surrounding tissues when worn out in the body. Extensive studies are being conducted to develop an alloy that does not contain Al and V elements [2,10]. Additionally, Wang et al. [11] discussed that the toxicity levels of Al, V, and Fe are higher than those of other elements, such as nickel (Ni), niobium (Nb), and tin (Sn). The addition of Sn to the titanium matrix improves hardness and corrosion resistance and helps reduce the modulus of elasticity [5,12]. The addition of Nb not only stabilizes the beta phase but also helps decrease the modulus of elasticity of the alloy by about 24% [13,14,15]. The mechanical properties of β-type Ti-based alloys, such as an elastic modulus of 30 GPa and a strength of up to 160 MPa, make them suitable for orthopedic applications, especially hip joint implants, as discussed by Yuhua Li et al. [16,17].
In their study, Khorasan et al. [18] analyzed various manufacturing processes for titanium alloys used in biomedical applications. The most commonly used techniques include additive manufacturing, casting, and powder metallurgy [18]. Additive manufacturing, also known as 3D printing, is an automated process that utilizes computer-aided design (CAD) to manufacture complex geometries. Although it has the potential to produce high-quality parts, further research is necessary to standardize the process and ensure accuracy in the final product. This is because finishing processes are still necessary to achieve the desired level of precision [18,19]. Casting, on the other hand, is the oldest method of fabricating implant materials. This involves selecting appropriate chemical compositions and melting them in a vacuum furnace before casting them into suitable molds. Although casting is a well-established technique, additional procedures, such as machining, are often necessary, which can increase the overall cost of the component [20,21,22]. Additionally, maintaining the correct chemical composition during the casting process can be challenging, which may require additional processing steps. To reduce processing costs, powder metallurgy technology is widely used to generate near-net parts or components. However, machining may still be necessary in some cases, such as when grooves or tapered holes are required. Despite these challenges, the use of titanium alloys in biomedical applications continues to grow [23].
This study aims to produce and compare the properties of three different alloys, Ti6Al4V, Ti5Sn, and Ti5SnNb, that were created using a powder metallurgy route. The objective of this research is to examine the effectiveness of using Nb and Sn as substitutes for Al in the alloy Ti6Al4V. To achieve this objective, the properties of each alloy were extensively analyzed and compared to each other. Powder metallurgy was used to produce these alloys in a cost-effective manner. This technique involves blending the raw materials in powder form and then compacting them under high pressure to create a solid billet. The billet is then heated to a high temperature to sinter the powder particles together into a solid mass. This method allows for precise control over the composition of the alloy and the resulting microstructure. The alloys were characterized using a variety of techniques, including X-ray diffraction (XRD), optical microscopy (OM), scanning electron microscopy (SEM), potentiostat, and nanoindentation. XRD was used to identify the phases present in each alloy, while OM and SEM were used to examine the microstructure. Potentiostat measurements were used to evaluate the corrosion resistance of each alloy, and nanoindentation was used to measure the mechanical properties of the alloys. The results of this study will help to determine whether Ti5Sn and Ti5SnNb can be used as substitutes for Al in the alloy Ti6Al4V. By comparing the properties of each alloy, this research will provide valuable insights into the effectiveness of using Nb and Sn as replacements for Al. The findings of this study will be of interest to material scientists, engineers, and manufacturers who are interested in developing new alloys with improved properties.
2. Experimental Section
2.1. Material
Ti, Sn, Nb, Al, and V were purchased from Sigma Aldrich, St. Louis, MO, USA in powder forms. Purchased powders were used in as-received condition without any treatment. The purity level as indicated by the manufacturer is shown in Table 1.

Table 1.
Raw powders with their purity.
2.2. Method
The powders were properly mixed (compositions are shown in Table 2) in a mixer (four tank mixer: MTI Corporation, Richmond, CA, USA) for 1 h at room temperature (25 °C) at 200 rpm for homogenization that enhanced the interfacial reaction of the powders with the titanium matrix. Conventional sintering was done for consolidation of the powders using the D2 die with dimensions of 1 cm inner diameter and outer diameter 12.5 cm at a pressure of 500 MPa and was sintered at 1200 °C in an argon environment using a tube furnace (SG-GS1200). The heating and cooling rate of 5 °C/min and the holding time of 4 h were followed. The proportion of alloying elements were selected as per our previous work [6]. It is worth mentioning that for each characterization technique at least 3–5 samples were utilized so margin of error could be minimized.

Table 2.
Composition of the alloys.
3. Characterization
3.1. Analysis of Powders
3.1.1. X-ray Fluorescence
X-ray fluorescence (XRF, ZSX primus, Rigaku, Tokyo, Japan) was utilized to confirm the purity level of the as-received powders. These results were also re-affirmed by using XRD.
3.1.2. SEM with EDX Analysis
SEM (TESCAN, Series; Vega-3, Prague, Czech Republic) with EDX was used to study and analyze the geometrical shapes of the powders.
3.2. Analysis of Developed Alloys
3.2.1. Density
The relative density and porosity of the newly developed alloys were determined using Equations (1) and (2), respectively [5,24].
Hence, the (relative, sintered), (green density), and (sintered density) of alloys, respectively, are presented as:
where Pt is the total porosity present in the alloys.
3.2.2. Microstructural Examination
For the microstructural examination, the surface was grounded with the help of silicon carbide (120 to 1000 grit paper), polished (using alumina solution 1 and 0.5 microns), and etched with the help of Kroll’s reagent (92 mL H2O, 6 mL HNO3 and 2 mL HF) [25]. A light (optical) microscope (IM-901) (Metkon Instruments Inc., Bursa, Turkey) was used to study the microstructure of the samples.
3.2.3. XRD Analysis
XRD (Amsterdam, The Netherlands X-Pert Pro XRD DY3313) was utilized for the study of different phases and compounds present in the sample. The diffracted peaks were analyzed and recorded in the 2θ range (i.e., 10 to 79°); the scan rate of about 0.1 with a step time is about 2 s using the Cu (kα) radiation.
3.2.4. Corrosion Resistance
Computerized potentiate Model G-750 (Gamry instruments, Warminster, PA, USA) was utilized for the determination of the corrosion rate. The electrolytic cell was comprised of the reference electrode (i.e., sutured calomel electrode), the working electrode (i.e., sample alloys prepared according to the ASTM Standard G 108-94), and the counter electrode (i.e., platinum). The scan rate of 0.05 mV/s was used to estimate the rate of corrosion of the alloys, and the temperature of the SBF solution [26] was maintained at 37 °C. The polarization curves were obtained between the potential and the current. The intersection of the anodic and the cathodic points reveals the potential (Ecorr) and the current density (Icorr) that were measured using the intersection method [27]. As a minimum, one span of the segment in Tafel extrapolation is necessary to confirm the accuracy. The corrosion rate is determined using Equation (3) [28].
3.2.5. Nanoindentation
Nanoindentation (Anton Paar UHT3, Graz, Austria) was conducted with the Berkovich indenter and was used to analyze the samples. The indenter is made up of a diamond (equipped with a three-sided pyramid). The nanoindentation technique was performed on three different sets of samples having different compositions. To reduce the error and sustain the accuracy, each sample was tested at least 10 times, and the reported data is averaged out. The test was performed according to the standard ISO 14577. Comparatively, this technique was used to measure the depth of penetration again the load applied on the mirror-like surface of the alloys. For accuracy and quality maintenance of the test, the nanoinductor was calibrated using silica [29]. The parameters used during the test are listed in Table 3.

Table 3.
Parameters used during the nanoindentation.
4. Results and Discussion
4.1. Analysis of Powder
4.1.1. Chemical Composition
The as-received powders were firstly analyzed via XRF to determine the chemical composition of the powders. The purities of the powders are given in Table 4. This test confirmed the materials used in this study were of a purified nature and free from inclusions.

Table 4.
Purities of the powders.
4.1.2. XRD of Pure Powders
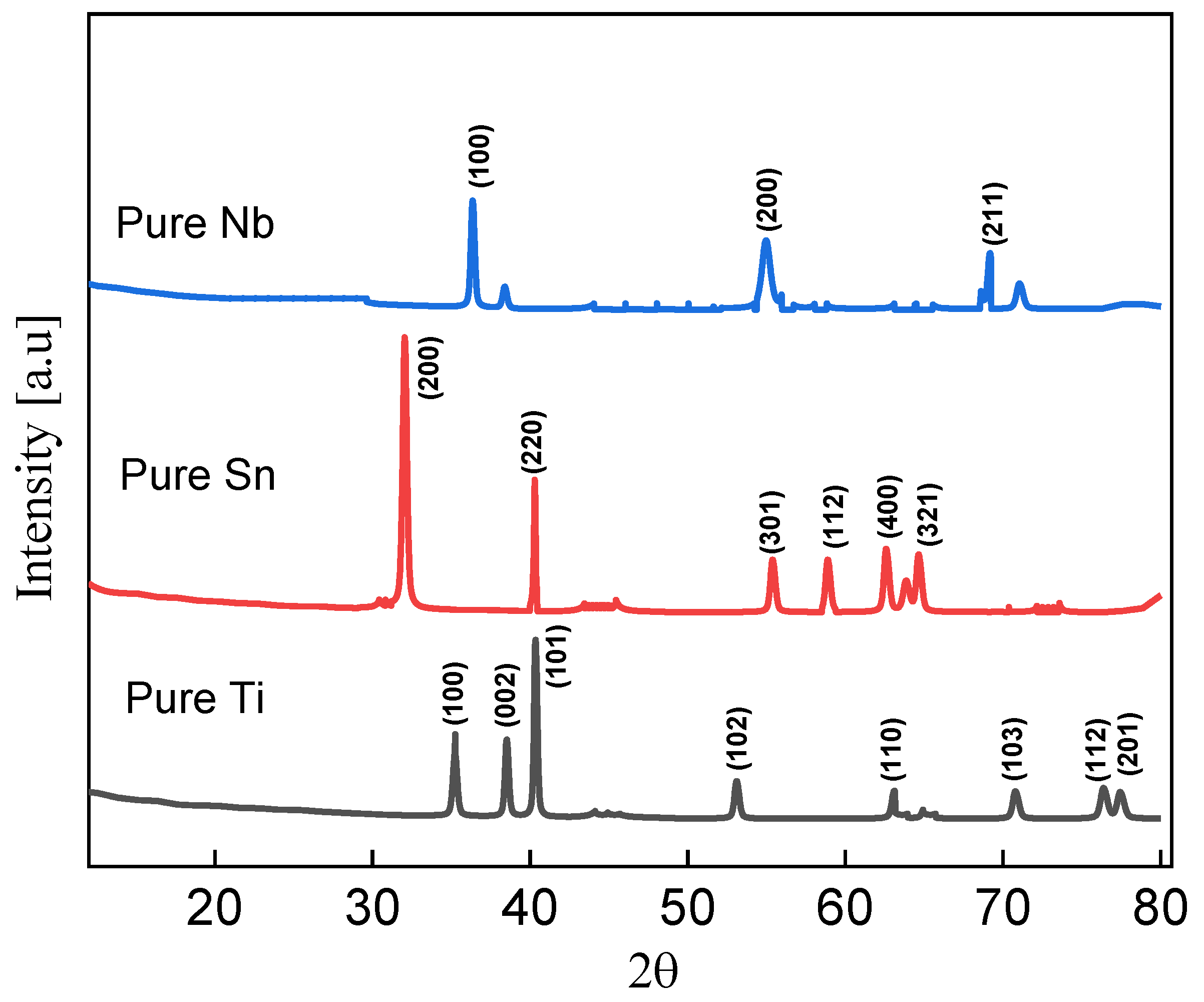
Figure 1 displays the X-ray diffraction (XRD) spectra of pure Nb, Sn, and Ti powders (JCPDS no. 035-0789, 01-086-2264, and 01-1197 for Nb, Sn, and Ti, respectively) [30,31,32]. The strongest diffraction peak, which indicates the satisfied orientation of niobium, was found to be at (100) and 54° [20]. The pure Sn powder sample’s sharp diffraction peaks at 31° and 40° in Figure 1 [33]. The Ti diffraction peak identified belongs to the (100) plane, and the plane distance is 2.56 Å throughout the range of the two diffracted (15°–85°) angles [34].
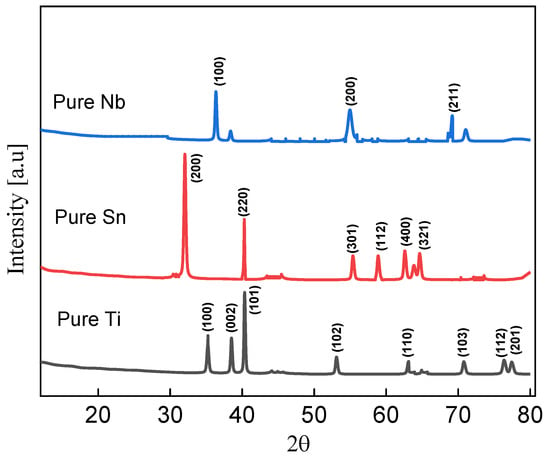
Figure 1.
XRD of pure Nb shown as blue line, XRD of pure Sn shown as red line, and XRD of pure Ti indicated by black line.
4.1.3. SEM with EDX Analysis
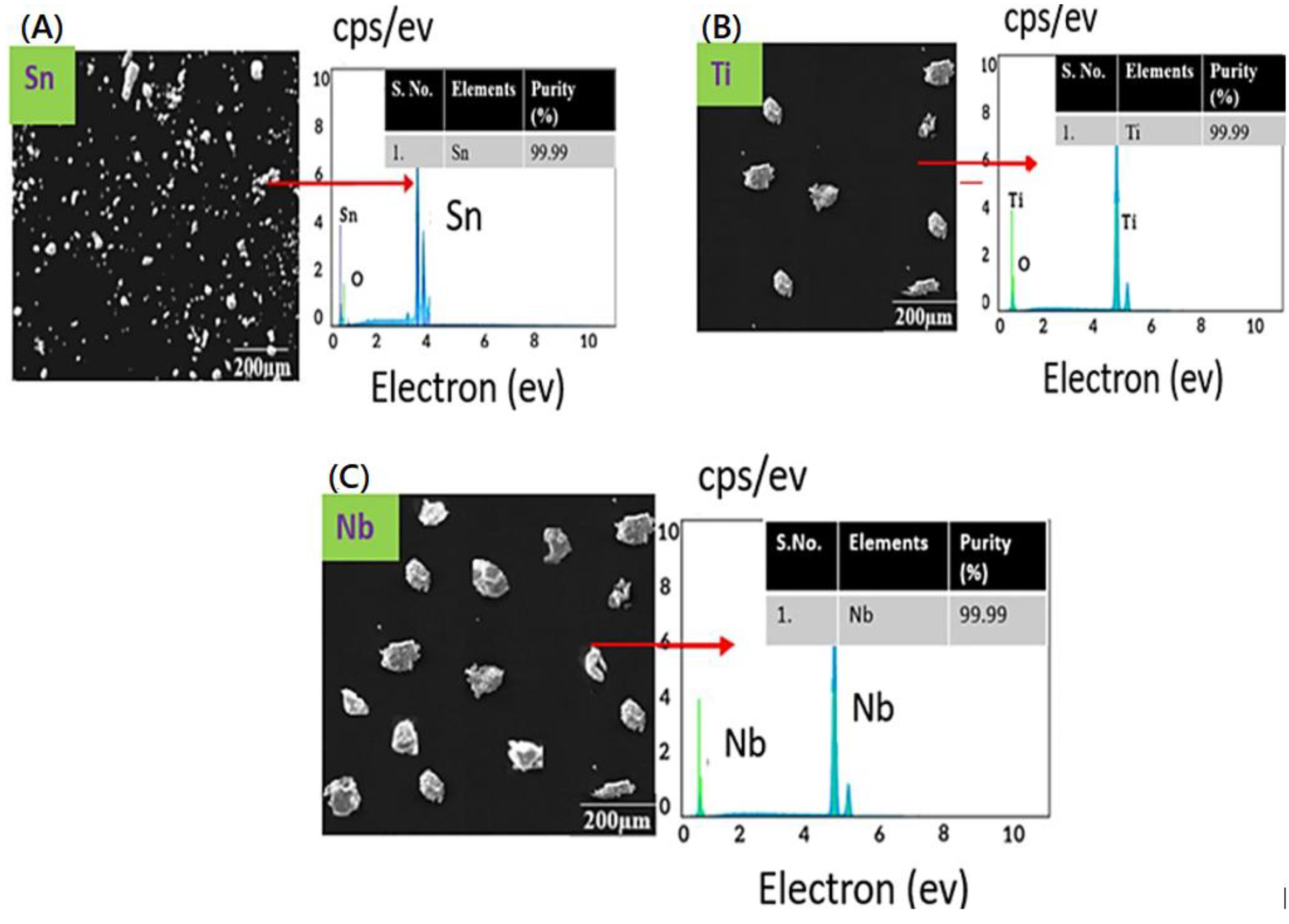
The microstructure analysis of the received powders are shown in Figure 2. The powders were found to be irregular in shape, and the sizes were found to be less than 100 microns. The elemental compositions of the titanium, tin, and niobium powders obtained from the energy dispersive spectroscopy are shown in Figure 1. Moreover, EDS analysis confirms the purity of powders, i.e., 99.9% pure (Figure 2A–C).
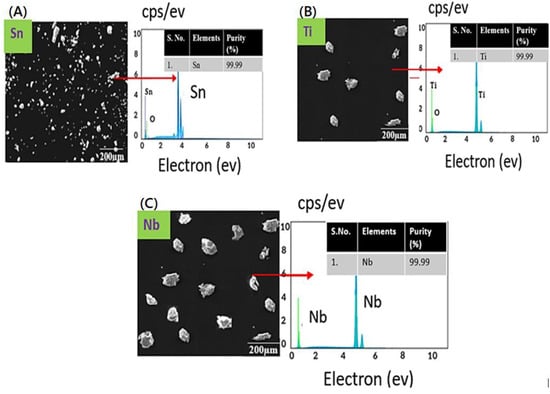
Figure 2.
SEM micrographs and EDS spectra of the samples, (A) SEM image and EDS spectra of tin, (B) SEM image and EDS spectra of titanium, (C) SEM image and EDS spectra of niobium.
4.2. Analysis of Developed Alloy
4.2.1. Green and Sintered Density
The measured densities of the Ti-based alloys are shown in Table 5, wherein the densities were enhanced after sintering, owing to densification up to 95%. The densities of the Ti5Sn, Ti5Sn5Nb, and Ti6Al4V reached up to 68%, 79%, and 84%, respectively. The theoretical density of the Ti-based alloy was 60–85 g/cm3 in this experiment, and the sintered density steadily enhanced as the sintering temperature increased [11,35]. With the increasing sintering temperature, the powder particles border developed from weak mechanical bonding to strong metallurgical bonding, and the interior pores of the green became smaller or disappeared [5,36].

Table 5.
Green and sintered densities of the alloys.
4.2.2. Relative Density (
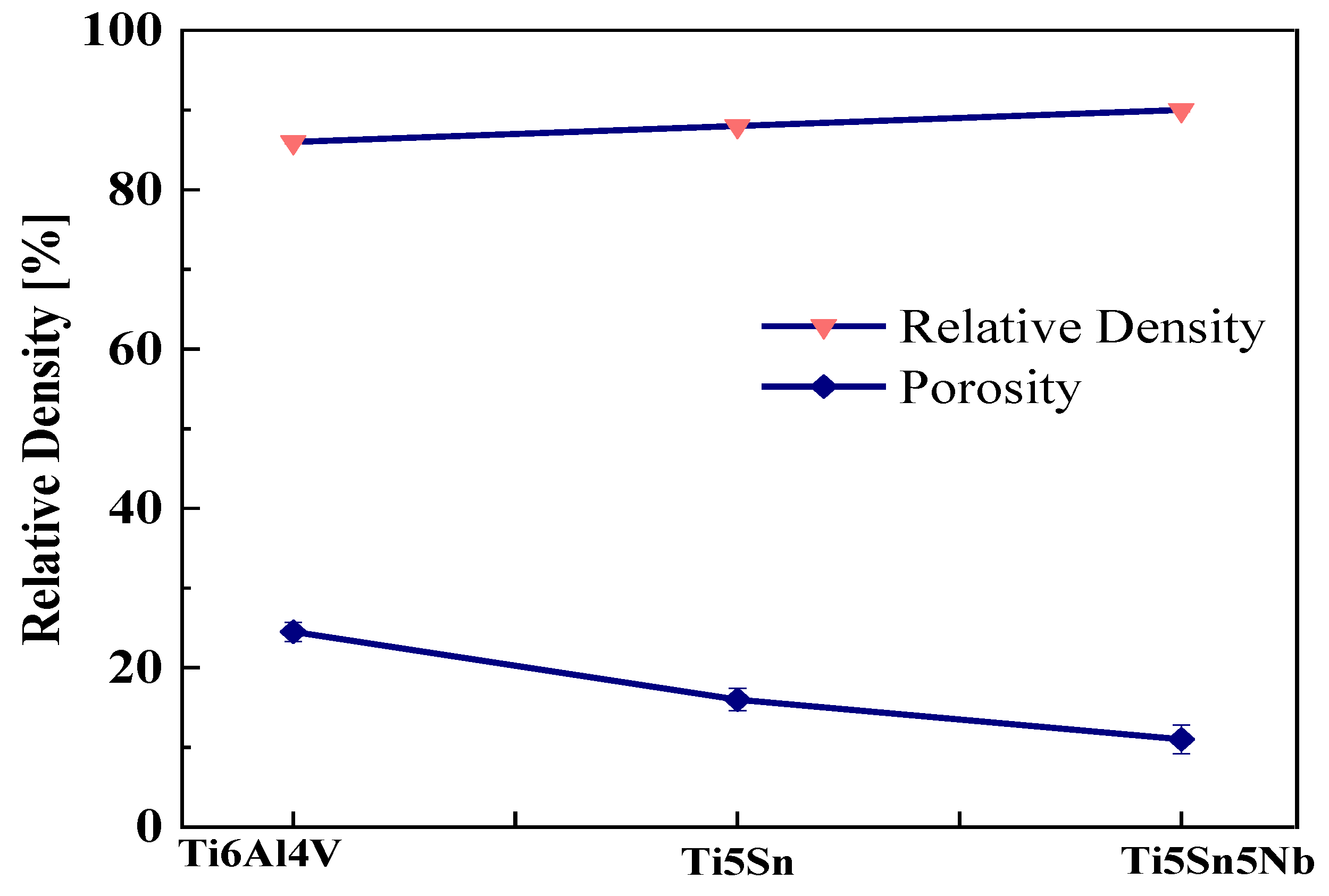
Figure 3 shows the of the Ti5Sn, Ti5Nb5Sn, and Ti6Al4V alloys. The effect of niobium was observed when added to Ti5Sn, and it was found that the addition of niobium does not significantly reduce the porosity level in the samples when compared with the Ti5Sn. The irregular morphology of Nb contributes to its higher density. in the Ti5Sn5Nb alloy [37]. Mahundla et al. [38] also discussed the similar phenomena of densification. The higher the densification level, the lower the chance of porosity present in the sample. The increased relative density of these samples may be attributed to the surface that has a minor number of holes, which may maximize the densification of the material [39,40].
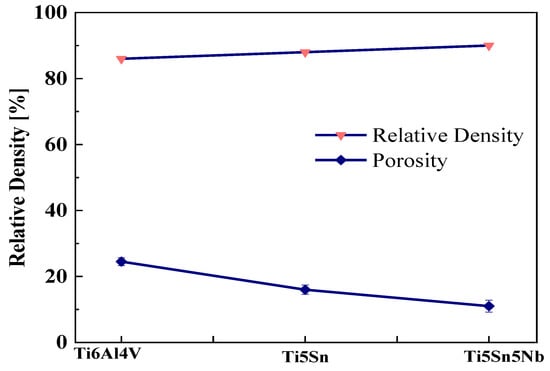
Figure 3.
Relative density and porosity of Ti-based alloy. Upper line shows relative density, and lower line shows porosity.
4.2.3. Microstructural Analysis
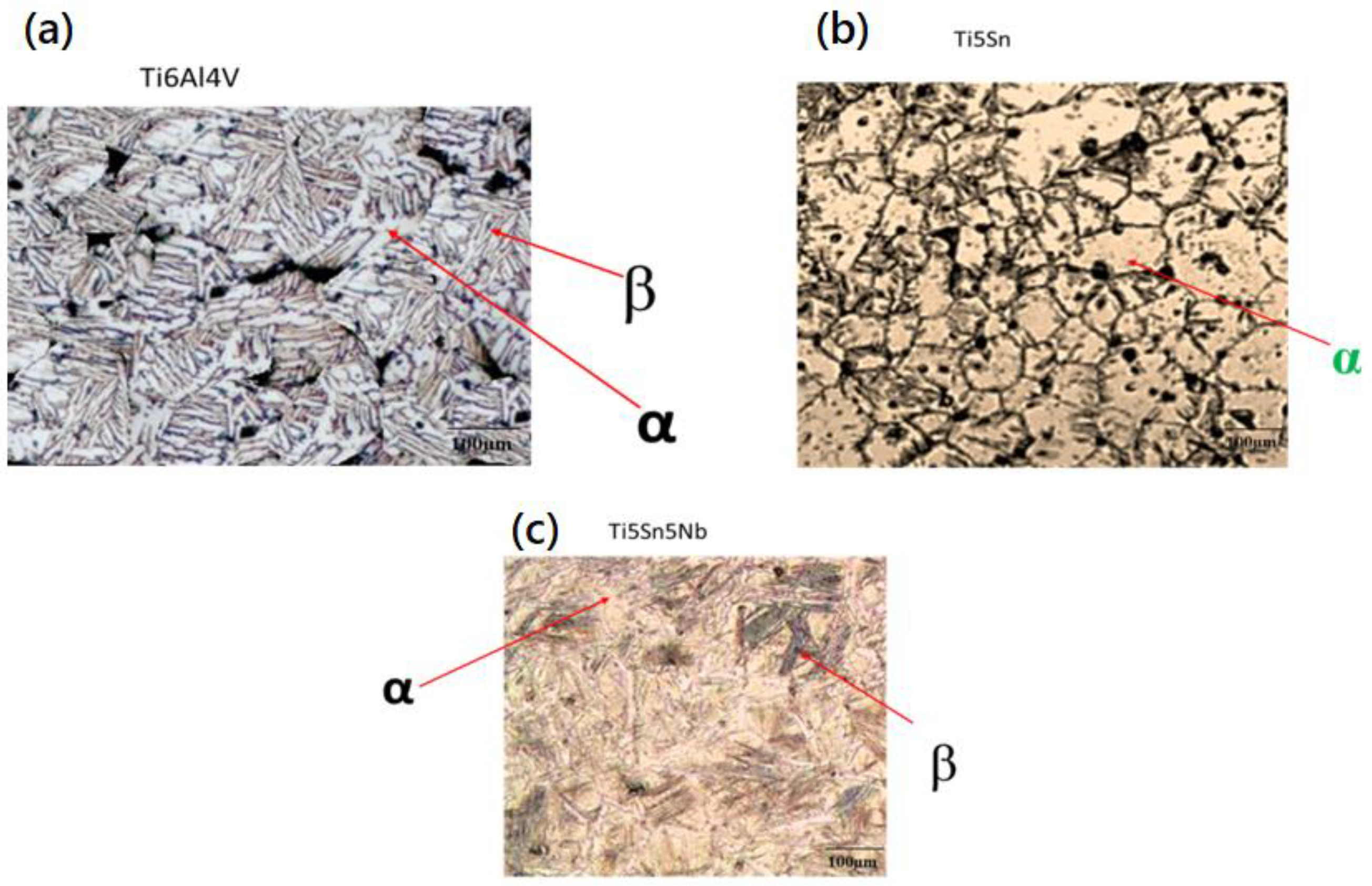
Figure 4 shows the microstructural examination of Ti5Sn, Ti5Nb5Sn, and Ti6Al4V [41]. Dual phases (α and β) were observed in Ti5Nb5Sn and Ti6Al4V due to the presence of the beta stabilizers, i.e., Nb and V in Figure 4a and 4b, respectively [42]. Furthermore, the Widsmanstatten structure (due to growth of β phases within the grain boundaries) and porosity (because of low densification of the alloys) were also observed in both alloys [43]. Figure 4c shows the micrograph of Ti5Sn. It consists of the equiaxed grains alpha phase, and the dark spots show the presence of porosity in the network. This alpha phase reveals the addition of tin in the titanium matrix does not affect the phase as the difference in the atomic radius (not greater than 15%). The alloy has an alpha phase, which was also confirmed with the phase diagram of Ti-Sn. The XRD was used for the confirmation of the α and β phases in Ti5Nb5Sn and Ti6Al4V and only the alpha phase in Ti5Sn [29,44,45,46,47,48,49,50].
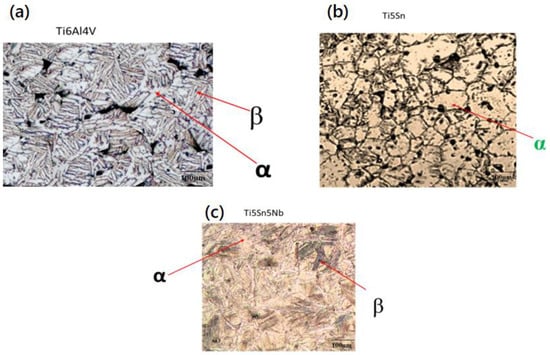
Figure 4.
Optical micrographs of the synthesized alloys via powder metallurgy route. (a) Ti5Sn5Nb, (b) Ti6Al4V, and (c) Ti5Sn show alpha and beta regions.
4.2.4. Phase Analysis
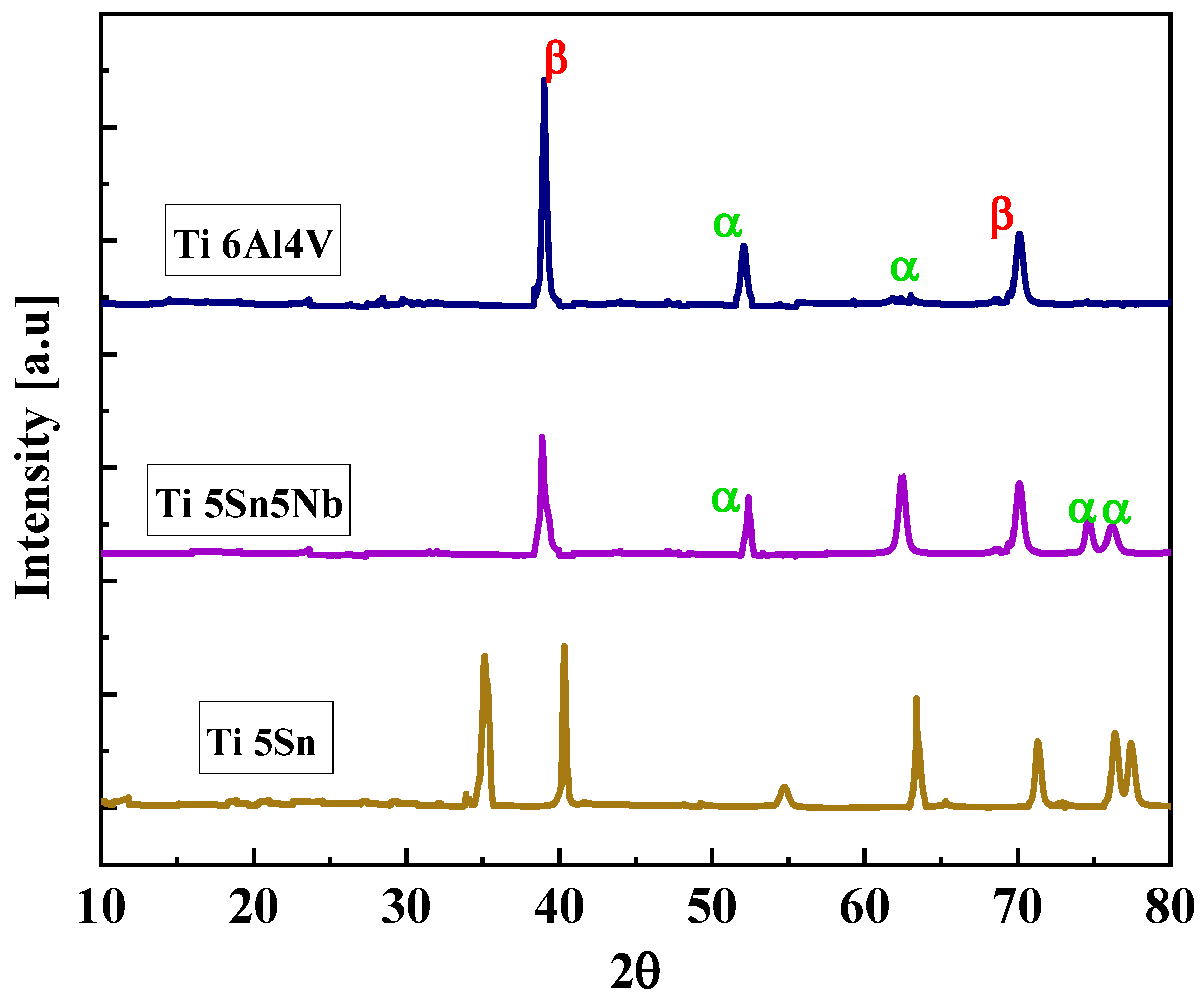
Figure 5 shows the XRD pattern of the prepared alloys, i.e., Ti5Sn, Ti6Al4V, and Ti5Nb5Sn. The diffracted peaks of Ti6Al4V and Ti5Nb5Sn show the alpha and beta phases when matched with the standard JCPDS file (JCPDS file #44-1294) and for the cubic β-Ti (JCPDS file #44-1288) [51], while Ti5Sn exhibits the alpha phase. Because Sn is a neutral stabilizer, it is not affected by the transition temperature [52,53].
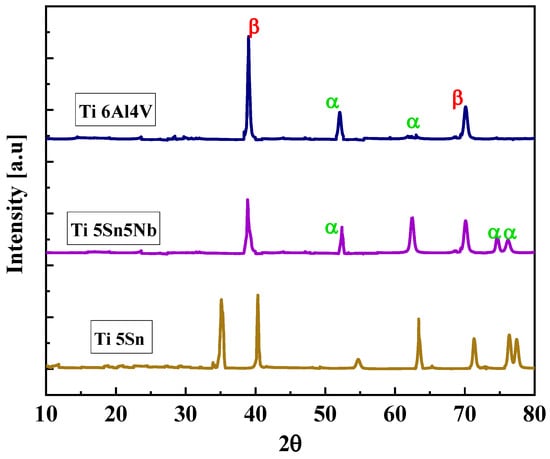
Figure 5.
Diffracted peaks of Ti5Sn, Ti5Sn 5Nb and, Ti6Al4V. Brown line indicates Ti6Al4V. Purple line shows Ti5Sn5Nb, and pink line represents Ti5Sn.
4.2.5. Corrosion Rate
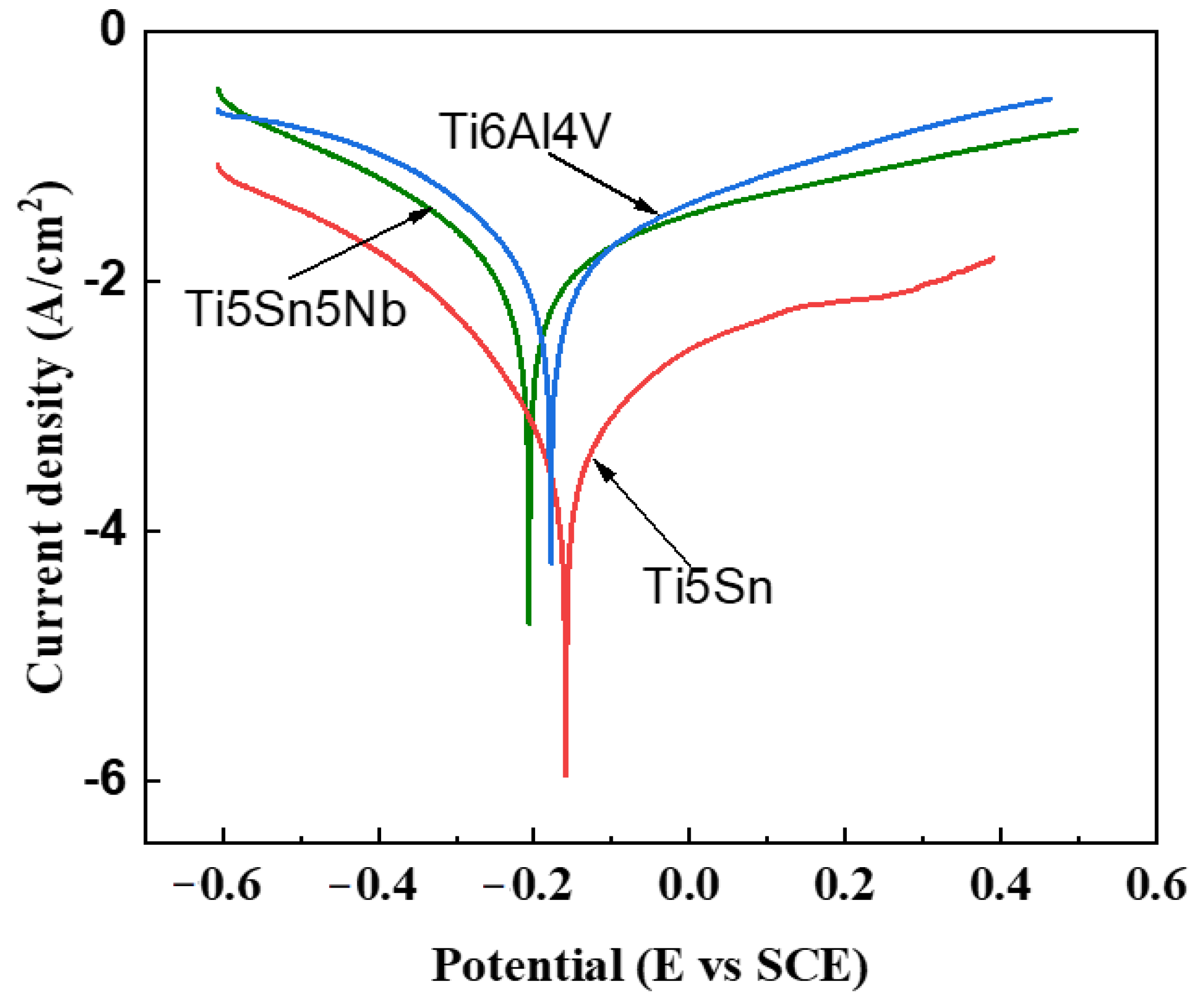
Corrosion resistance is a critical property for orthopedic implants to survive in the human body. When these implants are inserted, they can release metallic ions due to the unstable oxide film on their surface. This can result in severe reactions with the surrounding tissues, leading to various diseases. The strength of the oxide layer is influenced by the alloy composition. Tafel curves are used to study the electrochemical behavior of these alloys [54,55]. In this research work, Tafel curves were generated for three alloys, TiAl4V, Ti5Sn, and Ti5Sn5Nb, which were analyzed in the SBF fluid at 37 °C with pH of 7.4 (see Figure 6). The voltage range of −0.6 to 0 V was examined, and the ASTM G 108-94 standard was used to prepare the anode or working electrode. The current density (Icorr) was calculated from the Tafel curves, and the corrosion rate was determined using Equation (3) and is presented in Table 6. The results showed Ti5Sn had the lowest Icorr and highest Ecorr, while Ti6Al4V had the highest Icorr and lowest Ecorr. In contrast, Ti5Sn5Nb had moderate Icorr and Ecorr values. The addition of niobium in Ti5Sn5Nb prevented the ion exchange with the fluid, leading to the formation of a Nb205 layer at the surface, which improved the resistance to corrosion. The composition of the alloy influences the strength of the oxide layer, which in turn, affects the implant’s ability to resist corrosion in the human body. Understanding this behavior can help improve the design and selection of materials for orthopedic implants, reducing the risk of adverse reactions in patients [56].
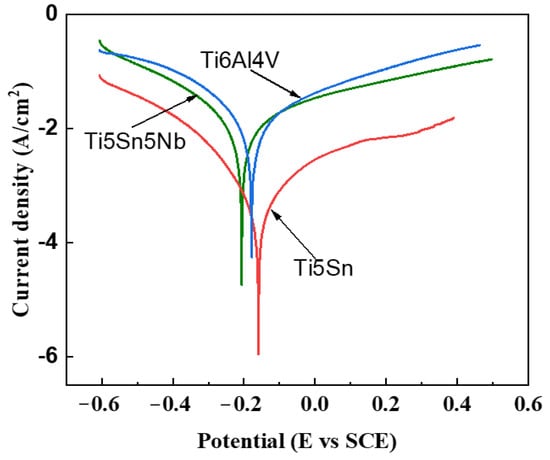
Figure 6.
Tafel curves of the alloys in SBF solution maintained at PH of 7.4 and a temperature range of 37 °C. Red line shows Ti5Sn. Blue line represents Ti6Al4V, and green line indicates Ti5Sn5Nb.

Table 6.
Parameters of corrosion found via Tafel extrapolation.
4.2.6. Nanoindentation
Load versus Displacement (L vs. D) Curves of the Ti6Al4V, Ti5Sn, and Ti5Sn5Nb Alloys
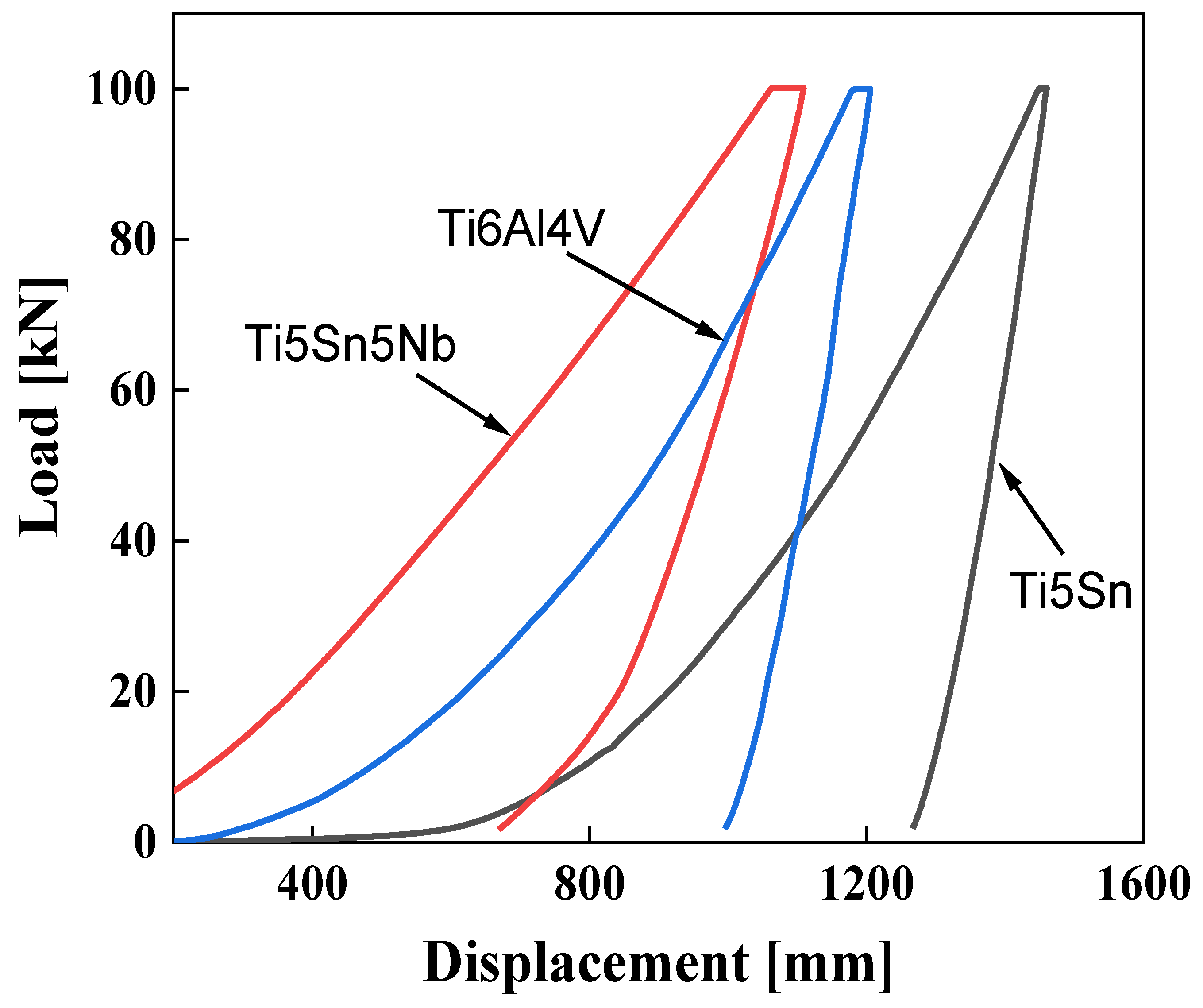
Nanoindentation tests comprise three major stages, (i) loading, (ii) holding, and (iii) unloading. The curve of loading is further comprised of two different stages; t first, the loading is elastic, which shows the elastic region (i.e., elastic deformation) in the material and secondly, the plastic region, which shows the plastic region in the material shown in Figure 7. The holding stage shows the plastic region, i.e., the time-dependent deformation of the alloys. In the last stage, the recovery of the elastic deformation is observed. The O-P (Oliver and Pharr) method is utilized for direct measurement of the H and E values of the alloys. Figure 7 reveals the load (P) versus displacement (h) curves of Ti6Al4V Ti5Sn and Ti5Sn5Nb are respectively found during the nanoindentation. Basically, the curves, which are based on load and displacement, depend upon various factors, such as the environment and the applied load. The curve P and H was observed for the alloys, and it was found the depth decreases in the case of Ti5Sn 5Nb as the hardness value increases [57]. This considerable low depth is related to the higher hardness when compared with Ti5Sn and Ti6Al4V. The curve towards the left side indicates the materials exhibit higher hardness. Comparatively, Ti5Nb5Sn shows the minimum displacement with minimum elastic modulus. However, the hardness of Ti6Al4V and Ti5Sn indicates the lower hardness and higher Young’s modulus.
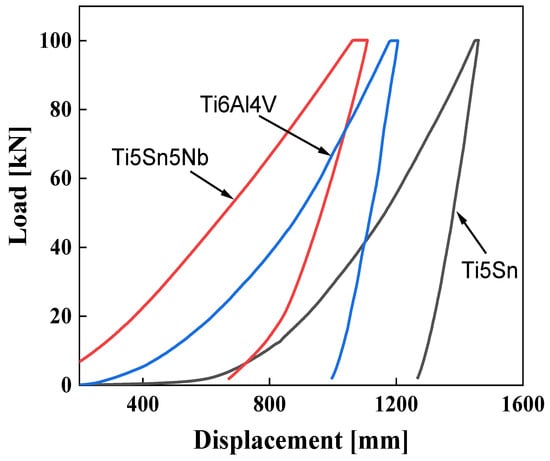
Figure 7.
Nano hardness curves of Ti6Al4V, Ti5Sn, and Ti5Sn5Nb. Red line indicates Ti5Sn5Nb alloy. Blue line represents Ti6Al4V alloy, and black line shows Ti5Sn alloy.
The freighting part of the load, i.e., P curve vs. H curve, are related to the multifaceted reaction of the material to strain, including elastic, though the unloading section of the curve for most alloys comprises elastic recovery. The slope of the unloading and modulus are firmly related to the stiffness, which results in the enhancement in modulus [57] The shifting of the Ti5Sn5Nb curve (from 1250 to 700 nm) towards the left is because of the enhanced strengthening mechanism and load-shearing capability.
Nano Hardness
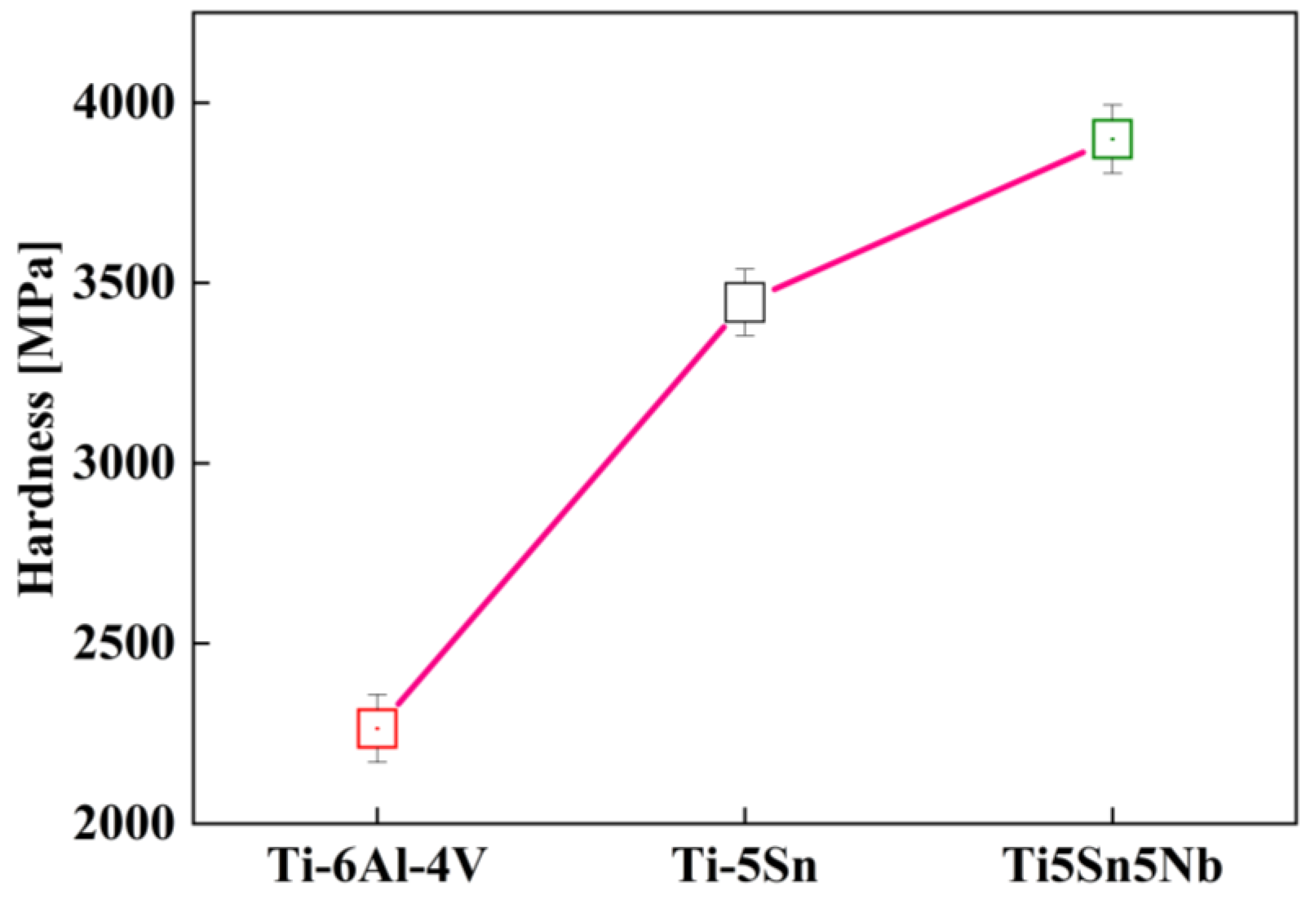
The advance nanoindentation technique is widely utilized to calculate the material hardness of the alloy at the determined load on the L vs. D curve [23]. Figure 8 shows the hardness trend of the alloys. The hardness values of Ti5Sn5Nb is 3899 GPa, whereas the hardness values of the other alloys (Ti6Al4V and Ti5Sn) are 2264 and 3446 GPa, respectively. However, the 72% increase in Ti5Sn5Nb hardness value was observed due to the addition of the niobium content [58,59]. Praveen Chapala et al. [31] reported the addition of Nb (β stabilizer) produces a higher beta fraction phase, which enhanced the overall hardness of the alloy.
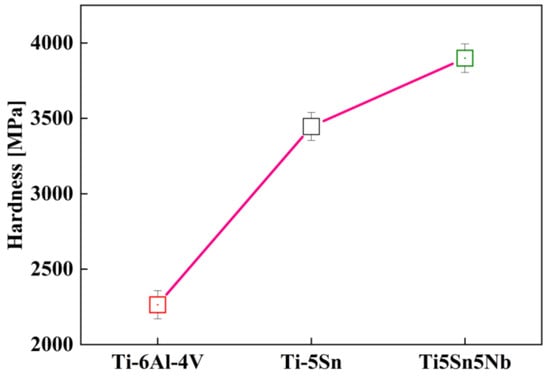
Figure 8.
Nanoindentation hardness versus Ti-based alloy composition at 50 Mn load with dwell time of 3 s, the red curve depicts the harness of tested samples.
Reduced Elastic Modulus of the Ti6Al4V, Ti-5Sn, and Ti-5Sn5Nb Alloys
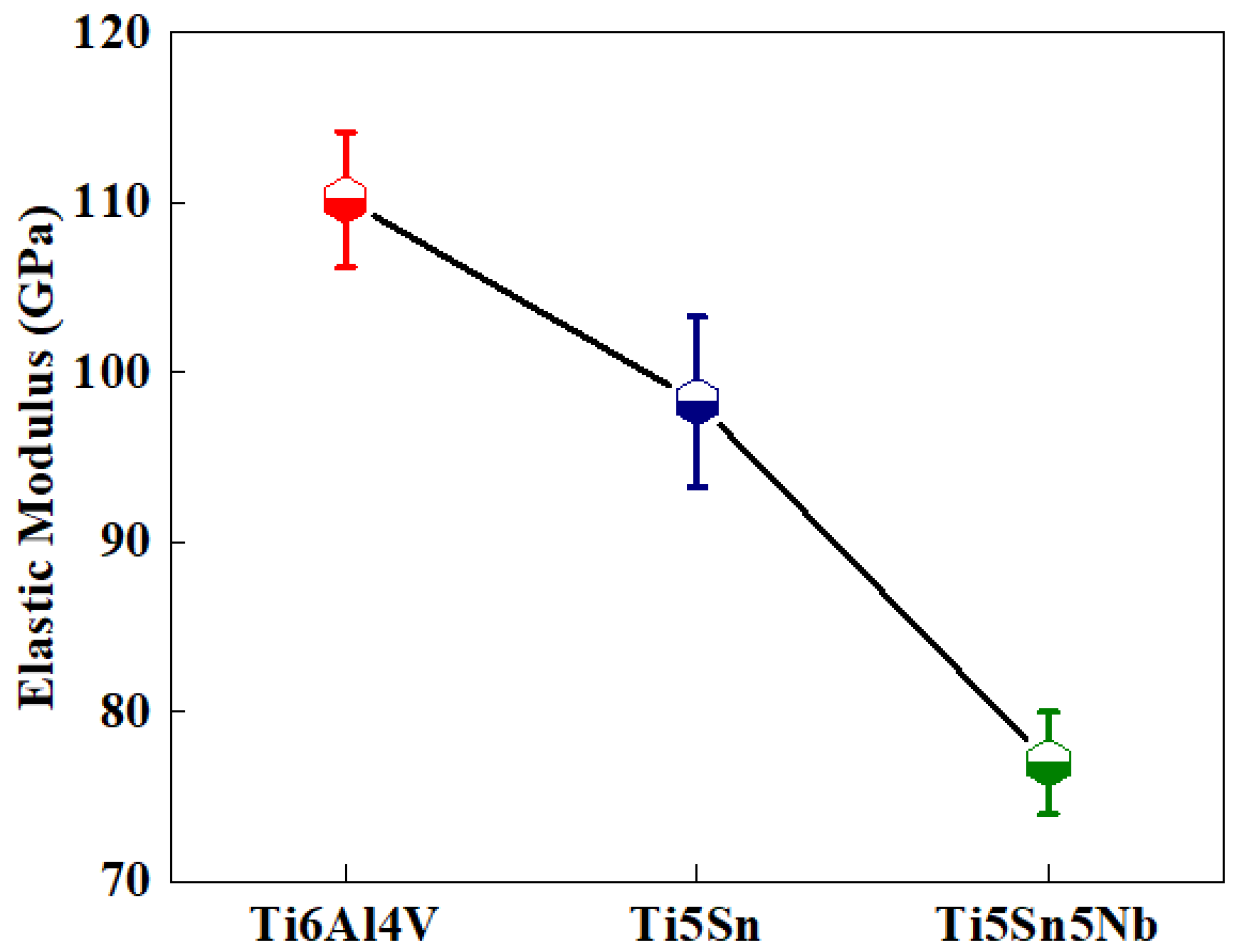
The Figure 9 depicts the elastic moduli of Ti5Sn, Ti6Al4V and Ti5Sn5Nb. The reduction in elastic moduli was observed when compared with the Ti6Al4V. The reduced modulus of elasticity helped design the orthopedic implants as it helps in reducing the phenomena of stress shielding, which led to enhancements in the life of the implant. Another important factor on the material hardness and modulus of Ti5Sn Ti6Al4V and Ti5Nb5Sn is the solid solution strengthening of the alloy, i.e., Ti5Nb5Sn. The reduced modulus of the alloy refers to the presence of the beta phase because Nb reduces the proportion of the alpha phase [60,61]. A 31% reduction in modulus of elasticity of Ti5Sn5Nb was observed when compared to the Ti6Al4V. Therefore, it is considered as the best alternate for biomedical applications.
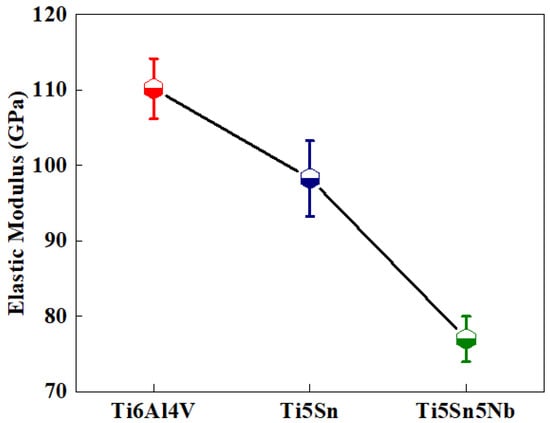
Figure 9.
Reduced elastic modulus of the three titanium-based alloys, the black curve depicts the elastic modulus of tested samples where red represents Ti6Al4V, blue represents Ti5Sn and green represents Ti5Sn5Nb alloys.
Wear Resistance
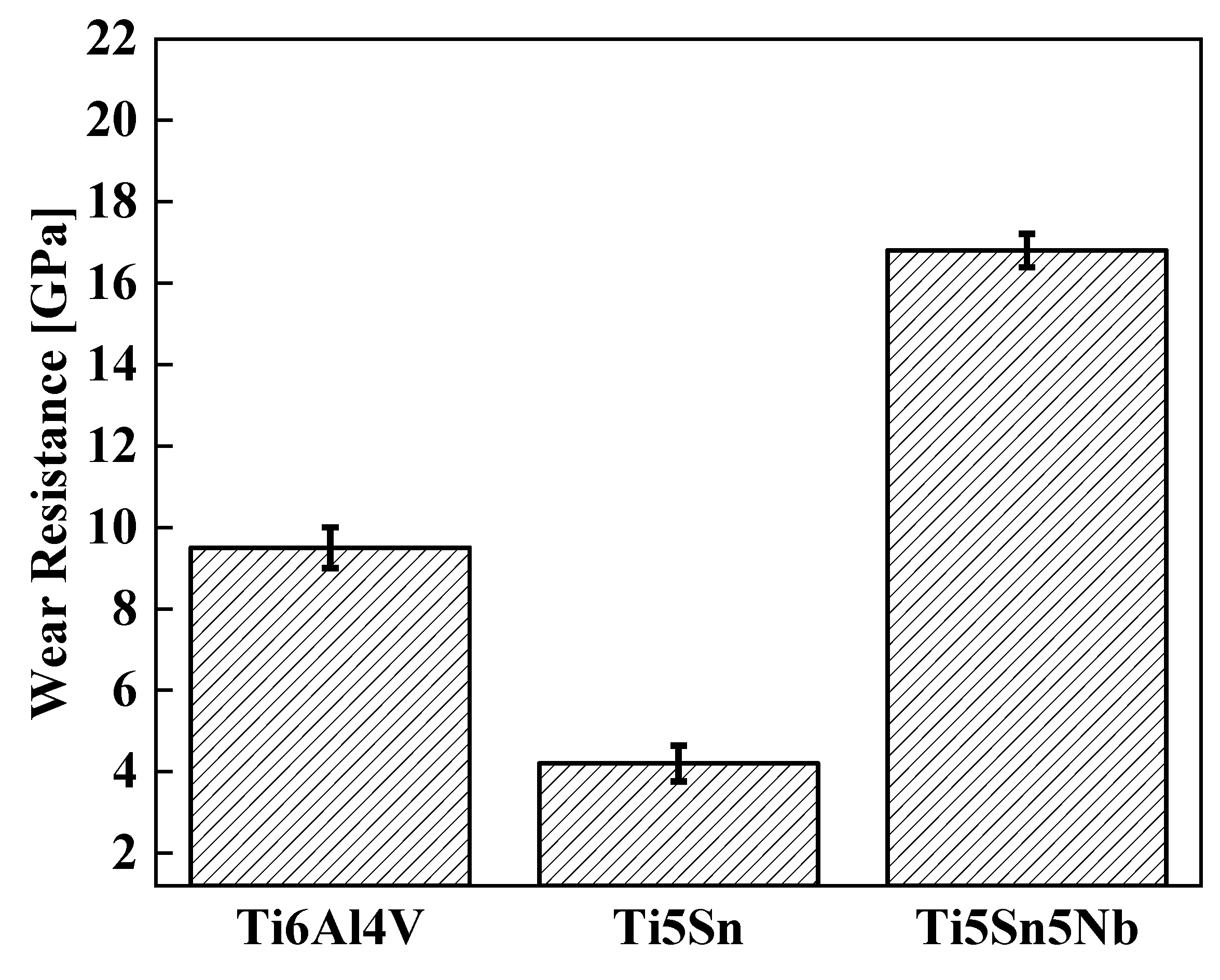
Improved wear is one of the important factors for biomedical applications, as orthopedic implants are inherently susceptible to wear from adjacent bone and tissue. Consequently, higher wear resistance can reduce the formation of wear particles that cause non-allergic reactions, thus necessitating implant loosening and re-operation. Figure 10 shows the comparative wear graph of alloys. The wear resistance of Ti5Sn5Nb is 16.5 GPa, whereas the wear resistances of other alloys (Ti6Al4V and Ti5Sn) are 9.5 and 4.2, respectively. Thus, a 70% increment in wear resistance of Ti5Sn5Nb was observed due to the increase in hardness value [62,63].
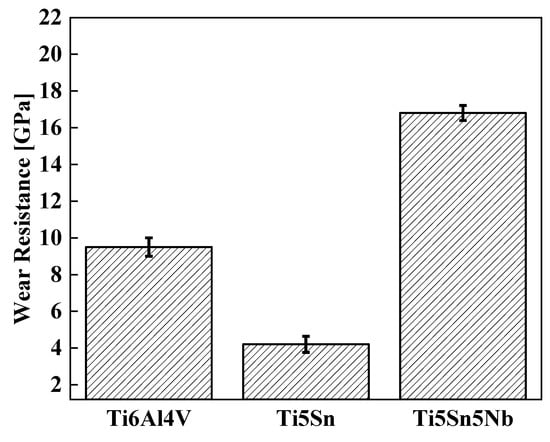
Figure 10.
Wear resistance of the Ti-based alloys at room temperature.
5. Conclusions
This research aims to investigate the potential of using Sn and Nb as alternative alloying elements to replace Al and V in Ti6Al4V biocompatible alloys. This is because Al-based alloys have been found to cause severe health issues when implanted in the human body. Sn and Nb, on the other hand, are known to be biocompatible and safe for use in biomedical applications. To explore this possibility, three different alloys were fabricated in the laboratory: Ti6Al4V, Ti5Sn, and Ti5Sn5Nb. Based on the experimental results obtained under the given set of conditions, the following conclusions were drawn. The alloys Ti5Sn5Nb and Ti6Al4V both contain alpha (α) and beta (β) phases, whereas Ti5Sn only contains an alpha phase. The nano hardness of Ti5Sn5Nb is higher compared to the other two alloys (Ti5Sn and Ti6Al4V), and it also has a lower Young’s modulus. Furthermore, Ti5Sn5Nb has the lowest corrosion rate among all the alloys studied. Therefore, it can be concluded that Ti5Sn5Nb has great potential for use in biomedical applications. The wear resistance of the newly developed alloy, Ti5Sn5Nb, is higher than that of the reference alloys, Ti6Al4V and Ti5Sn. This characteristic reduces the chance of wear in the body and makes it an attractive option for use in orthopedic applications. Thus, the addition of niobium in the Ti5Sn alloy has been found to be the most promising alloy for use in orthopedic applications. Overall, the findings of this research provide valuable insights into the potential of using Sn and Nb as alternative alloying elements to replace Al in Ti6Al4V biocompatible alloys. The results suggest Ti5Sn5Nb has excellent biocompatibility, corrosion resistance, and wear resistance, making it a promising candidate for use in orthopedic applications. This research will be of interest to material scientists, biomedical engineers, and manufacturers who are interested in developing new alloys with improved properties for biomedical applications.
Author Contributions
Conceptualization, A.D.C. and I.A.C. (Iftikhar Ahmed Channa); methodology, A.A. and S.A.; writing, I.A.C. (Irfan Ali Chandio), M.A.S. and J.A.; validation, I.A.C. (Irfan Ali Chandio) and S.A.; investigation, J.A., I.A.C. (Iftikhar Ahmed Channa), S.A., M.S.A. and S.D.; supervision, A.D.C. and I.A.C. (Iftikhar Ahmed Channa); funding acquisition, M.S.A., S.D. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their sincere appreciation to the Researchers Supporting Project number (RSP2023R68) at King Saud University, Riyadh, Saudi Arabia and the APC was funded by RSP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the corresponding authors.
Acknowledgments
The authors express their sincere appreciation to the Researchers Supporting Project number (RSP2023R68) at King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Zain-ul-abdein, M.; Channa, I.A.; Yaseen, M.K.; Gilani, S.J.; Makhdoom, M.A.; Mansoor, M.; Shahzad, U.; Jumah, M.N. bin Effect of Ultrasonic Surface Mechanical Attrition Treatment-Induced Nanograins on the Mechanical Properties and Biocompatibility of Pure Titanium. Materials 2022, 15, 5097. [Google Scholar] [CrossRef] [PubMed]
- Anene, F.A.; Aiza Jaafar, C.N.; Zainol, I.; Azmah Hanim, M.A.; Suraya, M.T. Biomedical Materials: A Review of Titanium Based Alloys. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Azmat, A.; Tufail, M.; Chandio, A.D. Synthesis and Characterization of Ti-Sn Alloy for Orthopedic Application. Materials 2021, 14, 7660. [Google Scholar] [CrossRef]
- Affi, J.; Gunawarman; Yetri, Y.; Fajri, H.; Juliadmi, D.; Nuswantoro, N.F.; Nurbaiti; Fonna, S.; Tjong, D.H.; Manjas, M. Corrosion Resistance of β Type Titanium (TNTZ) in 3%NaCl Solution. IOP Conf. Ser. Mater. Sci. Eng. 2019, 602, 012070. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.-H. A Review on Alloy Design, Biological Response, and Strengthening of β-Titanium Alloys as Biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Saini, A.; Pabla, B.S.; Dhami, S.S. Developments in Cutting Tool Technology in Improving Machinability of Ti6Al4V Alloy: A Review. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2016, 230, 1977–1989. [Google Scholar] [CrossRef]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short Term Exposure to Titanium, Aluminum and Vanadium (Ti 6Al 4V) Alloy Powder Drastically Affects Behavior and Antioxidant Metabolites in Vital Organs of Male Albino Mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef]
- Batool, S.A.; Liaquat, U.; Channa, I.A.; Gilani, S.J.; Makhdoom, M.A.; Yasir, M.; Ashfaq, J.; bin Jumah, M.N.; Rehman, M.A. Development and Characterization of Zein/Ag-Sr Doped Mesoporous Bioactive Glass Nanoparticles Coatings for Biomedical Applications. Bioengineering 2022, 9, 367. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xu, L.; Xiao, S.L.; Kong, F.; Woo, K. Ti–Nb–Sn–Hydroxyapatite Composites Synthesized by Mechanical Alloying and High Frequency Induction Heated Sintering. J. Mech. Behav. Biomed. Mater. 2011, 4, 2074–2080. [Google Scholar] [CrossRef]
- Makhdoom, M.A.; Sgobba, V.; Channa, I.A.; Ghewins, N. Producing Oxide Free Silicon Nanocrystals—A Novel & Benign Approach. Optik 2021, 246, 167789. [Google Scholar] [CrossRef]
- Okazaki, Y. A New Ti-15Zr-4Nb-4Ta Alloy for Medical Applications. Curr. Opin. Solid State Mater. Sci. 2001, 5, 45–53. [Google Scholar] [CrossRef]
- Marsumi, Y.W.; Pramono, A.W. Influence of Niobium or Molybdenum in Titanium Alloy for Permanent Implant Application. Adv. Mater. Res. 2014, 900, 53–63. [Google Scholar] [CrossRef]
- Cardoso, G.C.; de Almeida, G.S.; Corrêa, D.O.G.; Zambuzzi, W.F.; Buzalaf, M.A.R.; Correa, D.R.N.; Grandini, C.R. Preparation and Characterization of Novel As-Cast Ti-Mo-Nb Alloys for Biomedical Applications. Sci. Rep. 2022, 12, 11874. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Shamail., A.; Channa, I.A. Mathematical relationship between ferritic, pearlitic and average grain size of steel. J. Mod. Scie. Technol. 2013, 1, 1–18. [Google Scholar]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications—Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Ali, M.; Khalida, B.; Almaani, F.; Ahmed, A.; Aneela, S.; Dad, A.; Abdul, C.; Mugheri, Q.; Baradi, B.; Shymaa, W. Low Temperature Aqueous Chemical Growth Method for the Doping of W into ZnO Nanostructures and Their Photocatalytic Role in the Degradration of Methylene Blue. J. Clust. Sci. 2021, 6, 1445–1456. [Google Scholar] [CrossRef]
- Miura, K.; Yamada, N.; Hanada, S.; Jung, T.-K.; Itoi, E. The Bone Tissue Compatibility of a New Ti–Nb–Sn Alloy with a Low Young’s Modulus. Acta Biomater. 2011, 7, 2320–2326. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Egelhaaf, H.; Brabec, C.J. Solution Coated Barriers for Flexible Electronics. In Organic Flexible Electronics, Fundamentals, Devices, and Applications; Cosseddu, P., Caironi, M., Eds.; Woodhead Publishing: Soston, UK, 2020; ISBN 9780128188903. [Google Scholar]
- Channa, I.A. Development of Solution Processed Thin Film Barriers for Encapsulating Thin Film Electronics Entwicklung von Lösungsprozessierten Dünnschichtbarrieren Für Die Verpackung von Dünnschichtelektronik. 2019. Available online: https://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/12583 (accessed on 20 November 2022).
- Barão, V.A.R.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.-C.; Wimmer, M.A.; Sukotjo, C. Stability of Cp-Ti and Ti-6Al-4V Alloy for Dental Implants as a Function of Saliva PH—An Electrochemical Study. Clin. Oral Implants Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Alabi, A.A.; Tahir, S.M.; Hanim, M.A.A.; Zahari, N.I.; Anuar, M.S. Relationship between Fracture Toughness and Relative Density in Iron and Copper Metal Powder Compacts. Niger. J. Eng. 2020, 26, 77–90. [Google Scholar]
- Santos, P.F.; Niinomi, M.; Liu, H.; Cho, K.; Nakai, M.; Itoh, Y.; Narushima, T.; Ikeda, M. Fabrication of Low-Cost Beta-Type Ti-Mn Alloys for Biomedical Applications by Metal Injection Molding Process and Their Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2016, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, Y.G.; An, B.; Xu, H.; Liu, Y.; Zhang, L.C.; Ma, H.Y.; Wang, W.M. A Practical Anodic and Cathodic Curve Intersection Model to Understand Multiple Corrosion Potentials of Fe-Based Glassy Alloys in OH- Contained Solutions. PLoS ONE 2016, 11, e0146421. [Google Scholar] [CrossRef]
- Rahul, B.; Bhola, S.; Brajendra, M.; Olson, D. Electrochemical Behavior of Titanium and Its Alloys as Dental Implants in Normal Saline. Res. Lett. Phys. Chem. 2009, 2009, 574359. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Plocek, J.; Ismagulov, B.; Kupčík, J.; Vacík, J.; Ceccio, G.; Lavrentiev, V.; Němeček, J.; Michna, Š.; Klie, R. The Key Role of Tin (Sn) in Microstructure and Mechanical Properties of Ti(2)SnC (M(2)AX) Thin Nanocrystalline Films and Powdered Polycrystalline Samples. Nanomaterials 2022, 12, 307. [Google Scholar] [CrossRef]
- Alaf, M.; Gultekin, D.; Akbulut, H. Tin/Tinoxide (Sn/SnO2) Nanocomposites Thin Films as Negative-Electrode Materials for Li-Ion Batteries. Acta Phys. Pol. A 2013, 123, 323–325. [Google Scholar] [CrossRef]
- Chapala, P.; Sunil Kumar, P.; Joardar, J.; Bhandari, V.; Acharyya, S.G. Effect of Alloying Elements on the Microstructure, Coefficient of Friction, in-Vitro Corrosion and Antibacterial Nature of Selected Ti-Nb Alloys. Appl. Surf. Sci. 2019, 469, 617–623. [Google Scholar] [CrossRef]
- Kalyoncuoglu, U.T.; Yilmaz, B.; Gungor, S.; Evis, Z.; Uyar, P.; Akca, G.; Kansu, G. Evaluation of the Chitosan-Coating Effectiveness on a Dental Titanium Alloy in Terms of Microbial and Fibroblastic Attachment and the Effect of Aging. Mater. Tehnol. 2015, 49, 925–931. [Google Scholar] [CrossRef]
- Hu, R.; Sun, W.; Zeng, M.; Zhu, M. Dispersing SnO2 Nanocrystals in Amorphous Carbon as a Cyclic Durable Anode Material for Lithium Ion Batteries. J. Energy Chem. 2014, 23, 338–345. [Google Scholar] [CrossRef]
- Yan, M.; Luo, S.D.; Schaffer, G.B.; Qian, M. TEM and XRD Characterisation of Commercially Pure α-Ti Made by Powder Metallurgy and Casting. Mater. Lett. 2012, 72, 64–67. [Google Scholar] [CrossRef]
- Qian, M.; Schaffer, G.; Bettles, C.J. Sintering of Titanium and Its Alloys. In Sintering of Advanced Materials; Woodhead Publishing: Cambridge, UK, 2010; pp. 324–355. [Google Scholar]
- Yuan, X.; Qu, X.; Yin, H.; Feng, Z.; Tang, M.; Yan, Z.; Tan, Z. Effects of Sintering Temperature on Densification, Microstructure and Mechanical Properties of Al-Based Alloy by High-Velocity Compaction. Metals 2021, 11, 218. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Özkan Gülsoy, H. Characterization of Biomedical Ti-16Nb-(0–4)Sn Alloys Produced by Powder Injection Molding. Vacuum 2017, 142, 164–174. [Google Scholar] [CrossRef]
- Mahundla, M.R.; Matizamhuka, W.R.; Machaka, R.; Yamamoto, A.; Shongwe, M.B. Biocompatibility Study of Ti-Based Alloys Fabricated by Spark Plasma Sintering. IOP Conf. Ser. Mater. Sci. Eng. 2019, 655, 012021. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Ige, O.O.; Olubambi, P.A.; Lethabane, M.L.; Bhero, S.W. Influence of Spark Plasma Sintering on Microstructure and Wear Behaviour of Ti-6Al-4V Reinforced with Nanosized TiN. Trans. Nonferrous Met. Soc. China 2018, 28, 47–54. [Google Scholar] [CrossRef]
- Ahmed, M.; Obeidi, M.A.; Yin, S.; Lupoi, R. Influence of Processing Parameters on Density, Surface Morphologies and Hardness of as-Built Ti-5Al-5Mo-5V-3Cr Alloy Manufactured by Selective Laser Melting. J. Alloys Compd. 2022, 910, 164760. [Google Scholar] [CrossRef]
- Sugahara, T.; Reis, D.A.P.; Moura Neto, C.; Barboza, M.J.R.; Perez, E.A.C.; Piorino Neto, F.; Hirschmann, A.C.O. The Effect of Widmanstätten and Equiaxed Microstructures of Ti-6Al-4V on the Oxidation Rate and Creep Behavior. Mater. Sci. Forum 2010, 636–637, 657–662. [Google Scholar] [CrossRef]
- Fujii, H. Continuous Cooling Transformation Characteristics of α + β Titanium Alloys. Nippon Steel Tech. Rep. 1994, 74–79. [Google Scholar]
- Zhong, C.; Liu, J.; Zhao, T.; Schopphoven, T.; Fu, J.; Gasser, A.; Schleifenbaum, J.H. Laser Metal Deposition of Ti6Al4V-A Brief Review. Appl. Sci. 2020, 10, 764. [Google Scholar] [CrossRef]
- Silva, A.; Azevedo, T.F.; Sampaio, W.R.V.; Pereira, L.C.; Griza, S. Study of α”-Martensitic Transformation in Ti35NbxSn Alloys Subjected to Cryogenic Heat Treatment. Adv. Mater. Res. 2020, 1158, 17–26. [Google Scholar] [CrossRef]
- Dai, Y.; Song, M. Microstructural Evolution and Phase Transformation of a Ti-5Nb-5Al Alloy during Annealing Treatment. Mater. Res. 2019, 7, 22. [Google Scholar] [CrossRef]
- Sri Maha Vishnu, D.; Sure, J.; Liu, Y.; Vasant Kumar, R.; Schwandt, C. Electrochemical Synthesis of Porous Ti-Nb Alloys for Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jiang, F.; Sun, X.; Chen, Z.; Tian, C.; Zhao, H. Microstructure and Mechanical Properties of Ti-6al-4v Fabricated by Electron Beam Melting. Crystals 2020, 10, 972. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.Y.; Tardelli, J.D.C.; Dos Reis, A.C.; Almeraya-Calderón, F.; Leo, P. Passive Layers and Corrosion Resistance of Biomedical Ti-6al-4v and β-Ti Alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Lin, H.-C.; Wu, S.-C.; Hong, Y.-S.; Ho, W.-F. Microstructure and Grindability of As-Cast Ti–Sn Alloys. J. Mater. Sci. 2010, 45, 1830–1836. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, L.B.; Zhang, X.D.; Bai, W.M.; Chen, C.P. Isothermal Section of the Ti-Nb-Sn Ternary System at 700 °C. J. Phase Equilibria Diffus. 2014, 35, 223–231. [Google Scholar] [CrossRef]
- Da Silva, S.L.R.; Kerber, L.O.; Amaral, L.; dos Santos, C.A. X-ray Diffraction Measurements of Plasma-Nitrided Ti–6Al–4V. Surf. Coatings Technol. 1999, 116–119, 342–346. [Google Scholar] [CrossRef]
- Klimova-Korsmik, O.G.; Turichin, G.A.; Shalnova, S.A.; Gushchina, M.O.; Cheverikin, V.V. Structure and Properties of Ti-6Al-4V Titanium Alloy Products Obtained by Direct Laser Deposition and Subsequent Heat Treatment. J. Phys. Conf. Ser. 2018, 1109, 012061. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.J.; Świeszkowski, W. Laser and Electron Beam Additive Manufacturing Methods of Fabricating Titanium Bone Implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Prestat, M.; Thierry, D. Corrosion of Titanium under Simulated Inflammation Conditions: Clinical Context and in Vitro Investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Takahashi, E.; Sakurai, T.; Watanabe, S.; Masahashi, N.; Hanada, S. Effect of Heat Treatment and Sn Content on Superelasticity in Biocompatible TiNbSn Alloys. Mater. Trans. 2002, 43, 2978–2983. [Google Scholar] [CrossRef]
- Garcia-Ramirez, M.J.; Lopez-Sesenes, R.; Rosales-Cadena, I.; Gonzalez-Rodriguez, J.G. Corrosion Behaviour of Ti–Ni–Al Alloys in a Simulated Human Body Solution. J. Mater. Res. Technol. 2018, 7, 223–230. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Rajendran, N. Effect of Plasma Nitriding on Structure and Biocompatibility of Self-Organised TiO2 Nanotubes on Ti-6Al-7Nb. RSC Adv. 2015, 5, 41763–41771. [Google Scholar] [CrossRef]
- Wen, Y.; Guo, T.; Zhou, Y.; Bai, H.; Wang, Z. Nanoindentation Characterization on Microhardness of Micron-Level TiC and TiB Reinforcements in in-Situ Synthesized (TiC+TiB)/Ti-6Al-4V Composite. Front. Mater. 2019, 6, 205. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Cao, G.; Zhang, L.-C. Nanoindentation Study of Mechanical Properties of Ti Based Alloys with Fe and Ta Additions. J. Alloys Compd. 2017, 692, 892–897. [Google Scholar] [CrossRef]
- Delannoy, S.; Baïz, S.; Laheurte, P.; Jordan, L.; Prima, F. Elastically Graded Titanium Alloy Produced by Mechanical Surface Deformation. Front. Mater. 2021, 8, 634236. [Google Scholar] [CrossRef]
- Majumdar, P.; Singh, S.B.; Chakraborty, M. Elastic Modulus of Biomedical Titanium Alloys by Nano-Indentation and Ultrasonic Techniques-A Comparative Study. Mater. Sci. Eng. A 2008, 489, 419–425. [Google Scholar] [CrossRef]
- Revankar, G.D.; Shetty, R.; Rao, S.S.; Gaitonde, V.N. Wear Resistance Enhancement of Titanium Alloy (Ti–6Al–4V) by Ball Burnishing Process. J. Mater. Res. Technol. 2017, 6, 13–32. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Paramesh, T.; Sunita, K. A Review on Enhancement of Wear Resistance Properties of Titanium Alloy Using Nano-Composite Coating. IOP Conf. Ser. Mater. Sci. Eng. 2018, 455, 012120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).