Abstract
Novel Ag3PO4/Bi2WO6 catalysts with enhanced visible-light performance were synthesized using a hydrothermal method and characterized to investigate their morphology, microscopic structure, and binding energies. Photoluminescence spectrum (PL) and electrochemical impedance spectroscopy (EIS) data demonstrate that the formed Ag3PO4/Bi2WO6 heterojunction effectively promotes hole (h+)–electron (e−) separation and transfer efficiency, resulting in the enhancement of photocatalytic activity. Ag3PO4/Bi2WO6 displays higher photocatalytic activity than pure Bi2WO6 or Ag3PO4 alone. Photogenerated holes (h+), ·O2−, and ·OH were found to be the main active species for the degradation of malachite green (MG), methylene blue (MB), and Rhodamine B (RhB). The DFT calculation explains the photostability of Ag3PO4/Bi2WO6 from the perspective of electronic structure. The bandgap of Ag3PO4/Bi2WO6 between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) is 1.41 eV, compared with that of Ag3PO4 at 0.91 eV and Bi2WO6 at 2.59 eV. Ag–O–Bi hybridization and the wide HOMO–LUMO bandgap lead to difficulty in electron transfer. As a consequence, Ag+ is difficult to obtain electrons and difficult to convert into Ag0, which makes the catalyst stable.
1. Introduction
In recent years, semiconductor photocatalysis has gained attention as an efficient and environmentally friendly technology for tackling current environmental issues. The photocatalytic process offers several benefits, including simplicity, energy efficiency, and the elimination of organic pollutants, without producing secondary pollution [1,2,3,4]. Conventional photocatalysts, including TiO2 and ZnO, have been extensively researched for their ability to degrade organic pollutants [5]. However, they are limited to absorbing ultraviolet light and cannot fully utilize sunlight [6]. Recently, visible-light-response photocatalysts, such as Bi2WO6, BiOBr, BiVO4, Bi2O2CO3, Ag3PO4, AgBr, Ag3VO4, and AgCl, have been widely studied for their capacity to break down toxic dyes and organic pollutants [7,8,9,10,11,12,13,14,15]. Among these materials, Bi2WO6 has garnered significant attention due to its capacity to degrade organic pollutants through visible-light irradiation [16,17,18,19]. However, its practical application is thwarted by its high recombination of h+–e− pairs and low absorption efficiency of visible light [20,21,22]. Similarly, the Ag3PO4 photocatalyst reveals exceptional potential in photocatalytic processes for organic dye degradation under visible-light irradiation [23,24]. Furthermore, Ag3PO4 is a viable option for creating heterostructure composites, effectively enhancing photocatalytic activity. Nevertheless, the overabundance of electrons in the valence band of Ag3PO4 may lead to severe photo-corrosion by Ag+ to Ag0 [25]. Recently, various methods, including particle size and morphology control [26], metal deposition modification, and doping, have been employed to enhance and optimize the photocatalytic activity and stability of Ag3PO4. One promising approach is the coupling of Ag3PO4 with Bi2WO6. Maryam Amiri et al. developed a Bi2WO6/Ag3PO4-Ag Z-scheme heterojunction catalyst, which utilized the surface plasmon resonance (SPR) effect of Ag to increase photocatalytic activity and stability [27]. Sittikorn Jonjana et al. discovered that a 10 wt% mixture of Ag3PO4/Bi2WO6 exhibited superior photocatalytic activity and stability compared with separate Ag3PO4 or Bi2WO6 samples. This finding highlights the lack of research focused on mixtures exceeding 10 wt% of the Ag3PO4/Bi2WO6 system [28].
In this study, we present a straightforward hydrothermal and in situ precipitation approach to create a composite photocatalyst of Ag3PO4/Bi2WO6 with a boosted Ag3PO4 concentration. We evaluated the catalytic performance of the obtained samples, described the structural features, and examined the connections between the physicochemical characteristics and catalytic performance. Furthermore, we employed DFT to ascertain the grounds for the stability of Ag3PO4/Bi2WO6 based on electronic structure analysis.
2. Materials and Methods
2.1. Reagents and Materials
All the reagents used in the experiment were of analytic grade and were commercially purchased without further purification. AgNO3; NaH2PO4·3H2O; Bi(NO3)3·5H2O; Na2WO4·2H2O; Isopropyl alcohol (IPA); and MG, MB, and RhB were obtained from Sinopharm Chemical Reagent. Ammonium oxalate (AO) was purchased from Xilong Chemical Reagent, while p-benzoquinone (BQ) was obtained from Aladdin.
2.2. Preparation of Photocatalysts
In a typical procedure, Bi(NO3)3 (2 mmol, 0.7899 g) was dissolved in 10 mL of glacial acetic acid and 10 mL of 2 mol/L HNO3 to prevent Bi3+ ion hydrolysis. Technical term abbreviations were explained when they were first used. Afterward, 1 mmol of Na2WO4 (20 mL) solution was gradually added dropwise to the Bi(NO3)3 solution. The mixture was continuously stirred for 4 h, then moved to a Teflon-coated autoclave and maintained at 160 °C for 20 h. Finally, it was cooled down to room temperature naturally. The Bi2WO6 obtained was washed several times with deionized water to eliminate any residual by-products or reactants. It was then dried at 80 °C for 24 h. The dried powder was further calcined in a muffle oven at a temperature of 400 °C for 12 h with a temperature elevator of 5 °C/min.
Ag3PO4/Bi2WO6 composites were prepared using an in situ precipitation method. Typically, 0.6977 g (1 mmol) of the previously obtained Bi2WO6 powder was dispersed into 40 mL of deionized water via ultrasound for 30 min. Next, 20 mL of AgNO3 solution (0.5096 g, 3 mmol) was added to the Bi2WO6 dispersion. Subsequently, the mixture was stirred for an hour; then, 20 mL of NaH2PO4 solution (0.1199 g, 1 mmol) was added dropwise with magnetic stirring. Finally, the resulting precipitate underwent washing with deionized water several times, centrifugation, and vacuum drying at 60 °C. Pure Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6 mixed composites were also synthesized following identical procedures for comparative analysis. Ag3PO4/Bi2WO6-9 indicates that the mass proportion of Ag3PO4 is 90%. Meanwhile, Ag3PO4/Bi2WO6-X with various mass proportions (90%, 80%, 70%, 60%, and 50%) was produced alike by controlling the Ag3PO4 dose. The resulting products were labeled as Ag3PO4/Bi2WO6-X, where X equals 9, 8, 7, 6, and 5, respectively.
2.3. Characterization
The crystal phases and structures of the samples were analyzed via powder X-ray diffraction (XRD) utilizing a D/max-2550 PC (RIGAKU, Tokyo, Japan) equipped with Cu Kα (λ = 1.54056 Å) radiation, set at 40 kV and 200 mA, and scanned from 15° to 85° at a rate of 6 °/min. The Thermo ESCALAB 250 X-ray photoelectron spectroscope (XPS, Thermo Fisher Scientific, Waltham, MA, USA) was used to assess surface chemical compositions. Additionally, morphologies were examined using a Hi-tachi S-4800 field emission microscope. The scanning electron microscope (FE-SEM) from Japan operated at 10 kilovolts for imaging. The High-Resolution Transmission Electron Microscopy (HR-TEM) images were obtained utilizing a JEOL JEM-2100F microscope from Tokyo, Japan. To determine the energy band structure of the photocatalysts, UV–visible Optical Diffuse Reflectance Spectra (DRS) were measured using BaSO4 as the background with a PerkinElmer Lambda850 instrument from MA, USA. The PerkinElmer LS55 MA USA’s PL spectra, with an excitation wavelength of 360 nm, evaluated the h+–e− pairs’ recombination degree.
The CHI660E Electrochemical Workstation (CH Instruments, Shanghai, China) measured the photocatalyst’s Electrochemical Impedance Spectroscopy (EIS) over a 100 Hz to 0.01 kHz frequency domain with a 5 mV perturbation potential. The EIS outcomes were fitted with Zview 3.0 software. The electrochemical electrode is constructed with a photocatalyst (approximately 3 mg in ethanol) deposition on an indium tin oxide glass, which serves as the working electrode. A Pt sheet acts as the counter electrode, a saturated calomel electrode (SCE) is used as the reference electrode, and a 0.1 M Na2SO4 solution acts as the electrolyte solution. For slight and viscous suspension solutions, 3 mg sample powders mixed with Nafion ionomer were dissolved in an ethanol aqueous solution. The suspension was evenly applied onto the clean electrode surface composed of indium-tin oxide (ITO) through drop-coating and left to air dry, according to reference [23].
2.4. Activity Test
Photocatalytic experiments were conducted using a slurry reaction reactor and a 500 W Xe lamp (300 mW/cm2) from Perfectlight Technology Co., Ltd., Beijing, China, which was equipped with a cutoff filter (>400 nm) as a visible-light source. The target pollutants used were Malachite Green (MG), Methylene Blue (MB), and Rhodamine B (RhB) dye, and 20 mg of photocatalyst was dispersed into 50 mL of pollutant solution (C = 10 mg/L, MG, MB, or RhB) under visible-light irradiation at 25 °C. Before irradiation, the suspension was stirred continuously in the dark for 30 min to create a finely dispersed solution and establish adsorption–desorption equilibrium. While being irradiated, each 0.5 mL suspension was collected in a plastic sample tube containing 2.5 mL deionized water every 10 min and then centrifuged. The supernatant was analyzed using a T9 UV–vis spectrophotometer. Figure 1 depicts the schematic diagram of the photocatalytic reactor.
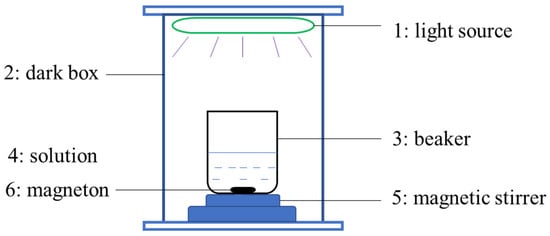
Figure 1.
The schematic diagram of photocatalytic reactor.
2.5. Calculation
DFT calculations were conducted using plane wave methods. We employed the Cambridge Sequential Total Energy Package (CASTEP) to perform the calculations. Our computational models consisted of tetragonal supercells (2 × 2 × 1) of Ag3PO4 and Bi2WO6. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) algorithm was utilized to optimize the lattice constants and atomic coordinates in these supercells. Subsequently, after acquiring the stable structure, we calculated the electronic properties of both supercells. The study utilized Generalized Gradient Approximation (GGA) to express exchange and correlation effects, which were parameterized by the PBE function developed by Perdew–Burke–Ernzerhof. Interactions between core and valence electrons were described using an ultrasoft pseudopotential. All geometry optimizations employ convergence thresholds of 5 × 10−6 eV/atom for total energies, a maximum force of 0.01 eV/Å, a maximum stress of 0.02 GPa, and a maximum displacement of 5 × 10−4 Å. Default values were utilized for other calculation parameters.
3. Results and Discussion
3.1. Physicochemical Structure
Figure 2 displays X-ray diffraction (XRD) patterns for various Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6 samples with varying ratios. The pure Ag3PO4 exhibits a cubic phase (JCPDS: 06-0505). The diffraction peaks of Ag3PO4 match crystal planes of (110), (200), (210), (211), (220), (310), (222), (320), (321), (400), (420), (421), and (332) at 2θ = 20.91°, 29.75°, 33.24°, 36.46°, 42.54°, 47.80°, 52.69°, 54.87°, 55.02°, 61.58°, 69.91°, 71.88°, and 73.88°, respectively, as depicted in Figure 2 [29]. The pure Bi2WO6 crystal (JCPDS 39-0256) can be clearly identified as orthorhombic. The perfectly matching characteristic peaks at 2θ = 28.29°, 32.79°, 47.14°, 55.99°, 58.54°, 68.75°, 76.08°, and 78.53° with Bi2WO6 (JCPDS No. 39-0256, space group: Pbca) can be indexed to the (131), (200), (202), (133), (262), (400), (193), and (204) crystal planes of orthorhombic Bi2WO6 [30,31], respectively. All diffraction peaks of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6 composites exhibit a well-matched two-phase composition. None of the diffraction peaks for impurities like Ag, Ag2O, Bi2O3, or WO3 are observed. The strong and sharp peaks assigned to Ag3PO4/Bi2WO6 composites display favorable crystal structures. Notably, the peak position of Ag3PO4 remains relatively unchanged, indicating that Bi2WO6 only adheres to the surface of Ag3PO4 without penetrating the lattice.
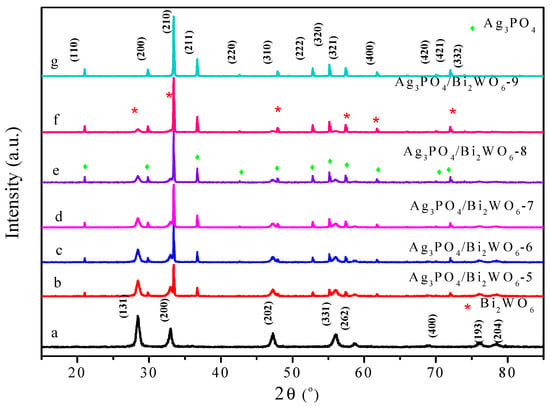
Figure 2.
The X-ray diffraction patterns obtained for the samples prepared with varying Ag3PO4/Bi2WO6 ratios. The asterisk symbol (*) indicates the Bi2WO6 peak positions, while the diamond symbol (◆) represents the peak positions of Ag3PO4.
The SEM was used to characterize the morphology and microscopic structure of the as-prepared samples, including Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6-9. In Figure 3a,b, Ag3PO4 exhibits cubic-shaped nanoparticles ranging between 100 and 400 nm in diameter, while the Bi2WO6 sample presents an irregular cubical shape between 30 and 100 nm. The SEM image of Ag3PO4/Bi2WO6-9 shows that the Bi2WO6 nanoparticle tightly adheres to the surface of Ag3PO4 (Figure 3c). Figure 3d displays the TEM pattern of Ag3PO4/Bi2WO6-9. The regular shape of the material is Ag3PO4, while the amorphous shape is usually Bi2WO6. An amplified image of the local area can be found in Figure 3e, which highlights the heterojunction between Ag3PO4 and Bi2WO6 within a green frame. The magnified part of the interface is displayed in Figure 3f. Additionally, the HR-TEM pattern of Ag3PO4 in Ag3PO4/Bi2WO6-9 heterojunction can be found in Figure 3g. The lattice fringe of 0.246 nm corresponds to the (211) plane of cubic Ag3PO4 phase and coincides well with reported literature [32]. The HR-TEM image of Bi2WO6 is shown in Figure 3h. The particle is confirmed to be Bi2WO6 with space group B2cb [33] by the (200) crystal spacing (0.273 nm). In Figure S1, the elemental maps of Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6-9 demonstrate uniform distribution of all elements without agglomeration.
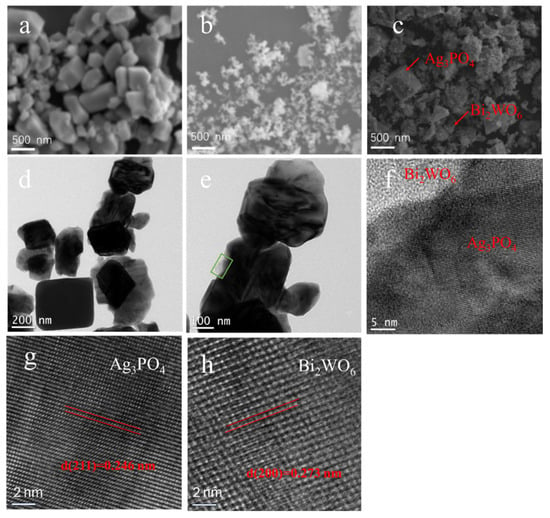
Figure 3.
(a) SEM of Ag3PO4; (b) SEM of Bi2WO6; (c) SEM image of Ag3PO4/Bi2WO6-9; (d) TEM image of Ag3PO4/Bi2WO6-9; (e) magnified TEM image ofAg3PO4/Bi2WO6-9; (f) HR-TEM of Ag3PO4/Bi2WO6-9 heterojunction structure; (g) HR-TEM of Ag3PO4 in Ag3PO4/Bi2WO6-9; (h) HR-TEM of Bi2WO6 in Ag3PO4/Bi2WO6-9.
XPS was utilized to determine the electronic structures of Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6-9 composites. Figure 4a displays the XPS spectra of Ag 3d for both the Ag3PO4 and Ag3PO4/Bi2WO6-9 composite catalysts. The characteristic peaks of Ag 3d at 368.0 eV (Ag 3d5/2) and 374.0 eV (Ag 3d3/2) are attributed to the Ag+ ions in Ag3PO4. After the introduction of Bi2WO6, the binding energy of spin-orbit Ag 3d splits into two peaks at 368.3 and 374.3 eV, which is 0.3 eV higher than that of Ag3PO4. This indicates that the electronic clouds on Ag+ in Ag3PO4 shift to Bi2WO6 after combination. Furthermore, compared with pure Ag3PO4, the binding energy of P 2p in Ag3PO4/Bi2WO6-9 is higher, with a value of 133.7 eV instead of 132.9 eV (Figure 4b). The peaks observed at 159.4 eV and 164.7 eV correspond to the Bi 4f7/2 and Bi 4f5/2 sublevels of Bi2WO6, respectively. On the other hand, the peaks at 159.9 eV and 165.2 eV are associated with the Bi 4f7/2 and Bi 4f5/2 levels of Ag3PO4/Bi2WO6-9, respectively, suggesting that the bismuth species in the composite are Bi3+ cations. Furthermore, the peaks observed at 35.98 eV and 37.98 eV for the W 4f7/2 and W 4f5/2 sublevels, respectively, can be attributed to a six-valent oxidation state for W6+ in Ag3PO4/Bi2WO6-9. These findings indicate a 0.18 eV deviation of 4f7/2 and 4f5/2 levels relative to the values in pure Bi2WO6 (refer to Figure 4d for more details).
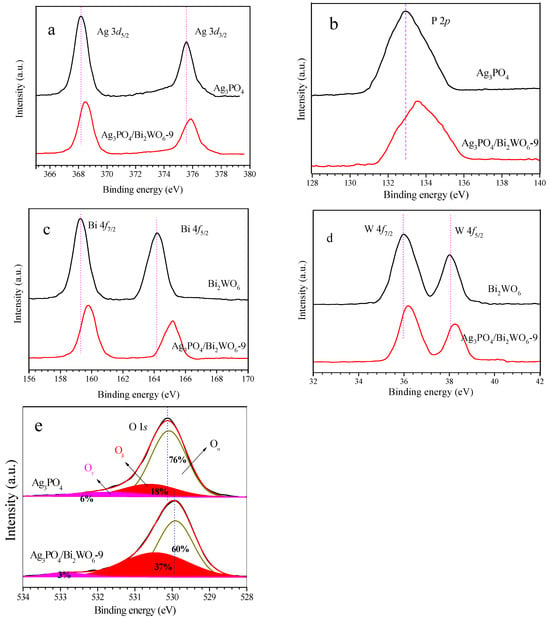
Figure 4.
XPS spectra of the catalysts. (a) Ag 3p; (b) P 2p; (c) Bi 4f; (d) W 4f; (e) O 1s.
The O 1s XPS spectra for the samples are presented in Figure 4e. The spectra for Ag3PO4 indicate three distinct peaks with binding energies of 530.1 eV (identified as Oα), 531.6 eV (531.3 eV, identified as Oβ), and 533.1 eV (533.1 eV, identified as Oγ). These peaks are attributed to lattice oxygen, adsorbed oxygen species, and defect oxygen located on the surface of Ag3PO4, respectively [33,34,35]. In the case of Ag3PO4/Bi2WO6-9, the binding energies for O 1s orbits are 529.9 eV, 531.4 eV, and 532.8 eV. The composites also exhibit significantly lower energy shifts compared to their individual constituents. It is apparent that the (Oβ + Oγ)/Oα ratio of Ag3PO4 (24%:76%) is appreciably lower than that of the Ag3PO4/Bi2WO6-9 composite (40%:60%). The augmented ratio of active oxygen (Oβ + Oγ) is beneficial in enhancing photocatalytic performance. The chemical interactions between Bi2WO6 and Ag3PO4 have resulted in variations in the binding energies of Ag 3d, P 2p, Bi 4f, W 4f, and O 1s, indicating the formation of heterostructures and the promotion of interfacial charge transfer. As a result, the photocatalytic activity of Ag3PO4/Bi2WO6 nanocomposites has improved. An increase in binding energy typically leads to the loss of an atom’s charge and a subsequent decrease in the density of the surrounding electron cloud. This reduction enhances the attraction between the nucleus and electrons, thereby increasing the electron binding energy. This leads to an increase in reactive oxygen species and a decrease in electron density of Ag+ ions, ultimately improving the catalytic performance and stability of Ag3PO4.
UV–vis DRS was conducted to examine the optical properties of relevant samples. All samples displayed visible-light absorbance. Figure S2 displays the results of UV–vis DRS for Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6 with differing composite ratios. Ag3PO4/Bi2WO6 exhibits varying absorption band edges according to the composite ratio. The composites demonstrate notable absorption within the visible-light range (760 nm > λ > 400 nm). In Figure 5, the fundamental absorption band edge of pure Bi2WO6 is measured at 459 nm. Additionally, the strong absorption band edge of Ag3PO4 is observed at approximately 523 nm, in line with previous findings [29]. The absorption of the composite material Ag3PO4/Bi2WO6-9 demonstrates infrared transfer to 493 nm compared with Bi2WO6. Based on Figure 5a, the direct optical band gap energies (Eg) of pure Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6-9 composites are estimated at 2.70, 2.37, and 2.51 eV, respectively. Tauc plots of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6-9 are displayed in Figure 5b for better comprehension. This demonstrates the formation of heterostructure in Ag3PO4/Bi2WO6-9.
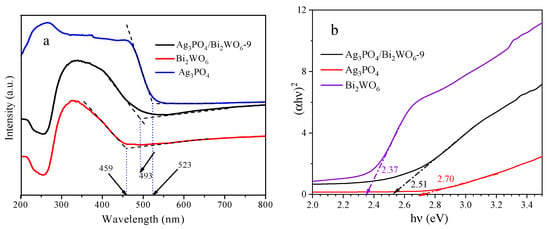
Figure 5.
(a) UV–vis DRS of as-synthesized samples of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6-9. (b) Tauc plots of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6-9.
3.2. Photocatalytic Performance under Visible-Light Irradiation
The catalytic performances of visible-light-driven catalysts were evaluated through the degradation of aqueous dyes with different structures, including triphenylmethane MG, phenothiazine group MB, and triphenyl RhB. To ensure adsorption–desorption equilibrium, the mixture containing dye solution and catalyst was kept in the dark for 30 min before irradiation. As depicted in Figure 6(a1,b1,c1), Ag3PO4/Bi2WO6-9 shows a good adsorption capacity for MG, MB, and RhB.
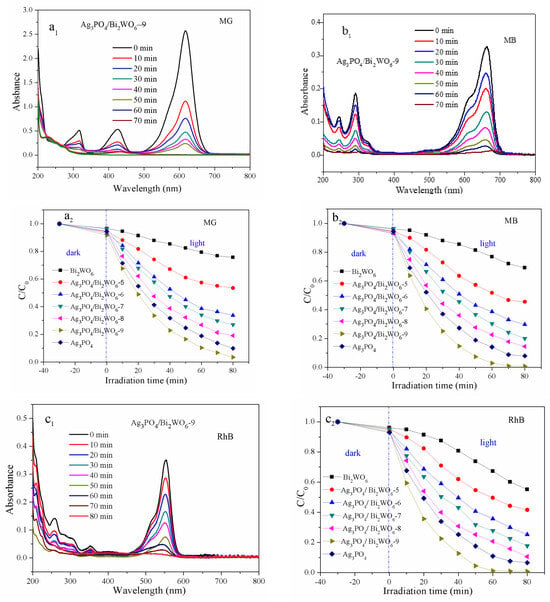
Figure 6.
(1) Time-dependent UV–vis absorption spectra of the MG (a1), MB (b1), and RhB (c1) solution in the presence of Ag3PO4/Bi2WO6-9. (2) Degradation curves of MG (a2), MB (b2), and RhB (c2) with Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6 composites under visible-light irradiation.
Figure 6(a2,b2,c2) display the decolorization efficiencies of Ag3PO4/Bi2WO6-X (X = 5, 6, 7, 8, 9), pure Ag3PO4, and Bi2WO6 for the dyes MG, MB, and RhB. The activity of these catalysts follows the trend of Bi2WO6 < Ag3PO4/Bi2WO6-5 < Ag3PO4/Bi2WO6-6 < Ag3PO4/Bi2WO6-7 < Ag3PO4/Bi2WO6-8 < Ag3PO4 < Ag3PO4/Bi2WO6-9 for the three dyes. The photocatalytic activity of the Ag3PO4/Bi2WO6-9 composite displays the best catalytic performance.
The stability of Ag3PO4 and Ag3PO4/Bi2WO6-9 was assessed by studying the degradation of RhB dyes under visible-light irradiation (see Figure 7). Following five cycles of the photodegradation process, the degradation efficiency of Ag3PO4 decreased from 86% to 30%. In contrast, the degradation efficiency of Ag3PO4/Bi2WO6-9 decreased from 97% to 76%, indicating that Ag3PO4/Bi2WO6-9 displays little activity loss and good photochemical stability. In contrast, the degradation efficiency of Ag3PO4/Bi2WO6-9 decreased from 97% to 76%, indicating that Ag3PO4/Bi2WO6-9 displays little activity loss and good photochemical stability.
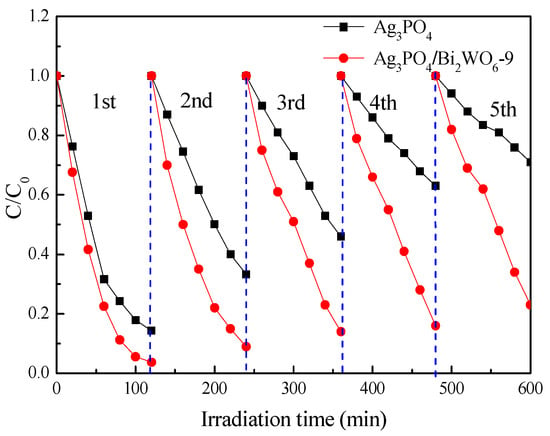
Figure 7.
Photocatalytic cycle performance of Ag3PO4 andAg3PO4/Bi2WO6-9.
3.3. The Relationship of Structure and Activity
3.3.1. Main Free Radicals
Ag3PO4/Bi2WO6-9 was utilized to photodegrade RhB, and scavengers—namely, AO, IPA, and BQ—were applied to test the ·OH, h+, and·O2− free radicals, correspondingly. Photodegradation efficiencies with different scavengers are presented in Figure 8a. Notably, the efficiency of Ag3PO4/Bi2WO6-9 without any scavengers is high, while the efficiencies decrease after adding scavengers. Figure 8b displays the reaction rate constant k. Based on the aforementioned results, the primary free radicals were ·OH, while the other free radicals consisted of ·O2− and hole. These highly reactive free radicals are potentially accountable for the deterioration of RhB.
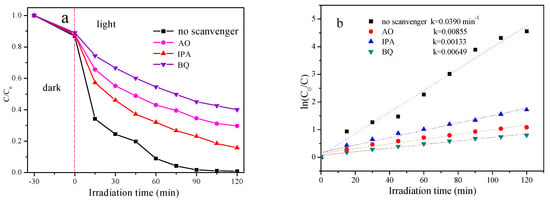
Figure 8.
Photodegradation efficiencies of RhB as a function of irradiation time with different scavengers. (a) Photodegradation efficiencies with different scavengers. (b) Reaction rate constants k with different scavengers.
3.3.2. PL Analysis
Figure S3 displays the photoluminescence (PL) spectra of Ag3PO4, Ag3PO4/Bi2WO6-5, Ag3PO4/Bi2WO6-6, Ag3PO4/Bi2WO6-7, Ag3PO4/Bi2WO6-8, Ag3PO4/Bi2WO6-9, and Bi2WO6 composites. The PL intensities follow the order Bi2WO6 > Ag3PO4/Bi2WO6-5 > Ag3PO4/Bi2WO6-6 > Ag3PO4/Bi2WO6-7 > Ag3PO4/Bi2WO6-8 > Ag3PO4/Bi2WO6-9 > Ag3PO4. The PL intensity of the Ag3PO4/Bi2WO6-9 composite exhibits an obvious decrease compared with pure Bi2WO6 (as shown in Figure 9). The Ag3PO4/Bi2WO6-9 composite exhibits a decreased PL intensity compared with Ag3PO4 and Bi2WO6, suggesting efficient inhibition of photoexcited electron–hole recombination through the formation of the Ag3PO4/Bi2WO6 heterojunction.
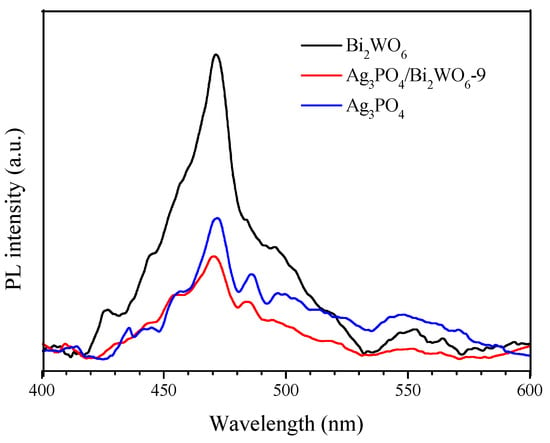
Figure 9.
PL spectra of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6-9 composites.
3.3.3. EIS Analysis
EIS was utilized to examine the charge transfer resistance and segregation of photogenerated h+–e− pairs at solid/electrolyte interfaces in the photocatalyst. Figure 10 illustrates the EIS Nyquist plots of Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6-9 blends under visible-light irradiation. The corresponding circuit model employed to fit the obtained data is included, where Rs indicates solution resistance, CPE denotes the constant phase angle element, and Rct refers to reaction resistance. This is indicated by the smallest arc radius belonging to Ag3PO4/Bi2WO6-9. In Figure 10a, the EIS plot shows that Ag3PO4/Bi2WO6-9 had the fastest interfacial electron transfer and more separation of photogenerated h+–e− pairs compared with Bi2WO6 and Ag3PO4. The simulated data can be found in Table S1. The combination of PL and EIS data indicates that creating a Ag3PO4/Bi2WO6 heterojunction effectively enhances charge separation and transfer efficiency, consequently resulting in an improvement in photocatalytic activity.
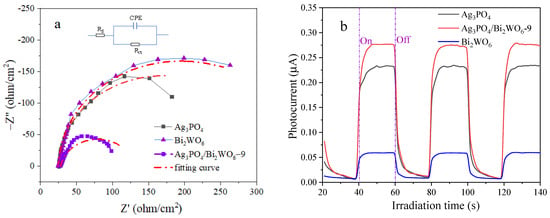
Figure 10.
(a) EIS plots of the samples under irradiation with visible light. Insert: equivalent circuit used to fit the data; (b) transient photocurrent response curves for the three catalysts.
Figure 10b illustrates the transient photocurrent response of the three catalysts, measured over several intermittent irradiation cycles. Upon activation, the photocurrent rapidly increases to a stable current platform, which subsequently falls back to a small value once deactivated. Notably, the photocurrent observed for Ag3PO4/Bi2WO6-9 is the largest, followed by Ag3PO4, while that of Bi2WO6 is the smallest. These results suggest that more electrons and holes in Ag3PO4/Bi2WO6-9 are excited, whereby they subsequently participate in the photocatalytic degradation reaction.
3.3.4. DFT Analysis
To elucidate the electronic states of the catalytically active sites (CASs) in Ag3PO4/Bi2WO6, the density of states (DOS) of Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6 were calculated using DFT. Figure S4 illustrates simplified models (unit cell) depicting the crystal structures of Ag3PO4 (Figure S4a) and Bi2W2O6 (Figure S4b), with different elements represented by distinct colors. The simplified models are solely utilized to demonstrate the bonding environment of Ag3PO4 and Bi2W2O6. By optimizing the lattice constants, the Ag3PO4 and Bi2W2O6 supercells have dimensions of 6.026 Å × 6.026 Å × 6.026 Å and 5.5340 Å × 5.4998 Å × 16.5507 Å, respectively. Technical terms are clearly explained when first used. Each P atom coordinates with four O atoms, and each O atom coordinates with one P atom and three Ag atoms in Ag3PO4 (Figure 11a).
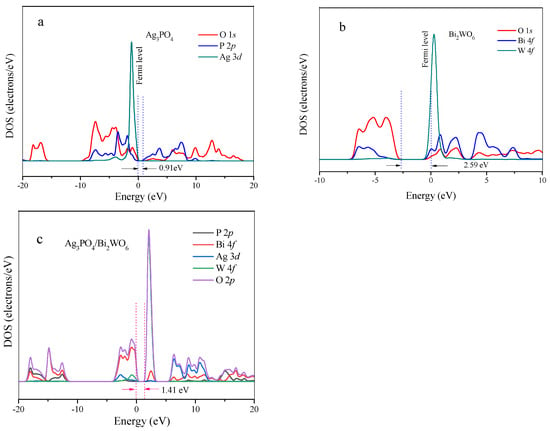
Figure 11.
The DOS patterns. (a) Ag3PO4; (b) Bi2WO6; (c) Ag3PO4/Bi2WO6.
The DOS of O 1s, P 2p, and Ag 3d appear in the same energy regions, indicating the presence of Ag-O and P-O hybridization. The bandgap between the HOMO and LUMO is approximately 0.91 eV. Mixing with Bi2WO6 (Figure 11b) caused an increase in the bandgap to 1.41 eV due to the upshift of HOMO energy (Figure 11c). This tendency is consistent with experimental results showing that the band gap energies of pure Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6 composites are 2.37, 2.7, and 2.51 eV, respectively. Therefore, due to the hybridization between Ag, O, and Bi, and the wide bandgap between the HOMO and LUMO, the transfer of electrons through bridging O between Ag and Bi may be hindered, resulting in difficulty obtaining electrons to reduce Ag+ into Ag0. Additionally, there is implicit hybridization between Bi and W cations through their interactions with bridging O atoms, as the DOS of Bi, O, and W in Bi2WO6 are in similar energy regimes. Due to the hybridization of Bi–O–W and the wide bandgap between the HOMO and LUMO, the transfer of electrons between W and Bi through the bridging O is expected to become relatively easy, resulting in the co-existence of multiple oxidation states of W and Bi. The composite Ag3PO4/Bi2WO6 increases the bandgap between the HOMO and LUMO, thereby enhancing the catalytic activity of the active O and improving the photostability of Ag3PO4.
3.3.5. Proposed Photocatalytic Degradation Mechanism
Based on the experimental data and literature review, we propose a mechanism for photocatalysis under visible-light irradiation (see Figure 12). When the catalyst is exposed to visible light, the electrons in the valence band (VB) of Ag3PO4 become excited and move to its conduction band (CB) orbital. Consequently, holes are formed in the VB of Ag3PO4, while the CB orbital of Ag3PO4 becomes occupied by electrons. The same behavior is observed in Bi2WO6. The efficient heterojunction between Ag3PO4 and Bi2WO6 results from the transfer of holes from Bi2WO6 to the VB orbital of Ag3PO4 and the migration of electrons from Ag3PO4 to the CB orbital of Bi2WO6. This can be attributed to the lower VB energy level of Ag3PO4 compared with that of Bi2WO6. Thus, the two materials are tightly bonded together. Furthermore, the dissolved oxygen molecules in water scavenge the electrons present on Ag3PO4 CB and produce highly oxidative ·O2− species, which could react with H2O to generate ·OH. The hole h+ also produces ·OH after reacting with water, while the formed ·OH, ·O2−, and h+ effectively break down organic substrates.
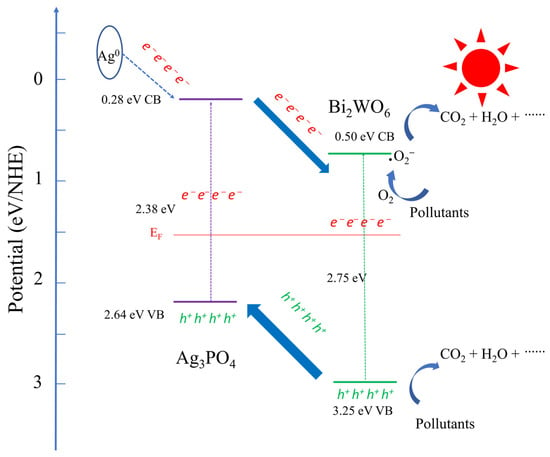
Figure 12.
Schematic diagram of the separation and transfer of photogenerated charges in the hybrid under visible-light irradiation.
4. Conclusions
A novel visible-light-driven photocatalyst composed of Ag3PO4/Bi2WO6 composites was synthesized and characterized successfully. X-ray diffraction analysis demonstrated that the Ag3PO4/Bi2WO6 composites possess crystal structures. The morphology and microscopic structures of Ag3PO4/Bi2WO6 were observed through scanning and transmission electron microscopy. X-ray photoelectron spectroscopy exhibited that the catalytic performance and stability of Ag3PO4 improved due to an increase in reactive oxygen species and a decrease in the electron density of Ag+ ions. The Ag3PO4/Bi2WO6 composites prepared demonstrate superior photodegradation activity for degrading MG, MB, and RhB in comparison with Bi2WO6 or Ag3PO4 under visible-light irradiation. The improved activity can be attributed to the effective separation of electron–hole pairs generated by light and the production of free radicals such as ·OH, ·O2−, and h+.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13111531/s1, Table S1. The EIS simulated data for Ag3PO4, Bi2WO6, and Ag3PO4/Bi2WO6-9. Figure S1. (a) Elemental mapping of Ag3PO4; (b) elemental mapping of Bi2WO6; (c) elemental mapping of Ag3PO4/Bi2WO6-9. Figure S2. UV–vis DRS of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6 composites with various ratios. Figure S3. PL spectra of Bi2WO6, Ag3PO4, and Ag3PO4/Bi2WO6 composites with various ratios. Figure S4. Crystal structures. (a) Ag3PO4; (b) Bi2W2O6.
Author Contributions
Conceptualization, J.W.; data curation, J.W. and H.P.; formal analysis, L.W. and J.W.; calculation, H.C.; experiment, Y.F. and C.W.; supervision, J.W. and H.P.; writing, L.W. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Research Key Project of Anhui Provincial Department of Education (KJ2021A1036, Junbo Wang), Zhejiang Provincial Natural Science Foundation of China (LGF22B070005, Hua Pan), the College Students’ Innovative Entrepreneurial Training Project (S202110375086, 202210375019, Junbo Wang), and the Special Innovation Foundation of Anhui Province (2020XZX005, Junbo Wang).
Data Availability Statement
Data are available from the authors.
Acknowledgments
We would like to thank Seyit YUZUAK for his assistance with language and grammar correction, editing, and advising, which greatly improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schipper, D.E.; Zhao, Z.; Leitner, A.P.; Xie, L.; Qin, F.; Alam, M.K.; Chen, S.; Wang, D.; Ren, Z.; Wang, Z.; et al. A TiO2/FeMnP core/shell nanorod array photoanode for efficient photoelectrochemical oxygen evolution. ACS Nano 2017, 11, 4051–4059. [Google Scholar] [CrossRef]
- Li, S.J.; Hu, S.W.; Jiang, W.; Liu, Y.; Liu, J.S.; Wang, Z.H. Facile synthesis of flower-like Ag3VO4/Bi2WO6 heterojunction with enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 501, 156–163. [Google Scholar] [CrossRef]
- Meng, F.P.; Liu, Y.Z.; Wang, J.; Tan, X.Y.; Sun, H.Q.; Liu, S.M.; Wang, S.B. Temperature dependent photocatalysis of g-C3N4, TiO2 and ZnO: Differences in photoactive mechanism. J. Colloid Interface Sci. 2018, 532, 321–330. [Google Scholar] [CrossRef]
- Yao, L.Z.; Wang, W.Z.; Wang, L.J.; Liang, Y.J.; Fu, J.L.; Shi, H.L. Chemical bath deposition synthesis of TiO2/Cu2O core/shell nanowire arrays with enhanced photoelectrochemical water splitting for H2 evolution and photostability. Int. J. Hydrogen Energy 2018, 43, 15907–15917. [Google Scholar] [CrossRef]
- Kosemen, A.; Kosemen, Z.A.; Canimkubey, B.; Erkovan, M.; Basarir, F.; San, S.E.; Ornek, O.; Tunc, A.V. Fe doped TiO2 thin film as electron selective layer for inverted solar cells. Sol. Energy 2016, 132, 511–517. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhu, S.F.; Xie, F.Z.; Zhang, J.; Meng, Z.D. Plate-on-plate structured Bi2MoO6/Bi2WO6 heterojunction with high-efficiently gradient charge transfer for decolorization of MB. Sep. Purif. Technol. 2013, 113, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Identification of Bi2WO6 as a highly selective visible-light photocatalyst toward oxidation of glycerol to dihydroxyacetone in water. Chem. Sci. 2013, 4, 1820–1824. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.S. Synthesis and characterization of high surface area CuWO4 and Bi2WO6 electrodes for use as photoanodes for solar water oxidation. J. Mater. Chem. A 2013, 1, 5006–5014. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Jiang, X.; Mei, J.F.; Li, Y.X.; Han, W.H.; Xie, M.Z.; Wang, F.C.; Xie, E.Q. Improved photoelectrocatalytic hydrogen generation through BiVO4 quantum-dots loaded on nano-structured SnO2 and modified with carbon quantum-dots. Chem. Eng. J. 2018, 331, 48–53. [Google Scholar] [CrossRef]
- Nualkaew, P.; Phuruangrat, A.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Synthesis of Ag3VO4 nanoparticles loaded on Bi2MoO6 nanoplates as heterostructure visible light driven photocatalyst by sonochemical method. J. Ceram. Soc. Jpn. 2016, 124, 1157–1160. [Google Scholar] [CrossRef]
- Kumar, S.; Surendar, T.; Baruah, A.; Shanker, V. Synthesis of a novel and stable g-C3N4-Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation. J. Mater. Chem. A 2013, 1, 5333–5340. [Google Scholar] [CrossRef]
- Yan, Q.S.; Li, C.; Lin, C.P.; Zhao, Y.L.; Zhang, M.H. Visible light response AgBr/Ag3PO4 hybrid for removal of anionic dye and tetracycline hydrochloride in water. J. Mater. Sci. Mater. Electron. 2018, 29, 2517–2524. [Google Scholar] [CrossRef]
- Qiao, R.; Mao, M.; Hu, E.; Zhong, Y.; Ning, J.; Hu, Y. Facile formation of mesoporous BiVO4/Ag/AgCl heterostructured microspheres with enhanced visible-light photoactivity. Inorg. Chem. 2015, 54, 9033–9039. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.F.; Dai, K.; Zhang, J.F.; Li, W.Y.; Liang, C.H. Construction of Ag SPR-promoted step-scheme porous g-C3N4/Ag3VO4 heterojunction for improving photocatalytic activity. Appl. Surf. Sci. 2019, 488, 151–160. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zhang, Y.F.; Lin, M.S.; Long, J.L.; Zhang, Z.Z.; Lin, H.X.; Wu, J.C.S.; Wang, X.X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Cui, Y.M.; Tan, X.Y.; Zheng, S.X.; Zhang, H.; Xu, J.W.; Wang, Q.Y. Construction of Ag3PO4/Ag4P2O7 nanospheres sensitized hierarchical titanium dioxide nanotube mesh for photoelectrocatalytic degradation of methylene blue. Sep. Purif. Technol. 2019, 215, 619–624. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Zeng, J.; Ramaraghavulu, R.; Shim, J.; Mauger, A.; Julien, C.M. High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts. Int. J. Mol. Sci. 2022, 24, 663. [Google Scholar] [CrossRef]
- Wang, R.; Xu, M.; Xie, J.W.; Ye, S.Y.; Song, X.L. A spherical TiO2-Bi2WO6 composite photocatalyst for visible-light photocatalytic degradation of ethylene. Colloid Surface A 2020, 602, 125048. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef]
- Wang, J.J.; Tang, L.; Zeng, G.M.; Deng, Y.C.; Liu, Y.N.; Wang, L.G.; Zhou, Y.Y.; Guo, Z.; Wang, J.J.; Zhang, C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 285–294. [Google Scholar] [CrossRef]
- Lu, X.Y.; Che, W.J.; Hu, X.F.; Wang, Y.; Zhang, A.T.; Deng, F.; Luo, S.L.; Dionysiou, D.D. The facile fabrication of novel visible-light-driven Z-scheme CuInS2/Bi2WO6 heterojunction with intimate interface contact by in situ hydrothermal growth strategy for extraordinary photocatalytic performance. Chem. Eng. J. 2019, 356, 819–829. [Google Scholar] [CrossRef]
- Ma, F.Y.; Yang, Q.L.; Wang, Z.J.; Liu, Y.H.; Xin, J.J.; Zhang, J.J.; Hao, Y.T.; Li, L. Enhanced visible-light photocatalytic activity and photostability of Ag3PO4/Bi2WO6 heterostructures toward organic pollutant degradation and plasmonic Z-scheme mechanism. RSC Adv. 2018, 8, 15853–15862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Dai, Y.Z.; Wang, X.Y. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, T.; Kuntalue, B.; Thongtem, S. Decolorization of rhodamine B photocatalyzed by Ag3PO4/Bi2WO6 nanocomposites under visible radiation. Mater. Lett. 2018, 218, 146–149. [Google Scholar] [CrossRef]
- Ma, P.Y.; Yu, Y.; Xie, J.J.; Fu, Z.Y. Ag3PO4/CuO composites utilizing the synergistic effect of photocatalysis and Fenton-like catalysis to dispose organic pollutants. Adv. Powder Technol. 2017, 28, 2797–2804. [Google Scholar] [CrossRef]
- Zheng, C.X.; Yang, H. Assembly of Ag3PO4 nanoparticles on rose flower-like Bi2WO6 hierarchical architectures for achieving high photocatalytic performance. J. Mater. Sci. Mater. Electron. 2018, 29, 9291–9300. [Google Scholar] [CrossRef]
- Amiri, M.; Dashtian, K.; Ghaedi, M.; Mosleh, S.; Jannesar, R. Bi2WO6/Ag3PO4-Ag Z-scheme heterojunction as a new plasmonic visible-light-driven photocatalyst: Performance evaluation and mechanism study. New J. Chem. 2019, 43, 1275–1284. [Google Scholar] [CrossRef]
- Qian, X.F.; Yue, D.T.; Tian, Z.Y.; Reng, M.; Zhu, Y.; Kan, M.; Zhang, T.Y.; Zhao, Y.X. Carbon quantum dots decorated Bi2WO6 nanocomposite with enhanced photocatalytic oxidation activity for VOCs. Appl. Catal. B Environ. 2016, 193, 16–21. [Google Scholar] [CrossRef]
- Hou, G.Q.; Zeng, X.F.; Gao, S.J. Fabrication and photocatalytic activity of core@shell Ag3PO4@Cu2O heterojunction. Mater. Lett. 2019, 238, 116–120. [Google Scholar] [CrossRef]
- Chi, C.Y.; Pan, J.Q.; You, M.Z.; Dong, Z.J.; Zhao, W.J.; Song, C.S.; Zheng, Y.Y.; Li, C.R. The porous TiO2 nanotubes/Ag3PO4 heterojunction for enhancing sunlight photocatalytic activity. J. Phys. Chem. Solids 2018, 114, 173–178. [Google Scholar] [CrossRef]
- Zhang, F.J.; Sun, R.; Li, R.S.; Song, N.N.; Feng, L.M.; Zhong, S.; Zhao, Z.Q. Novel La-doped Bi2WO6 photocatalysts with enhanced visible-light photocatalytic activity. J. Sol-Gel Sci. Technol. 2018, 86, 640–649. [Google Scholar] [CrossRef]
- Zhang, J.L.; Ma, Z. Ag3VO4/AgI composites for photocatalytic degradation of dyes and tetracycline hydrochloride under visible light. Mater. Lett. 2018, 216, 216–219. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.G.; Si, M.Z.; Yu, Y.F.; Zhang, H.Y. (Yb3+, Er3+) co-doped TiO2/Ag3PO4 hybrid photocatalyst with enhanced activity for photodegradation of phenol. Appl. Surf. Sci. 2019, 463, 159–168. [Google Scholar] [CrossRef]
- Tian, L.; Xian, X.Z.; Cui, X.K.; Tang, H.; Yang, X.F. Fabrication of modified g-C3N4 nanorod/Ag3PO4 nanocomposites for solar-driven photocatalytic oxygen evolution from water splitting. Appl. Surf. Sci. 2018, 430, 301–308. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Zhu, Z.D.; Wang, X.G.; Khan, A.; Gong, J.Y.; Zhang, Y.R. Synthesis of Z-scheme g-C3N4/Ag/Ag3PO4 composite for enhanced photocatalytic degradation of phenol and selective oxidation of gaseous isopropanol. Mater. Res. Bull. 2018, 107, 407–415. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).