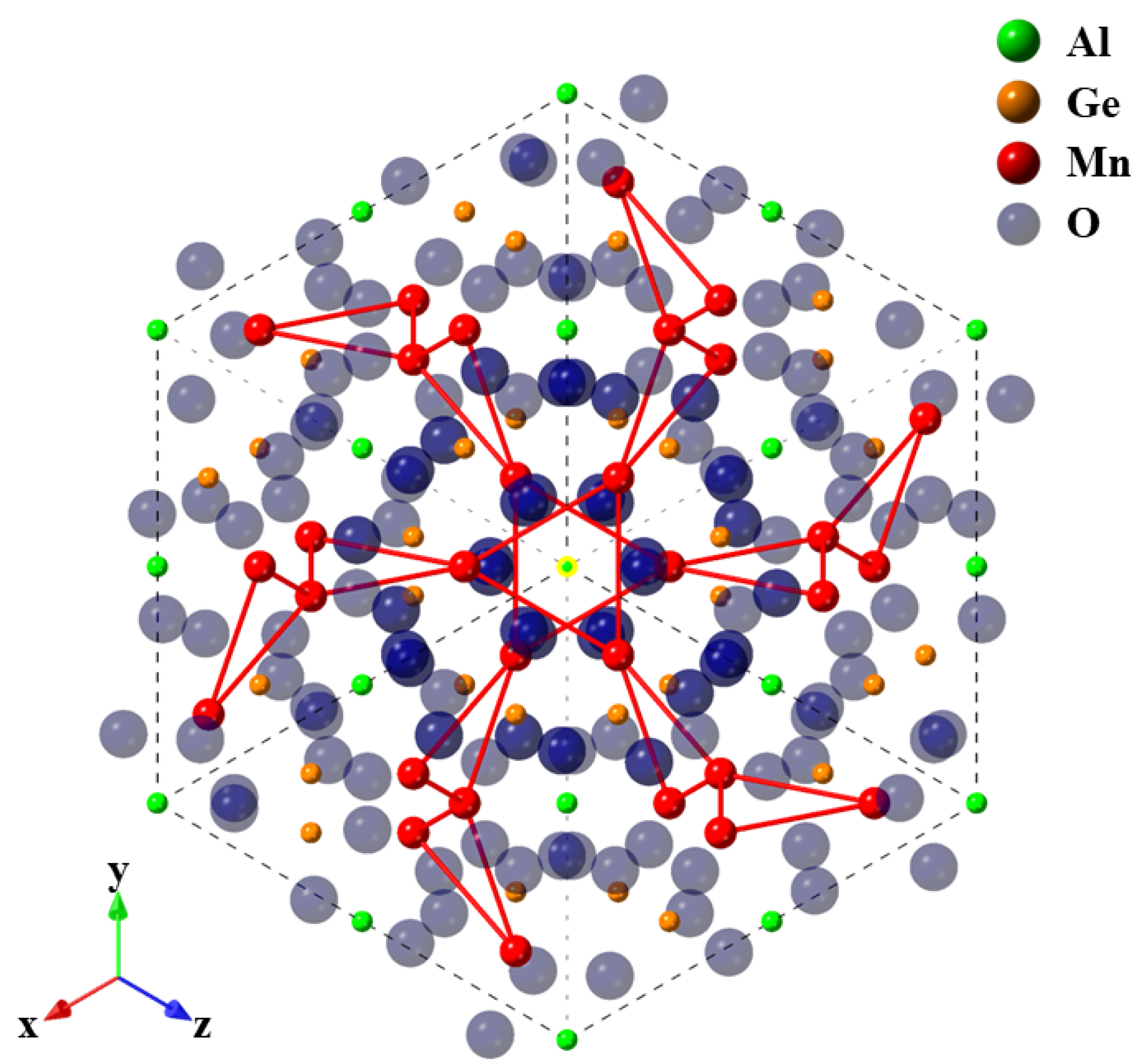
Frustrated Magnet Mn3Al2Ge3O12 Garnet: Crystal Growth by the Optical Floating Zone Method
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
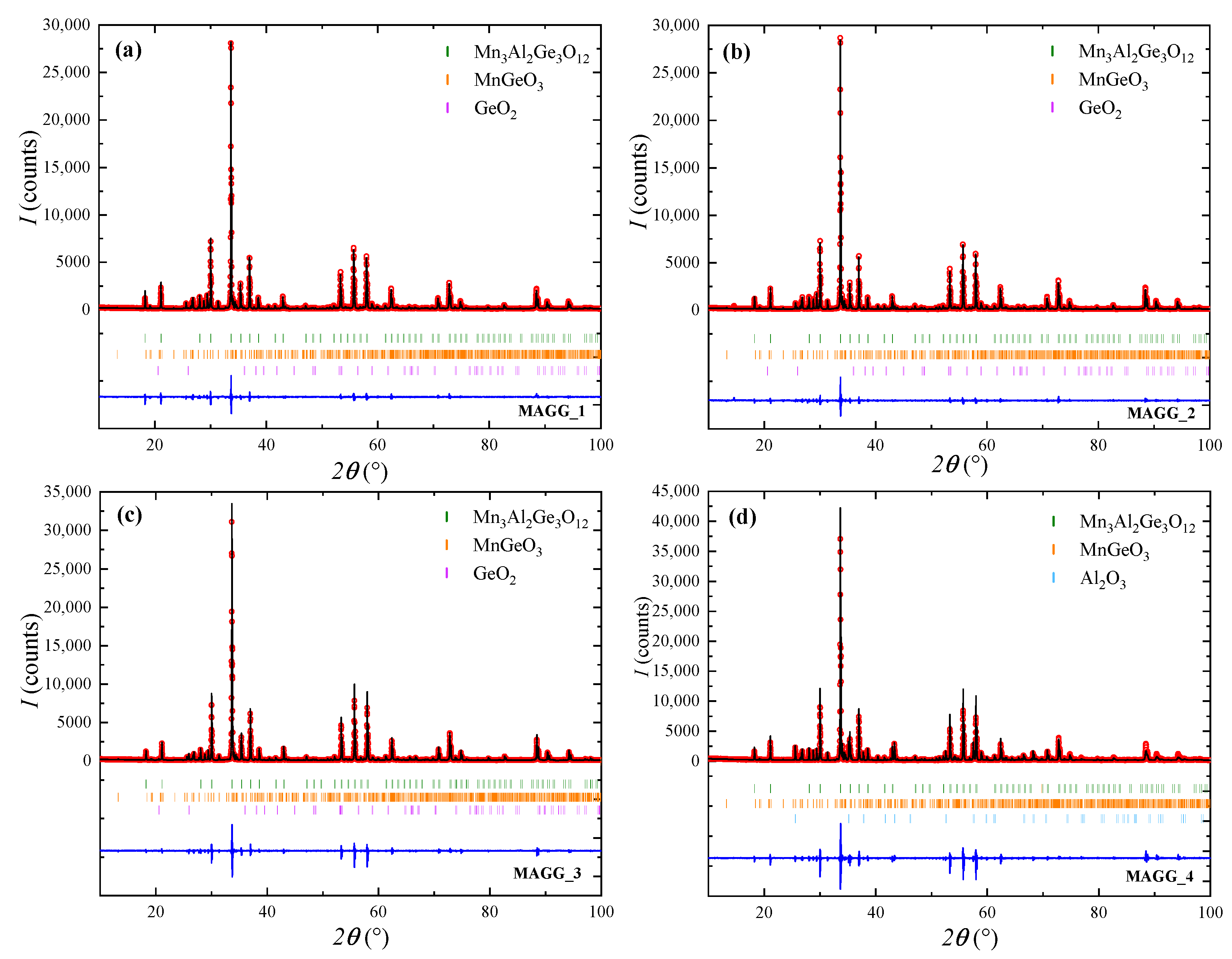
3.1. Polycrystalline Synthesis
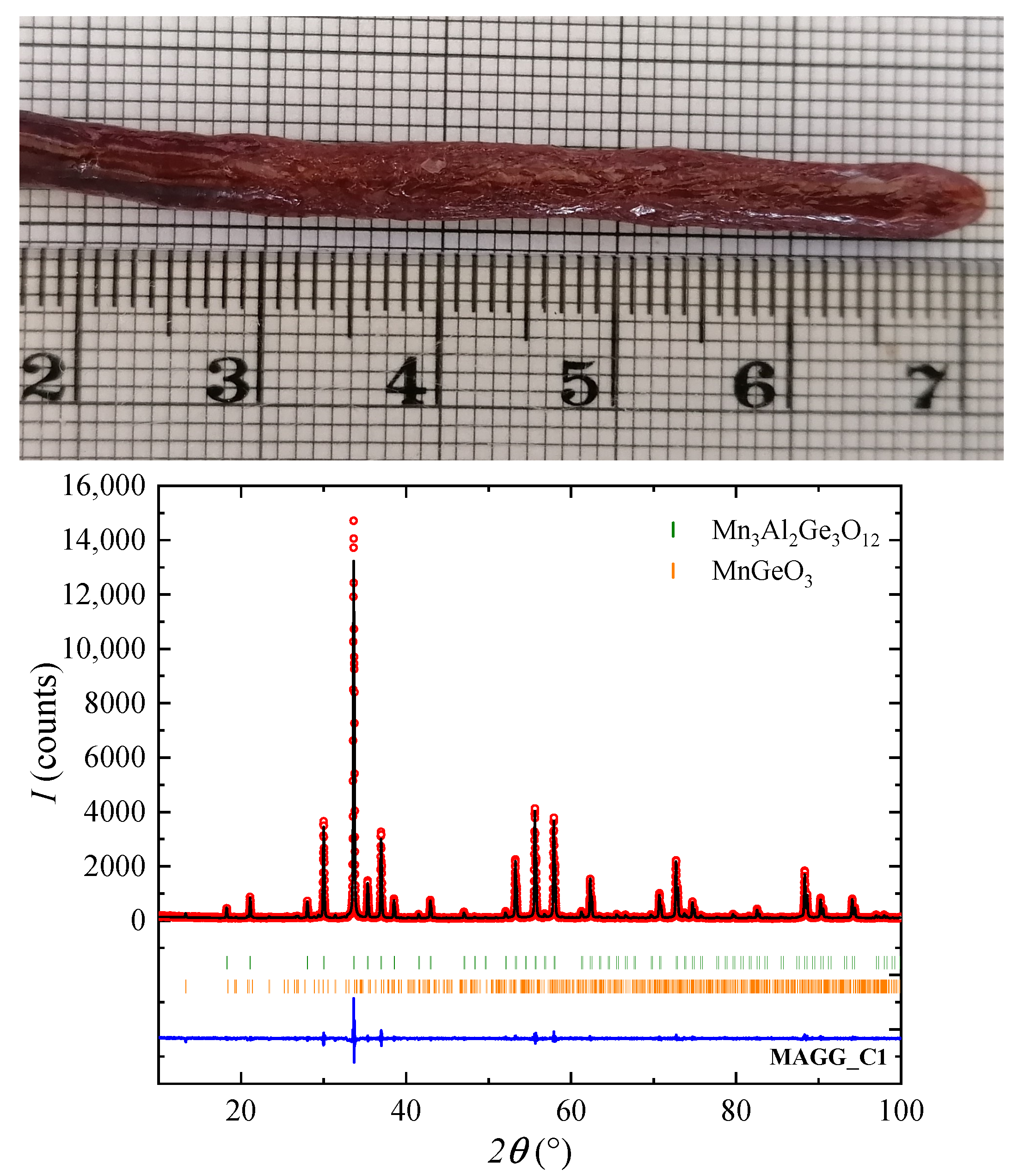
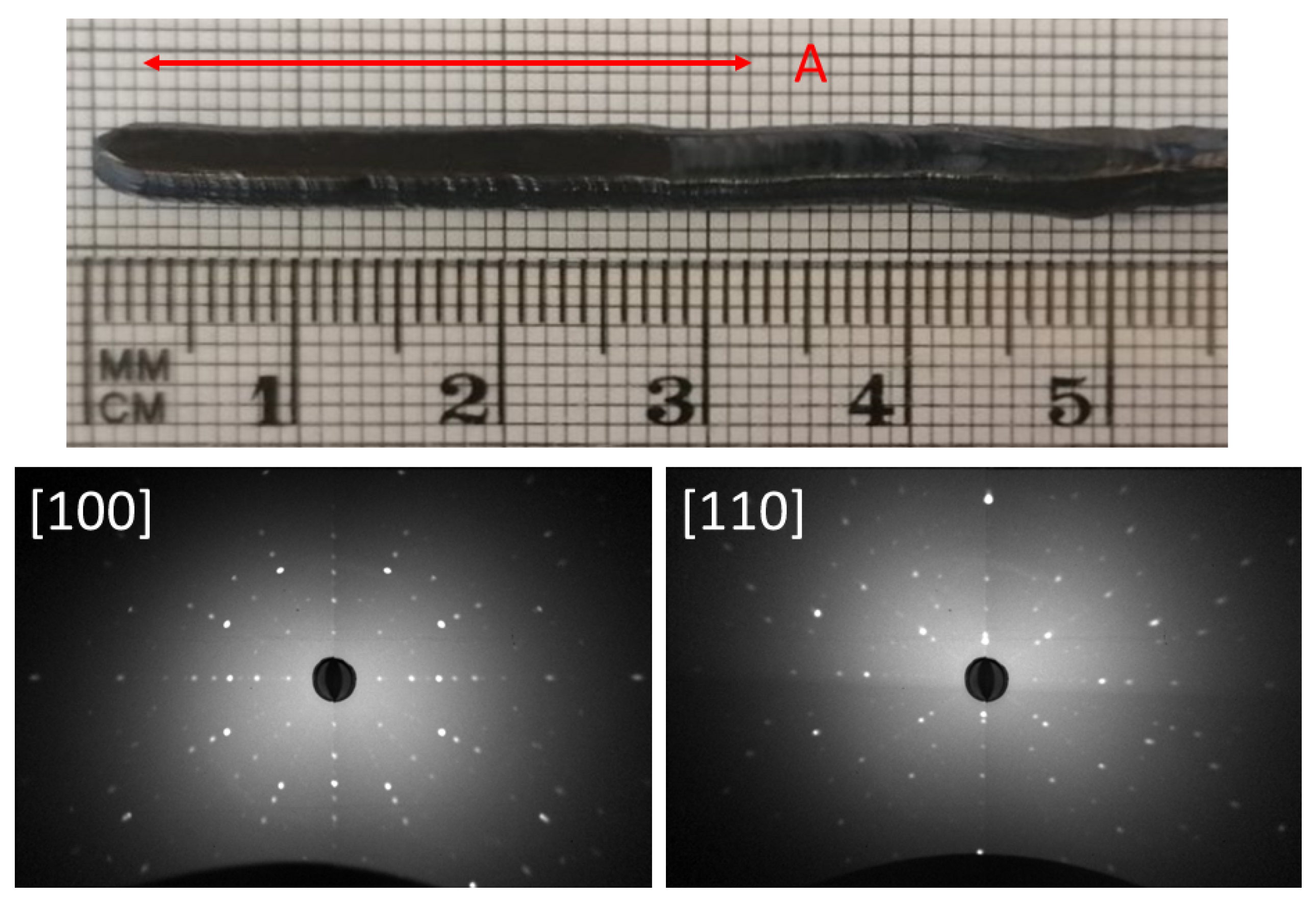
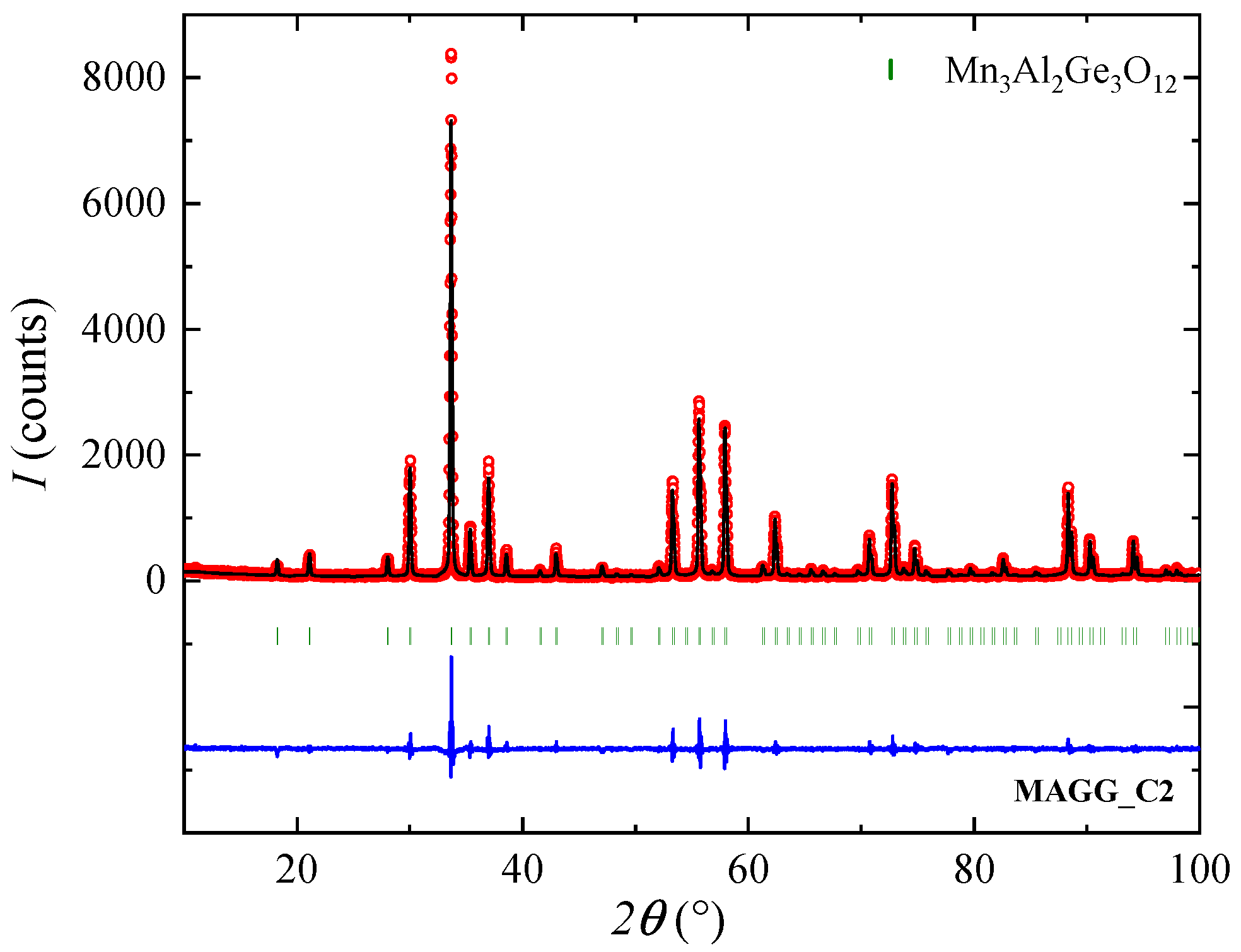
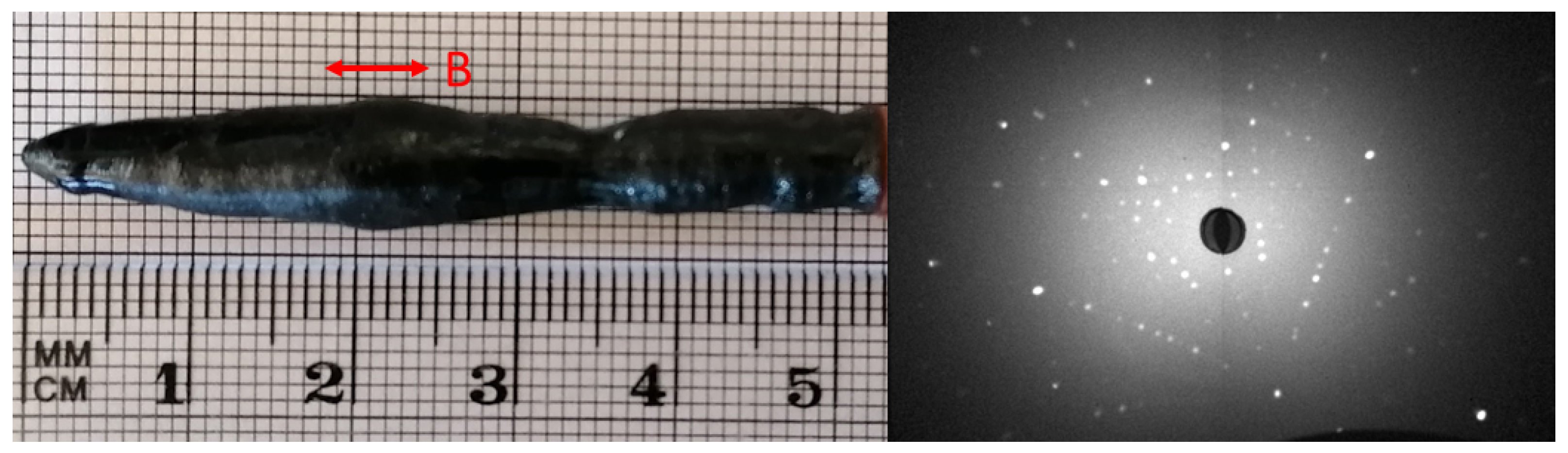
3.2. Crystal Growth
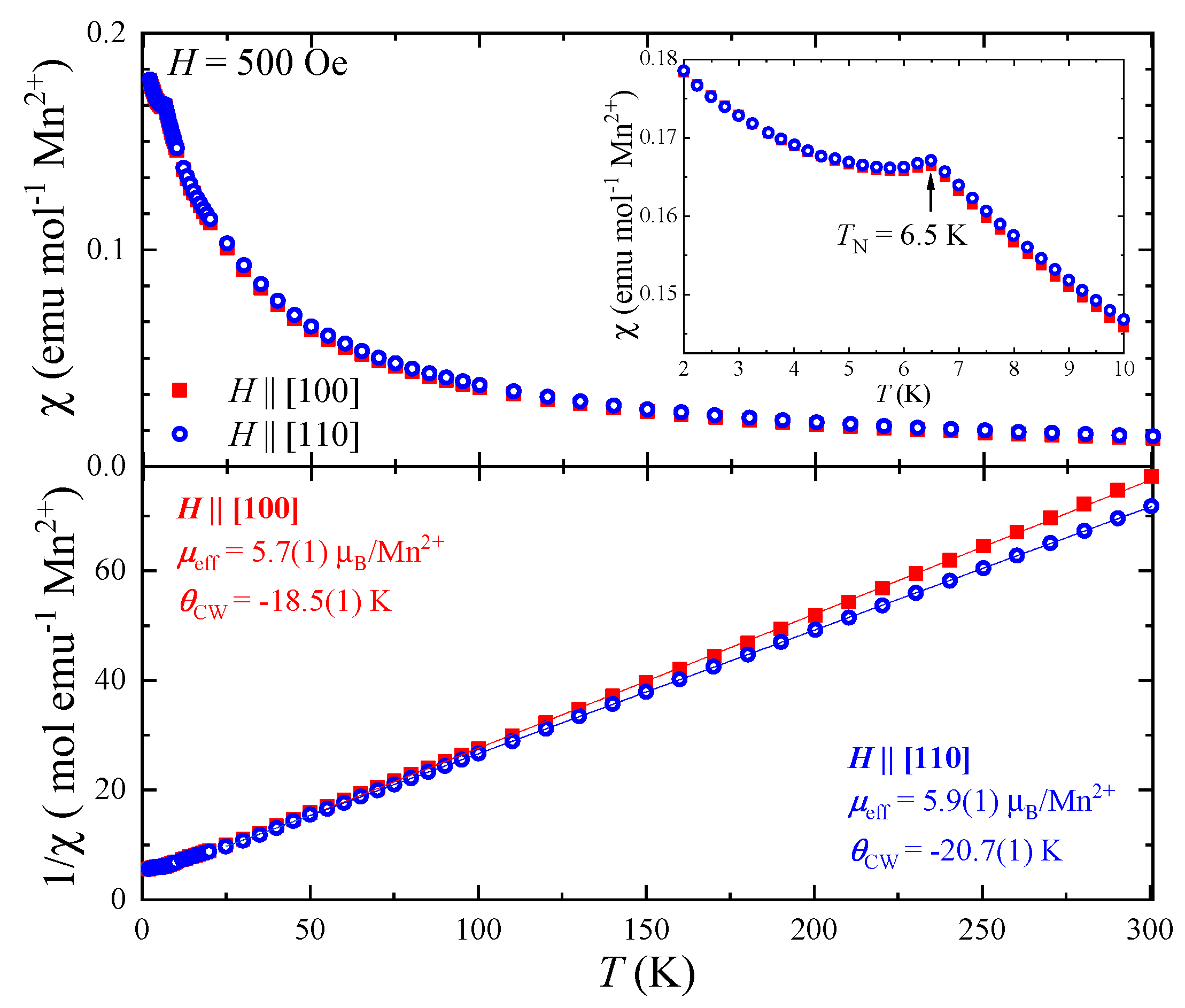
3.3. Magnetic Characterisation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Prandl, W. Rhombohedral Magnetic Structure in Spessartite Type Garnets. Phys. Status Solidi B 1973, 55, K159–K163. [Google Scholar] [CrossRef]
- Lau, G.C.; Klimczuk, T.; Ronning, F.; McQueen, T.M.; Cava, R.J. Magnetic properties of the garnet and glass forms of Mn3Al2Si3O12. Phys. Rev. B 2009, 80, 214414. [Google Scholar] [CrossRef]
- Geller, S. Crystal chemistry of the garnets. Z. Krist.-Cryst. Mater. 2014, 125, 1–47. [Google Scholar] [CrossRef]
- Deen, P.P. An Overview of the Director State in Gadolinium Gallate Garnet. Front. Phys. 2022, 10, 868339. [Google Scholar] [CrossRef]
- Ramirez, A.P. Strongly Geometrically Frustrated Magnets. Ann. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
- Greedan, J.E. Geometrically frustrated magnetic materials. J. Mater. Chem. 2001, 11, 37–53. [Google Scholar] [CrossRef]
- Moessner, R.; Ramirez, A.P. Geometrical frustration. Phys. Today 2006, 59, 24–29. [Google Scholar] [CrossRef]
- Canals, B.; Lacroix, C. Pyrochlore Antiferromagnet: A Three-Dimensional Quantum Spin Liquid. Phys. Rev. Lett. 1998, 80, 2933–2936. [Google Scholar] [CrossRef]
- Kitagawa, K.; Takayama, T.; Matsumoto, Y.; Kato, A.; Takano, R.; Kishimoto, Y.; Bette, S.; Dinnebier, R.; Jackeli, G.; Takagi, H. A spin-orbital-entangled quantum liquid on a honeycomb lattice. Nature 2018, 554, 341–345. [Google Scholar] [CrossRef]
- Bramwell, S.T.; Harris, M.J. The history of spin ice. J. Phys. Condens. Matter 2020, 32, 374010. [Google Scholar] [CrossRef]
- Gardner, J.S.; Gingras, M.J.P.; Greedan, J.E. Magnetic pyrochlore oxides. Rev. Mod. Phys. 2010, 82, 53–107. [Google Scholar] [CrossRef]
- Schiffer, P.; Ramirez, A.P.; Huse, D.A.; Valentino, A.J. Investigation of the Field Induced Antiferromagnetic Phase Transition in the Frustrated Magnet: Gadolinium Gallium Garnet. Phys. Rev. Lett. 1994, 73, 2500–2503. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyashita, S. Thermodynamic properties of the quantum Heisenberg antiferromagnet on the kagomé lattice. Phys. Rev. B 1995, 52, 9174–9177. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Miyashita, S. Existence of Phase Transition in Ising-like Heisenberg Antiferromagnets on the Kagome Lattice. J. Phys. Soc. Jpn. 1995, 64, 4509–4512. [Google Scholar] [CrossRef]
- d’Ambrumenil, N.; Petrenko, O.A.; Mutka, H.; Deen, P.P. Dispersionless Spin Waves and Underlying Field-Induced Magnetic Order in Gadolinium Gallium Garnet. Phys. Rev. Lett. 2015, 114, 227203. [Google Scholar] [CrossRef]
- Paddison, J.A.M.; Jacobsen, H.; Petrenko, O.A.; Fernández-Díaz, M.T.; Deen, P.P.; Goodwin, A.L. Hidden order in spin-liquid Gd3Ga5O12. Science 2015, 350, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Claudine, L.; Mendels, P.; Mila, F. Introduction to Frustrated Magnetism; Springer: Berlin/Heidelberg, Germany, 2011; Volume 164. [Google Scholar] [CrossRef]
- Plumier, R. Determination par diffraction des neutrons de la structure antiferromagnetique du grenat Mn3Al2Ge3O12. Solid State Commun. 1973, 12, 109–112. [Google Scholar] [CrossRef]
- Valyanskaya, T.V.; Plakhtii, V.P.; Sokolov, V.I. Antiferromagnetism of the garnet Mn3Al2Ge3O12. Sov. Phys. JETP 1976, 43, 1189. [Google Scholar]
- Min, J.; Zheng, S.; Gong, J.; Chen, X.; Liu, F.; Xie, Y.; Zhang, Y.; Ma, Z.; Liu, M.; Wang, X.; et al. Magnetoelectric Effect in Garnet Mn3Al2Ge3O12. Inorg. Chem. 2022, 61, 86–91. [Google Scholar] [CrossRef]
- Pajaczkowska, A.; Jasiołek, G.; Majcher, K. Crystal growth and some structural investigations of Mn3Al2Ge3O12 (MAGG) garnets, where A = Cr, Fe, Ga. J. Cryst. Growth 1986, 79, 417–420. [Google Scholar] [CrossRef]
- Tauber, A.; Banks, E.; Kedesdy, H.H. Magnetic Germanates Isostructural with Garnet. J. Appl. Phys. 1958, 29, 1123. [Google Scholar] [CrossRef]
- Kazei, Z.A.; Kolmakova, N.P.; Levanidov, M.V.; Mill, B.V.; Sokolov, V.I. Magnetoanisotropic properties of the exchange-noncollinear antiferromagnet Mn3Al2Ge3O12. Sov. Phys. JETP 1987, 65, 2277–2282. [Google Scholar]
- Krasnikova, Y.V.; Glazkov, V.N.; Soldatov, T.A. Experimental study of antiferromagnetic resonance in noncollinear antiferromagnetic Mn3Al2Ge3O12. JETP 2017, 125, 476–479. [Google Scholar] [CrossRef][Green Version]
- Tikhonov, A.M.; Pavlov, N.G.; Udalov, O.G. Nuclear magnetic resonance in noncollinear antiferromagnet Mn3Al2Ge3O12. JETP Lett. 2012, 96, 517–520. [Google Scholar] [CrossRef]
- Gukasov, A.; Plakhty, V.P.; Dorner, B.; Kokovin, S.Y.; Syromyatnikov, V.N.; Smirnov, O.P.; Chernenkov, Y.P. Inelastic neutron scattering study of spin waves in the garnet Mn3Al2Si3O12 with a triangular magnetic structure. J. Phys. Condens. Matter 1999, 11, 2869–2878. [Google Scholar] [CrossRef]
- Gardner, J.; Gaulin, B.; Paul, D. Single crystal growth by the floating-zone method of a geometrically frustrated pyrochlore antiferromagnet, Tb2Ti2O7. J. Cryst. Growth 1998, 191, 740–745. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Petrenko, O.A.; Lees, M.R.; Paul, D.M. Single crystal growth of rare earth titanate pyrochlores. J. Phys. Condens. Matter 1998, 10, L723–L725. [Google Scholar] [CrossRef]
- Brunt, D.; Ciomaga Hatnean, M.; Petrenko, O.A.; Lees, M.R.; Balakrishnan, G. Single-Crystal Growth of Metallic Rare-Earth Tetraborides by the Floating-Zone Technique. Crystals 2019, 9, 211. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Hayes, T.J.; Petrenko, O.A.; Paul, D.M. High quality single crystals of the SrR2O4 family of frustrated magnets. J. Phys. Condens. Matter 2008, 21, 012202. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Becker, U.; Felsche, J. Phases and structural relations of the rare earth germanates RE2Ge2O7, RE≡La-Lu. J. Less Common. Met. 1987, 128, 269–280. [Google Scholar] [CrossRef]
- Redhammer, G.J.; Senyshyn, A.; Tippelt, G.; Roth, G. Magnetic spin structure of pyroxene-type MnGeO3. J. Phys. Condens. Matter 2011, 23, 254202. [Google Scholar] [CrossRef] [PubMed]
- Herpin, P.; Whuler, A.; Boucher, B.; Sougi, M. Étude cristallographique et magnétique de MnGeO3. Phys. Status Solidi B 1971, 44, 71–84. [Google Scholar] [CrossRef]
- Finch, C.B.; Clark, G.W. Flux growth and characterization of hexagonal germanium dioxide single crystals. Am. Mineral. 1968, 53, 1394–1398. [Google Scholar]
- Lewis, J.; Schwarzenbach, D.; Flack, H.D. Electric field gradients and charge density in corundum, α-Al2O3. Acta Crystallogr. A 1982, 38, 733–739. [Google Scholar] [CrossRef]
- Dekker, E.H.L.J.; Rieck, G.D. Revised phase diagram and X-ray data of the Mn3O4Al2O3 system in air. Z. Anorg. Allg. Chem. 1975, 415, 69–80. [Google Scholar] [CrossRef]
- Kedesdy, H.; Katz, G.; Levin, S.B. Structural relationship between ramsdellite and some synthetic manganese dioxides. Acta Crystallogr. 1957, 10, 780–781. [Google Scholar]
- Lutts, G.B.; Denisov, A.L.; Zharikov, E.V.; Zagumennyi, A.I.; Kozlikin, S.N.; Lavrishchev, S.V.; Samoylova, S.A. GSAG and YSAG: A study on isomorphism and crystal growth. Opt. Quantum Electron. 1990, 22, S269–S281. [Google Scholar] [CrossRef]
- Kaurova, I.; Domoroshchina, E.; Kuz’micheva, G.; Rybakov, V. Evaluation of stability region for scandium-containing rare-earth garnet single crystals and their congruent-melting compositions. J. Cryst. Growth 2017, 468, 452–456. [Google Scholar] [CrossRef]
- Dabkowska, H.; Dabkowski, A. Crystal Growth of Oxides by Optical Floating Zone Technique. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 367–391. [Google Scholar]
- Navarro, R.; Gomez, A.; de Avillez, R. Heat capacity of stoichiometric Al2MnO4 spinel between 2 and 873 K. CALPHAD 2012, 37, 11–17. [Google Scholar] [CrossRef]


| Sample Label | Synthesis Conditions | GeO | Phase Composition Analysis | |
|---|---|---|---|---|
| Temperature | Duration | Excess | ||
| (°C) | (h) | (%) | ||
| MAGG_1 | 900 | 24 | 5 | mixture of cubic , orthorhombic MnGeO and trigonal GeO |
| 1100 | 24 | |||
| 1150 | 24 | |||
| MAGG_2 | 900 | 24 | 10 | mixture of cubic , orthorhombic MnGeO and trigonal GeO |
| 1100 | 24 | |||
| 1150 | 24 | |||
| MAGG_3 | 900 | 24 | 15 | mixture of cubic , orthorhombic MnGeO and trigonal GeO |
| 1100 | 24 | |||
| 1150 | 24 | |||
| MAGG_4 | 900 | 48 | 0 | mixture of cubic , orthorhombic MnGeO and rhombohedral AlO |
| 1050 | 48 | |||
| 1150 | 72 | |||
| MAGG_5 | 900 | 24 | 0 | mainly cubic , a few peaks of cubic MnAlO and orthorhombic MnO |
| 1100 | 24 | |||
| 1150 | 24 | |||
| Crystal Label | Growth | Gas Atmosphere/ | Feed & Seed | Remarks |
|---|---|---|---|---|
| Rate | Pressure | Rotation Rate | ||
| (mm/h) | (rpm) | |||
| MAGG_C1 | 20 | N, 3–3.5 bars | 6-6 | mainly cubic phase and a few peaks of orthorhombic MnGeO |
| MAGG_C2 | 20 | N, 1 bar | 10-16 | cubic phase ★ |
| grain ∼ 4–5 × 4–5 × 30 mm | ||||
| MAGG_C3 | 2-6 | N, 1 bar | 10-5 | mainly cubic phase and a few peaks of cubic MnAlO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Ciomaga Hatnean, M.; Balakrishnan, G.; Petrenko, O.A. Frustrated Magnet Mn3Al2Ge3O12 Garnet: Crystal Growth by the Optical Floating Zone Method. Crystals 2023, 13, 397. https://doi.org/10.3390/cryst13030397
Islam M, Ciomaga Hatnean M, Balakrishnan G, Petrenko OA. Frustrated Magnet Mn3Al2Ge3O12 Garnet: Crystal Growth by the Optical Floating Zone Method. Crystals. 2023; 13(3):397. https://doi.org/10.3390/cryst13030397
Chicago/Turabian StyleIslam, Manisha, Monica Ciomaga Hatnean, Geetha Balakrishnan, and Oleg A. Petrenko. 2023. "Frustrated Magnet Mn3Al2Ge3O12 Garnet: Crystal Growth by the Optical Floating Zone Method" Crystals 13, no. 3: 397. https://doi.org/10.3390/cryst13030397
APA StyleIslam, M., Ciomaga Hatnean, M., Balakrishnan, G., & Petrenko, O. A. (2023). Frustrated Magnet Mn3Al2Ge3O12 Garnet: Crystal Growth by the Optical Floating Zone Method. Crystals, 13(3), 397. https://doi.org/10.3390/cryst13030397







