Erbium-Doped LiYF4 as a Potential Solid-State Frequency Reference: Eligibility and Spectroscopic Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Design
- The radius of the cation to be substituted should be as close as possible to the REI radius (for the same coordination) in order to minimize strains due to doping, especially when considering higher concentrations;
- The cation to be substituted and the REI should have the same oxidation state to avoid charge compensation;
- The cation to be substituted should occupy a single crystalline site (i.e., all sites should be equivalent);
- A cationic site with high symmetry to minimize the crystal field’s splitting;
- Minimum magnetic moment; hence, elements with null or low magnetic spin are preferable;
- Isotropic linear coefficient of thermal expansion (CTE) to avoid excessive deformation during cooling;
- The lowest possible presence of defects; hence, its high purity that is both chemical and isotopic, and the best crystalline quality are obtained;
- Synthesis methods compatible with standard device fabrication techniques must be considered a plus.
- S1: 166Er:7LiYF4 with Er concentration of 100 ppm (molar fraction);
- S2: Er:LiYF4 with Er concentration of 100 ppm (molar fraction);
- S3: Er:LiYF4 with Er concentration of 35 ppm (molar fraction).
2.2. Material Realization and Experimental Setup
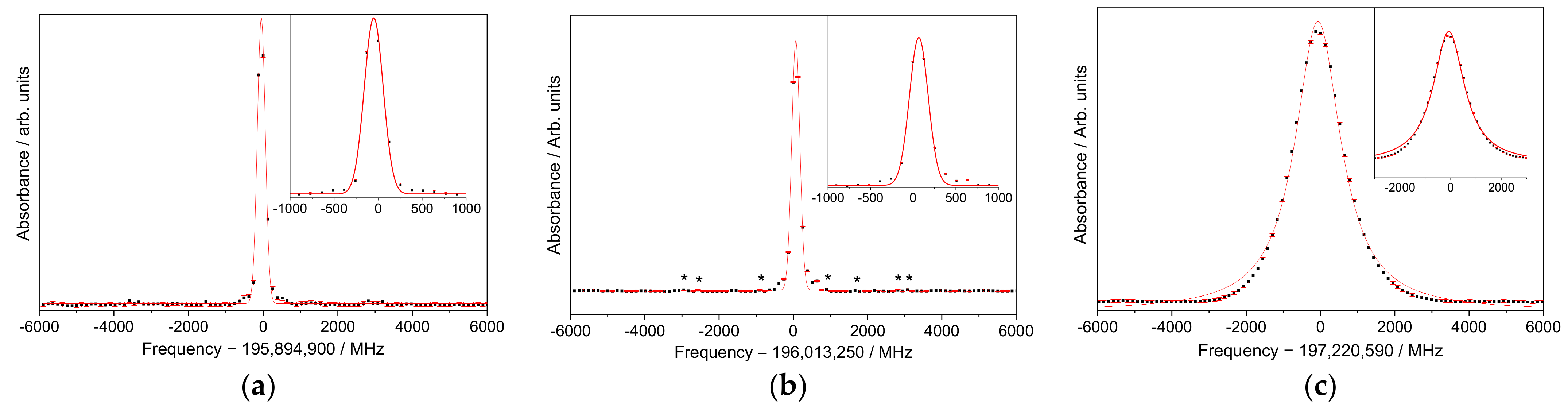
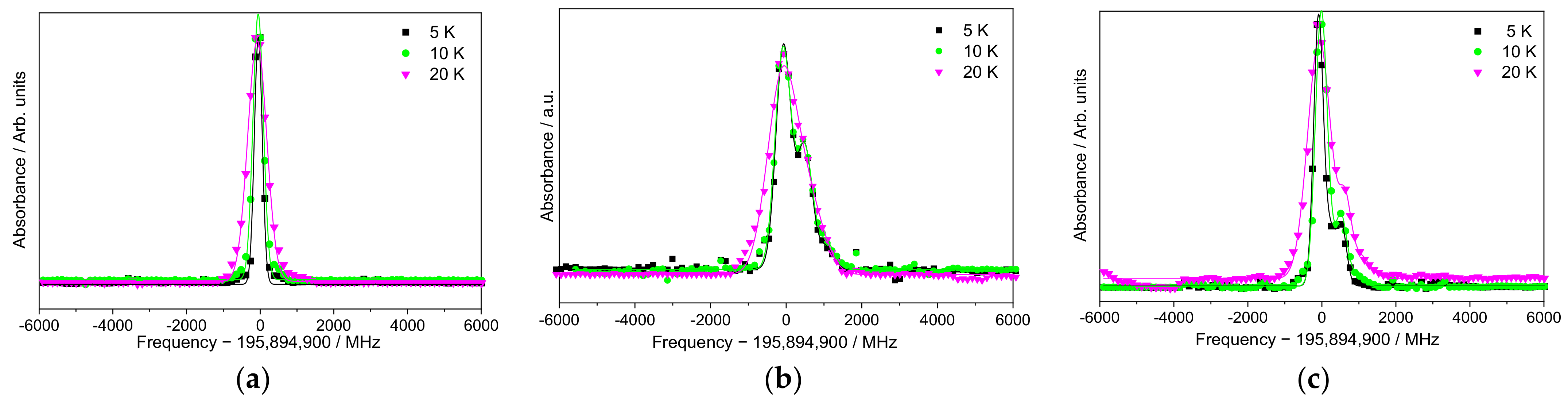
3. Results
High-Resolution Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pizzocaro, M.; Bregolin, F.; Barbieri, P.; Rauf, B.; Levi, F.; Calonico, D. Absolute Frequency Measurement of the 1S0-3P0 Transition of 171Yb with a Link to International Atomic Time. Metrologia 2020, 57, 035007. [Google Scholar] [CrossRef]
- Beloy, K.; Bodine, M.I.; Bothwell, T.; Brewer, S.M.; Bromley, S.L.; Chen, J.-S.; Deschênes, J.-D.; Diddams, S.A.; Fasano, R.J.; Fortier, T.M.; et al. Frequency Ratio Measurements at 18-Digit Accuracy Using an Optical Clock Network. Nature 2021, 591, 564–569. [Google Scholar] [CrossRef]
- Ushijima, I.; Takamoto, M.; Das, M.; Ohkubo, T.; Katori, H. Cryogenic Optical Lattice Clocks. Nat. Photonics 2015, 9, 185–189. [Google Scholar] [CrossRef]
- Brewer, S.M.; Chen, J.-S.; Hankin, A.M.; Clements, E.R.; Chou, C.W.; Wineland, D.J.; Hume, D.B.; Leibrandt, D.R. 27Al+ Quantum-Logic Clock with a Systematic Uncertainty below 10−18. Phys. Rev. Lett. 2019, 123, 33201. [Google Scholar] [CrossRef]
- Origlia, S.; Pramod, M.S.; Schiller, S.; Singh, Y.; Bongs, K.; Schwarz, R.; Al-Masoudi, A.; Dörscher, S.; Herbers, S.; Häfner, S.; et al. Towards an Optical Clock for Space: Compact, High-Performance Optical Lattice Clock Based on Bosonic Atoms. Phys. Rev. A 2018, 98, 53443. [Google Scholar] [CrossRef]
- Koller, S.B.; Grotti, J.; Vogt, S.; Al-Masoudi, A.; Dörscher, S.; Häfner, S.; Sterr, U.; Lisdat, C. Transportable Optical Lattice Clock with 7 × 1017 Uncertainty. Phys. Rev. Lett. 2017, 118, 73601. [Google Scholar] [CrossRef]
- Takamoto, M.; Ushijima, I.; Ohmae, N.; Yahagi, T.; Kokado, K.; Shinkai, H.; Katori, H. Test of General Relativity by a Pair of Transportable Optical Lattice Clocks. Nat. Photonics 2020, 14, 411–415. [Google Scholar] [CrossRef]
- Marlow, B.L.S.; Scherer, D.R. A Review of Commercial and Emerging Atomic Frequency Standards. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2007–2022. [Google Scholar] [CrossRef]
- Volpi, A.; Cittadino, G.; Di Lieto, A.; Tonelli, M. Anti-Stokes Cooling of Yb-Doped KYF4 Single Crystals. J. Lumin. 2018, 203, 670–675. [Google Scholar] [CrossRef]
- Cittadino, G.; Damiano, E.; Di Lieto, A.; Tonelli, M. First Demonstration of Optical Refrigeration Efficiency Greater than 4% at Room Temperature. Opt. Express 2020, 28, 14476–14489. [Google Scholar] [CrossRef]
- Chiossi, F.; Vasiukov, S.; Borghesani, A.F.; Braggio, C.; Di Lieto, A.; Tonelli, M.; Carugno, G. High Infrared Light Yield of Erbium-Doped Fluoride Crystals. J. Lumin. 2020, 219, 116883. [Google Scholar] [CrossRef]
- Tanner, P.A. Some Misconceptions Concerning the Electronic Spectra of Tri-Positive Europium and Cerium. Chem. Soc. Rev. 2013, 42, 5090–5101. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.W.; Bottger, T.; Cone, R.L. Rare-Earth-Doped Materials for Applications in Quantum Information Storage and Signal Processing. J. Lumin. 2011, 131, 353–361. [Google Scholar] [CrossRef]
- Welinski, S.; Thiel, C.W.; Dajczgewand, J.; Ferrier, A.; Cone, R.L.; Macfarlane, R.M.; Chanelière, T.; Louchet-Chauvet, A.; Goldner, P. Effects of Disorder on Optical and Electron Spin Linewidths in Er3+, Sc3+:Y2SiO5. Opt. Mater. 2017, 63, 69–75. [Google Scholar] [CrossRef]
- Macfarlane, R.M. High-Resolution Laser Spectroscopy of Rare-Earth Doped Insulators: A Personal Perspective. J. Lumin. 2002, 100, 1–20. [Google Scholar] [CrossRef]
- Chukalina, E.P.; Popova, M.N.; Korableva, S.L.; Abdulsabirov, R.Y. Fine Structure of Spectral Lines in LiYF4:Er3+ Due to Isotopic Disorder in the Lattice. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2000, 269, 348–350. [Google Scholar] [CrossRef]
- Kukharchyk, N.; Sholokhov, D.; Morozov, O.; Korableva, S.L.; Kalachev, A.A.; Bushev, P.A. Optical Coherence of 166Er:7LiYF4 Crystal below 1 Kelvin. New J. Phys. 2018, 20, 023044. [Google Scholar] [CrossRef]
- Kukharchyk, N.; Sholokhov, D.; Morozov, O.; Korableva, S.L.; Cole, J.H.; Kalachev, A.A.; Bushev, P.A. Optical Vector Network Analysis of Ultra-Narrow Transitions in 166Er3+:7LiYF4. Opt. Lett. 2018, 43, 935–938. [Google Scholar] [CrossRef]
- Phenicie, C.M.; Stevenson, P.; Welinski, S.; Rose, B.C.; Asfaw, A.T.; Cava, R.J.; Lyon, S.A.; De Leon, N.P.; Thompson, J.D. Narrow Optical Line Widths in Erbium Implanted in TiO2. ACS Nano Lett. 2019, 19, 8928–8933. [Google Scholar] [CrossRef]
- Thorpe, M.J.; Rippe, L.; Fortier, T.M.; Kirchner, M.S.; Rosenband, T. Frequency Stabilization to 6 × 10−16 via Spectral-Hole Burning. Nat. Photonics 2011, 5, 688–693. [Google Scholar] [CrossRef]
- Leibrandt, D.R.; Thorpe, M.J.; Chou, C.W.; Fortier, T.M.; Diddams, S.A.; Rosenband, T. Absolute and Relative Stability of an Optical Frequency Reference Based on Spectral Hole Burning in Eu3+:Y2SiO5. Phys. Rev. Lett. 2013, 111, 237402. [Google Scholar] [CrossRef]
- Galland, N.; Lucic, N.; Zhang, S.; Alvarez-Martinez, H.; Le Targat, R.; Ferrier, A.; Goldner, P.; Fang, B.; Seidelin, S.; Le Coq, Y. Double-Heterodyne Probing for an Ultra-Stable Laser Based on Spectral Hole Burning in a. Opt. Lett. 2020, 45, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Eliseeva, S.V. Basics of Lanthanide Photophysics. In Lanthanide Luminescence: Photophysical, Analytical and Biological Aspects; Hänninen, P., Härmä, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–45. ISBN 978-3-642-21023-5. [Google Scholar]
- Jia, Y.Q. Crystal Radii and Effective Ionic Radii of the Rare Earth Ions. J. Solid State Chem. 1991, 95, 184–187. [Google Scholar] [CrossRef]
- Singh, M.K.; Prakash, A.; Wolfowicz, G.; Wen, J.; Huang, Y.; Rajh, T.; Awschalom, D.D.; Zhong, T.; Guha, S. Epitaxial Er-Doped Y2O3 on Silicon for Quantum Coherent Devices. APL Mater. 2020, 8, 031111. [Google Scholar] [CrossRef]
- Böttger, T.; Pryde, G.J.; Thiel, C.W.; Cone, R.L. Laser Frequency Stabilization at 1.5 Microns Using Ultranarrow Inhomogeneous Absorption Profiles in Er3+:LiYF4. J. Lumin. 2007, 127, 83–88. [Google Scholar] [CrossRef]
- Burkhalter, R.; Dohnke, I.; Hulliger, J. Growing of Bulk Crystals and Structuring Waveguides of Fluoride Materials for Laser Applications. Prog. Cryst. Growth Charact. Mater. 2001, 42, 1–64. [Google Scholar] [CrossRef]
- Macfarlane, R.M.; Arcangeli, A.; Ferrier, A.; Goldner, P. Optical Measurement of the Effect of Electric Fields on the Nuclear Spin Coherence of Rare-Earth Ions in Solids. Phys. Rev. Lett. 2014, 113, 2–6. [Google Scholar] [CrossRef]
- Zhang, S.; Lučić, N.; Galland, N.; Le Targat, R.; Goldner, P.; Fang, B.; Seidelin, S.; Le Coq, Y. Precision Measurements of Electric-Field-Induced Frequency Displacements of an Ultranarrow Optical Transition in Ions in a Solid. Appl. Phys. Lett. 2020, 117, 221102. [Google Scholar] [CrossRef]
- Yang, H.S.; Hong, K.S.; Feofilov, S.P.; Tissue, B.M.; Meltzer, R.S.; Dennis, W.M. Electron-Phonon Interaction in Rare Earth Doped Nanocrystals. J. Lumin. 1999, 83–84, 139–145. [Google Scholar] [CrossRef]
- Bartholomew, J.G.; De Oliveira Lima, K.; Ferrier, A.; Goldner, P. Optical Line Width Broadening Mechanisms at the 10 KHz Level in Eu3+:Y2O3 Nanoparticles. Nano Lett. 2017, 17, 778–787. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Christensen, H.P. Spectroscopic Analysis of LiHoF4 and LiErF4. Phys. Rev. B 1979, 19, 6564. [Google Scholar] [CrossRef]
- Brown, M.R.; Roots, K.G.; Shand, W.A. Energy Levels of Er3+ in LiYF4. J. Phys. C Solid State Phys. 1969, 2, 593. [Google Scholar] [CrossRef]
- Heyde, K.; Binnemans, K.; Gorller-Walrand, C. Spectroscopic Properties of LiErF4. J. Chem. Soc. Faraday Trans. 1998, 94, 843–849. [Google Scholar] [CrossRef]
- Chukalina, E.P.; Popova, M.N. Hyperfine Structure of Infrared Transitions in LiYF4:Er3+. Phys. Lett. A 1999, 262, 191–194. [Google Scholar] [CrossRef]
- Macfarlane, R.M.; Cassanho, A.; Meltzer, R.S. Inhomogeneous Broadening by Nuclear Spin Fields: A New Limit for Optical Transitions in Solids. Phys. Rev. Lett. 1992, 69, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Beha, K.; Cole, D.C.; Yi, X.; Del’Haye, P.; Lee, H.; Li, J.; Oh, D.Y.; Diddams, S.A.; Papp, S.B.; et al. Broadband Dispersion-Engineered Microresonator on a Chip. Nat. Photonics 2016, 10, 316–320. [Google Scholar] [CrossRef]
- Briles, T.C.; Stone, J.R.; Drake, T.E.; Spencer, D.T.; Fredrick, C.; Li, Q.; Westly, D.; Ilic, B.R.; Srinivasan, K.; Diddams, S.A.; et al. Interlocking Kerr-Microresonator Frequency Combs for Microwave to Optical Synthesis. Opt. Lett. 2018, 43, 2933. [Google Scholar] [CrossRef]
- Papp, S.B.; Beha, K.; Del’Haye, P.; Quinlan, F.; Lee, H.; Vahala, K.J.; Diddams, S.A. Microresonator Frequency Comb Optical Clock. Optica 2014, 1, 10. [Google Scholar] [CrossRef]


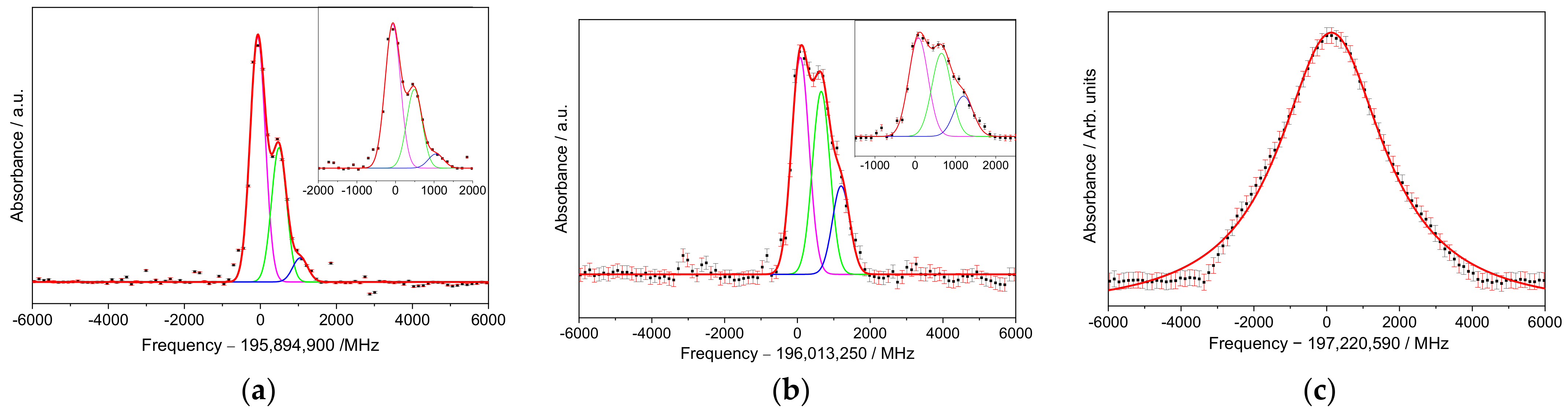
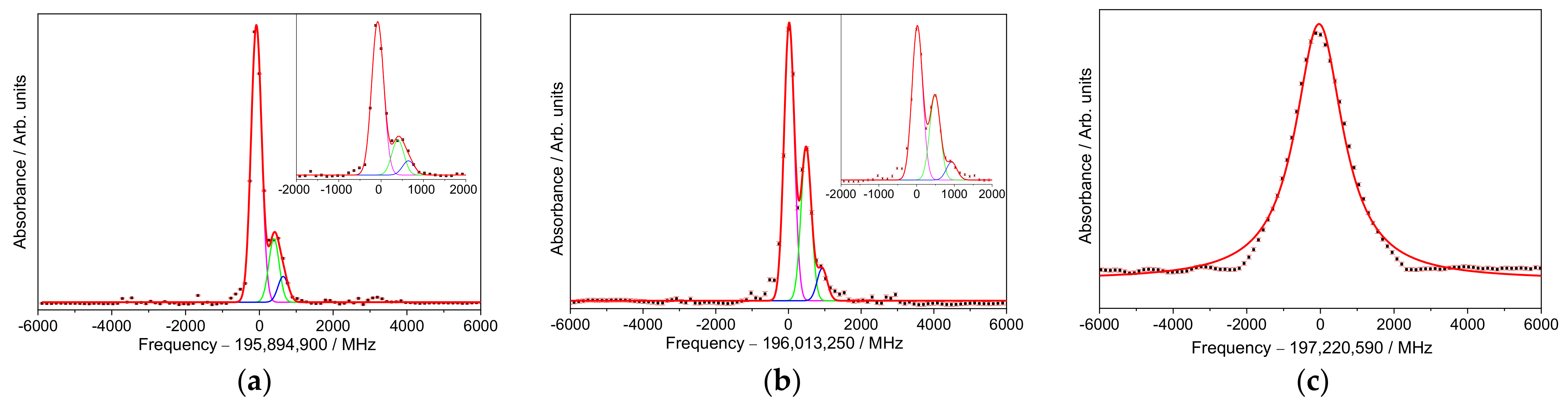
| Transition | Sample | Peak Centers 1,2,3/MHz | FWHM */MHz | R2,† | ||
|---|---|---|---|---|---|---|
| Peak 1 | Peak 2 | Peak 3 | ||||
| T1 1 | S1 | −50.3 ± 1.5 | / | / | 252 ± 4 | 0.99 |
| S2 | −71 ± 4 | 467 ± 8 | 1050 ± 40 | 397 ± 7 | 0.99 | |
| S3 | −80 ± 2 | 427 ± 9 | 760 ± 60 | 333 ± 3 | 0.99 | |
| T2 2 | S1 | 66 ± 1 | / | / | 258 ± 3 | 0.99 |
| S2 | 78 ± 9 | 653 ± 15 | 1200 ± 20 | 480 ± 13 | 0.99 | |
| S3 | 19 ± 3 | 487 ± 6 | 927 ± 25 | 330 ± 5 | 0.99 | |
| T3 3 | S1 | −68 ± 6 | / | / | 1486 ± 23 | 0.99 |
| S2 | 128 ± 16 | / | / | 3490 ± 70 | 0.99 | |
| S3 | −37 ± 10 | / | / | 1571 ± 34 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrato, E.; Gionco, C.; Rizzelli Martella, G.; Clivati, C.; Gaudino, R.; Calonico, D. Erbium-Doped LiYF4 as a Potential Solid-State Frequency Reference: Eligibility and Spectroscopic Assessment. Crystals 2023, 13, 1476. https://doi.org/10.3390/cryst13101476
Cerrato E, Gionco C, Rizzelli Martella G, Clivati C, Gaudino R, Calonico D. Erbium-Doped LiYF4 as a Potential Solid-State Frequency Reference: Eligibility and Spectroscopic Assessment. Crystals. 2023; 13(10):1476. https://doi.org/10.3390/cryst13101476
Chicago/Turabian StyleCerrato, Erik, Chiara Gionco, Giuseppe Rizzelli Martella, Cecilia Clivati, Roberto Gaudino, and Davide Calonico. 2023. "Erbium-Doped LiYF4 as a Potential Solid-State Frequency Reference: Eligibility and Spectroscopic Assessment" Crystals 13, no. 10: 1476. https://doi.org/10.3390/cryst13101476
APA StyleCerrato, E., Gionco, C., Rizzelli Martella, G., Clivati, C., Gaudino, R., & Calonico, D. (2023). Erbium-Doped LiYF4 as a Potential Solid-State Frequency Reference: Eligibility and Spectroscopic Assessment. Crystals, 13(10), 1476. https://doi.org/10.3390/cryst13101476








