Crystal Growth of RuS2 Using a Chemical Vapor Transport Technique and Its Properties
Abstract
:1. Introduction
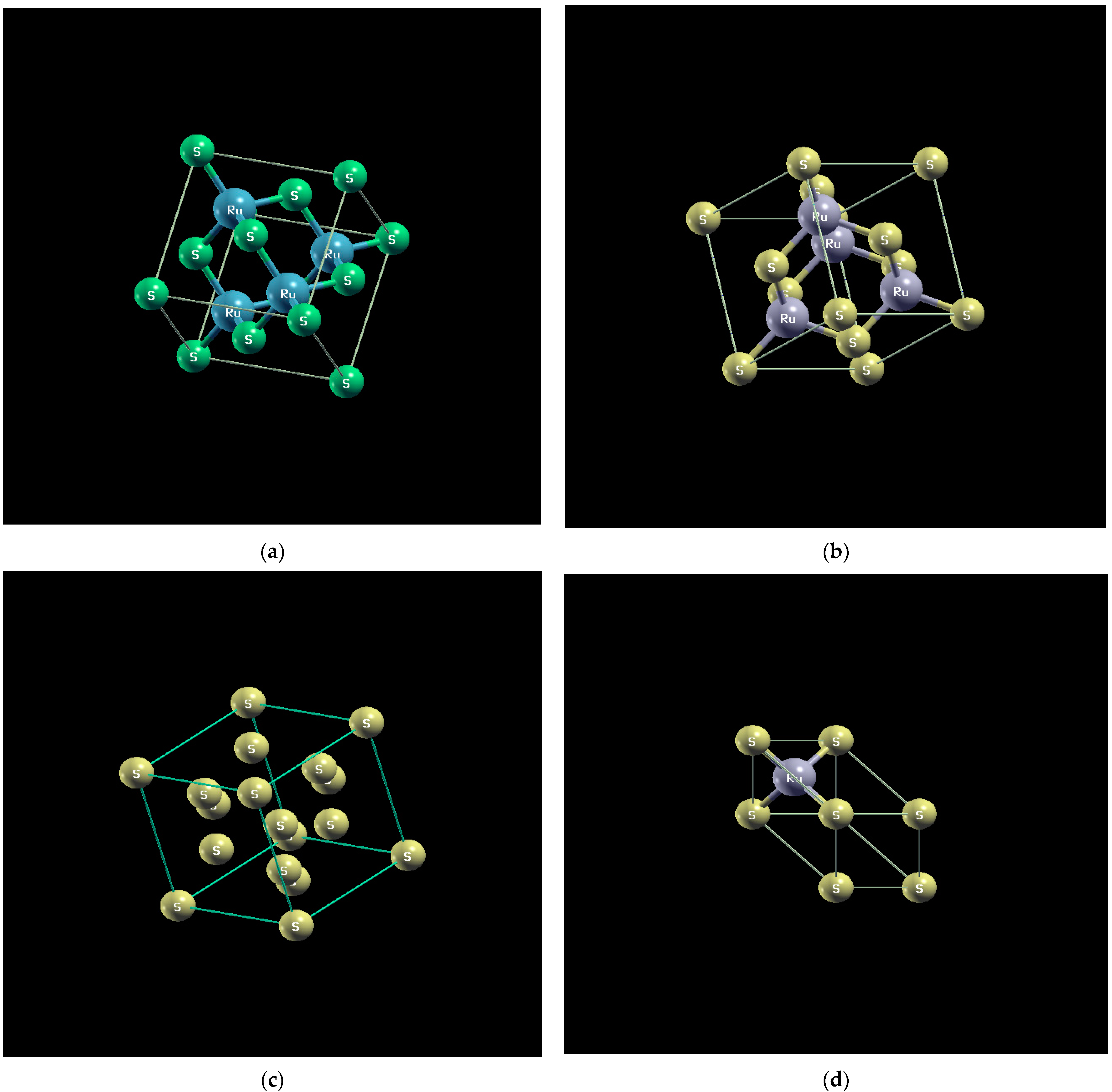
 is one of the interesting sulfur compounds from both fundamental and technological points of view. It is one of the semiconducting transition-metal dichalcogenide (TMDC) materials, with a reported band gap of 1.8 eV [2] and has a pyrite structure [3]. Ruthenium Sulfide, has several possible uses, including its use as a catalyst [4] and as a photoelectrode [5,6,7,8]. However, it is difficult to obtain the crystalline due to several facts, for instance we can obtain only at temperatures greater than 1000 °C. Therefore, obtaining its crystalline structure at low temperatures is practically impossible. Moreover, the physical vapor transport method is difficult to use because the vapor pressure of is very low, at temperatures between 800 and 1050 .
is one of the interesting sulfur compounds from both fundamental and technological points of view. It is one of the semiconducting transition-metal dichalcogenide (TMDC) materials, with a reported band gap of 1.8 eV [2] and has a pyrite structure [3]. Ruthenium Sulfide, has several possible uses, including its use as a catalyst [4] and as a photoelectrode [5,6,7,8]. However, it is difficult to obtain the crystalline due to several facts, for instance we can obtain only at temperatures greater than 1000 °C. Therefore, obtaining its crystalline structure at low temperatures is practically impossible. Moreover, the physical vapor transport method is difficult to use because the vapor pressure of is very low, at temperatures between 800 and 1050 .2. Experimental Section
3. Analysis
3.1. Analysis by Microprobe
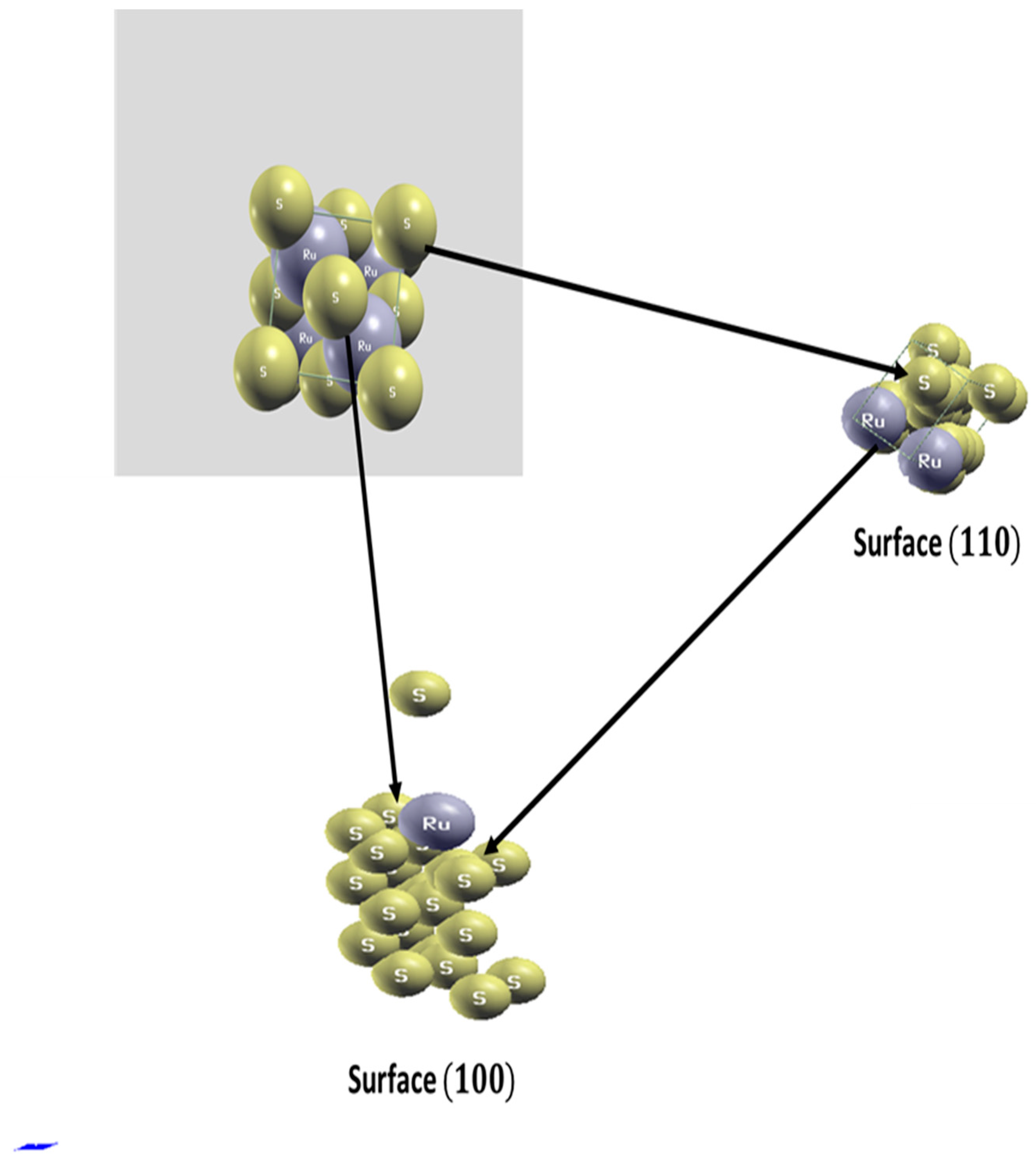
3.2. Analysis by X-ray
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topsoe, H.; Clausen, B.S.; Massoth, F.E. Hydrotreating Catalysis-Science and Technology. Springer 1996, 14, 1465. [Google Scholar] [CrossRef]
- Hulliger, F. Crystal Structure and Electrical Properties of Some Cobalt-Group Chalcogenides. Nature 1964, 204, 644–646. [Google Scholar] [CrossRef]
- Knop, O.; Reid, K.I.G.; Sutarno; Nakagawa, Y. Chalkogenides of the transition elements. VI. X-ray, neutron, and magnetic investigation of the spinels Co3O4, NiCo2O4, Co3S4, and NiCo2S4. Can. J. Chem. 1968, 46, 22. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.; Chianelli, R. Catalysis by transition metal sulfides: The relation between calculated electronic trends and HDS activity. J. Catal. 1984, 86, 400–412. [Google Scholar] [CrossRef]
- Ezzouia, H.; Heindl, R.; Parsons, R.; Tributsch, H. Visible light photo-oxidation of water with single-crystal RuS2 electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1983, 145, 279–292. [Google Scholar] [CrossRef]
- Heindl, R.; Parsons, R.; Redon, A.; Tributsch, H.; Vigneron, J. Photoelectrochemical behaviour of ruthenium disulphide electrodes in contact with aqueous electrolytes. Surf. Sci. 1982, 115, 91–103. [Google Scholar] [CrossRef]
- Ezzaouia, H.; Heindl, R.; Loriers, J. Synthesis of ruthenium and osmium dichalcogenide single crystals. J. Mater. Sci. Lett. 1984, 3, 625–626. [Google Scholar] [CrossRef]
- Ezzaouia, H.; Foise, J.W.; Gorochov, O. Crystal growth in tellurium fluxes and characterization of RuS2 single crystals. Mater. Res. Bull. 1985, 20, 1353–1358. [Google Scholar] [CrossRef]
- Fiechter, S.; Kuhne, H.-M. Crystal growth of RuX2 (X = 5, Se, Te) by chemical vapour transport and high temperature solution growth. J. Cryst. Growth 1987, 83, 517–522. [Google Scholar] [CrossRef]
- Hulliger, F. Electrical Properties of Pyrite-Type and Related Compounds with Zero Spin Moment. Nature 1963, 200, 1064–1065. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Gunjakar, J.L.; Inamdar, A.I.; Kim, H.; Im, H. One-step facile route to copper cobalt sulfide electrodes for supercapacitors with high-rate long-cycle life performance. J. Alloy. Compd. 2017, 724, 744–751. [Google Scholar] [CrossRef]
- Sai, R.; Gorochov, O.; Ezzaouia, H. The study of the electronic structure of RuS2. Results Phys. 2021, 26, 104393. [Google Scholar] [CrossRef]
- Castillo-Villalón, P.; Ramírez, J.; Maugé, F. Structure, stability and activity of RuS2 supported on alumina. J. Catal. 2008, 260, 65–74. [Google Scholar] [CrossRef]
- Spirkoska, D.; Efros, A.L.; Lambrecht, W.R.L.; Cheiwchanchamnangij, T.; Fontcuberta i Morral, A.; Abstreiter, G. Valence band structure of polytypic zinc-blende/wurtzite GaAs nanowires probed by polarization-dependent photoluminescence. Phys. Rev. B 2012, 85, 045309. [Google Scholar] [CrossRef] [Green Version]
- Cheiwchanchamnangij, T.; Lambrecht, W. Band structure parameters of wurtzite and zinc-blende GaAs under strain in the GW approximation. Phys. Rev. B 2011, 84, 035203. [Google Scholar] [CrossRef]
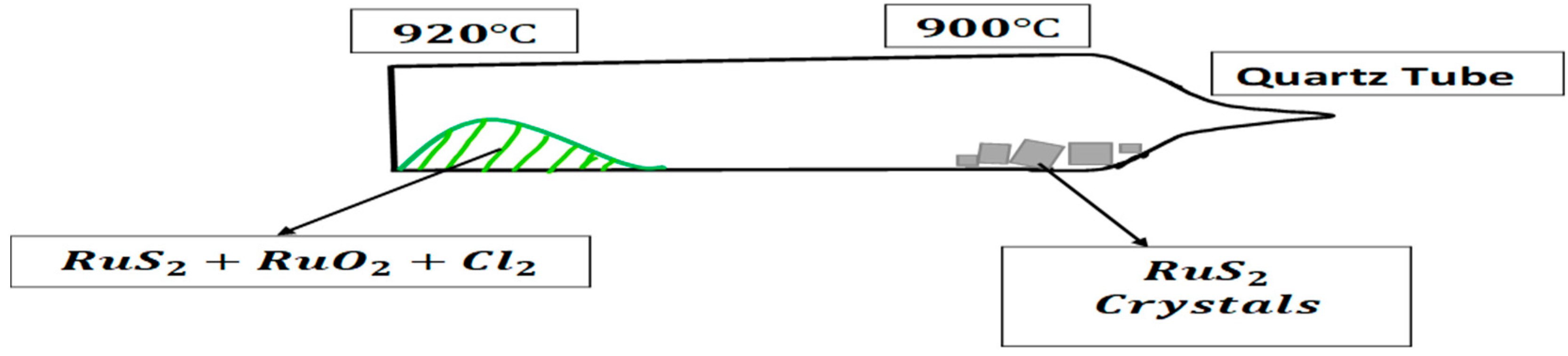
| Sample | Temperature | (%) of extra Sulfure in RuS2 | Color of Monocristal | |
|---|---|---|---|---|
| White and dull | ||||
| Dull gray | ||||
| Dull gray | ||||
| Dull gray | ||||
| Dull gray | ||||
| Light gray | ||||
| Shiny gray | ||||
| Shiny gray | ||||
| Very Shiny gray |
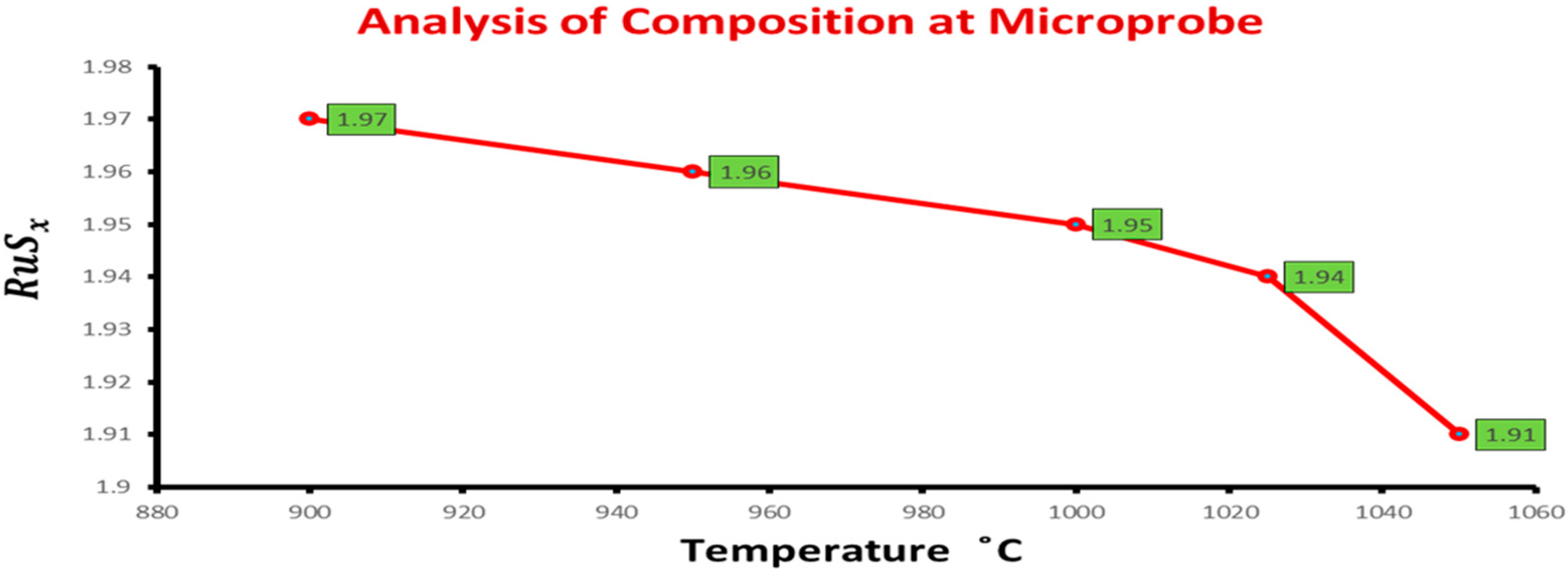
| Sample | Temperature | Excess of S in for RuS2 | Analysis of Composition at Microprobe | Amount of Precipitate O2 |
|---|---|---|---|---|
| CS1 | 1050 °C | 1 | RuS1.90 | 0.005 |
| CS2 | 1050 °C | 2 | RuS1.92 | 0.005 |
| CS4 | 1025 °C | 2 | RuS1.94 | - |
| CS5 | 1000 °C | 1 | RuS1.95 | - |
| CS7 | 950 °C | 1 | RuS1.96 | - |
| CS9 | 900 °C | 1 | RuS1.97 | - |
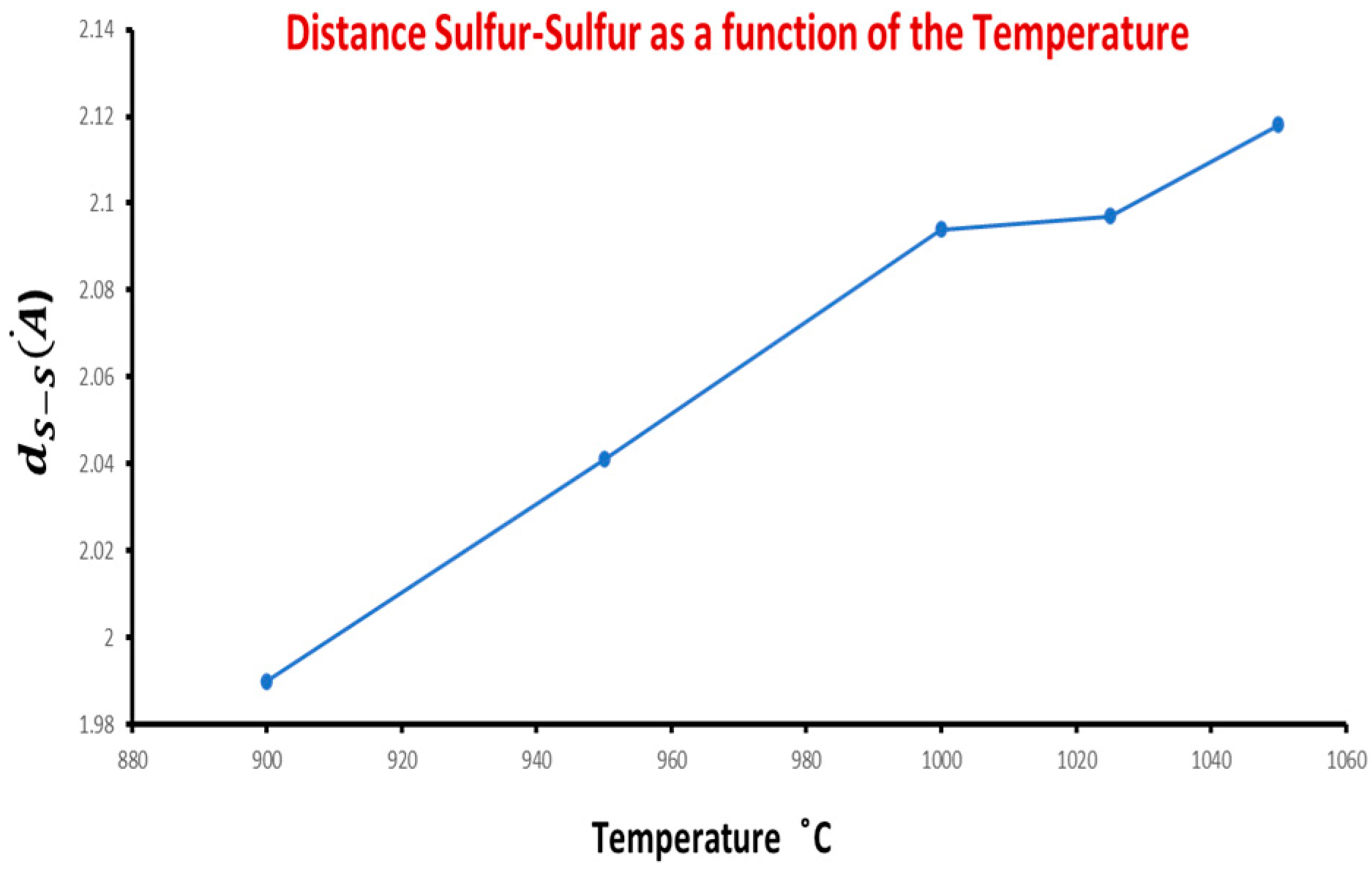
| Sample | Temperature | S2 (%) | ||||
|---|---|---|---|---|---|---|
| CS1 | 1050 °C | 1 | 5635 | 0.1085 | 2.118 | 2.369 |
| CS3 | 1025 °C | 1 | 5630 | 0.1072 | 2.097 | 2.370 |
| CS5 | 1000 °C | 1 | 5624 | 0.1075 | 2.094 | 2.367 |
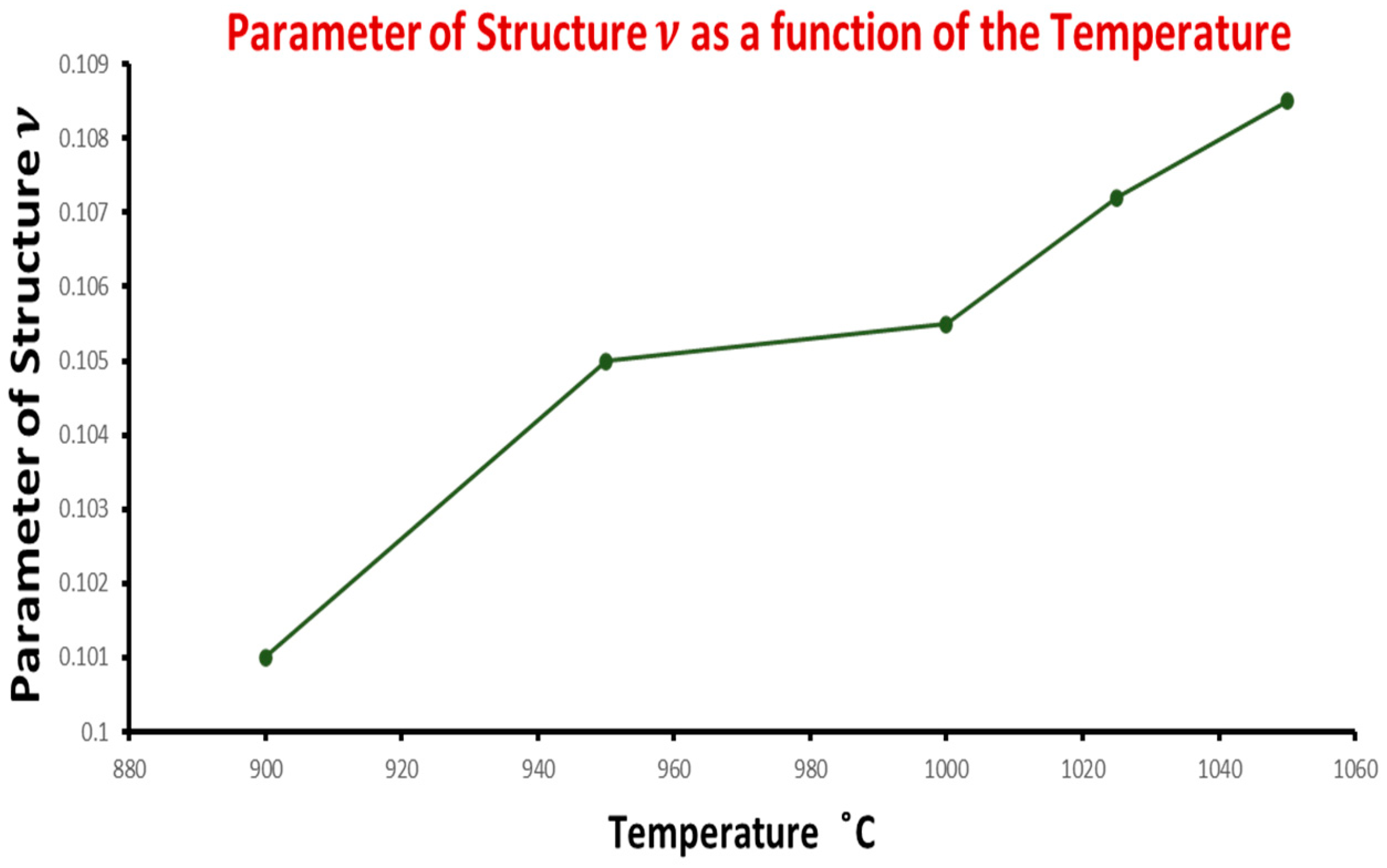
| CS6 | 1000 °C | 2 | 5617 | 0.1055 | 2.052 | 2.369 |
| CS7 | 950 °C | 1 | 5611 | 0.105 | 2.041 | 2.368 |
| CS9 | 900 °C | 1 | 5.609 | 0.101 | 1.990 | 2.373 |
| Sample | Temperature | (%)S | a in Å | Concentration of RuSx | Eg Experimental (eV) |
|---|---|---|---|---|---|
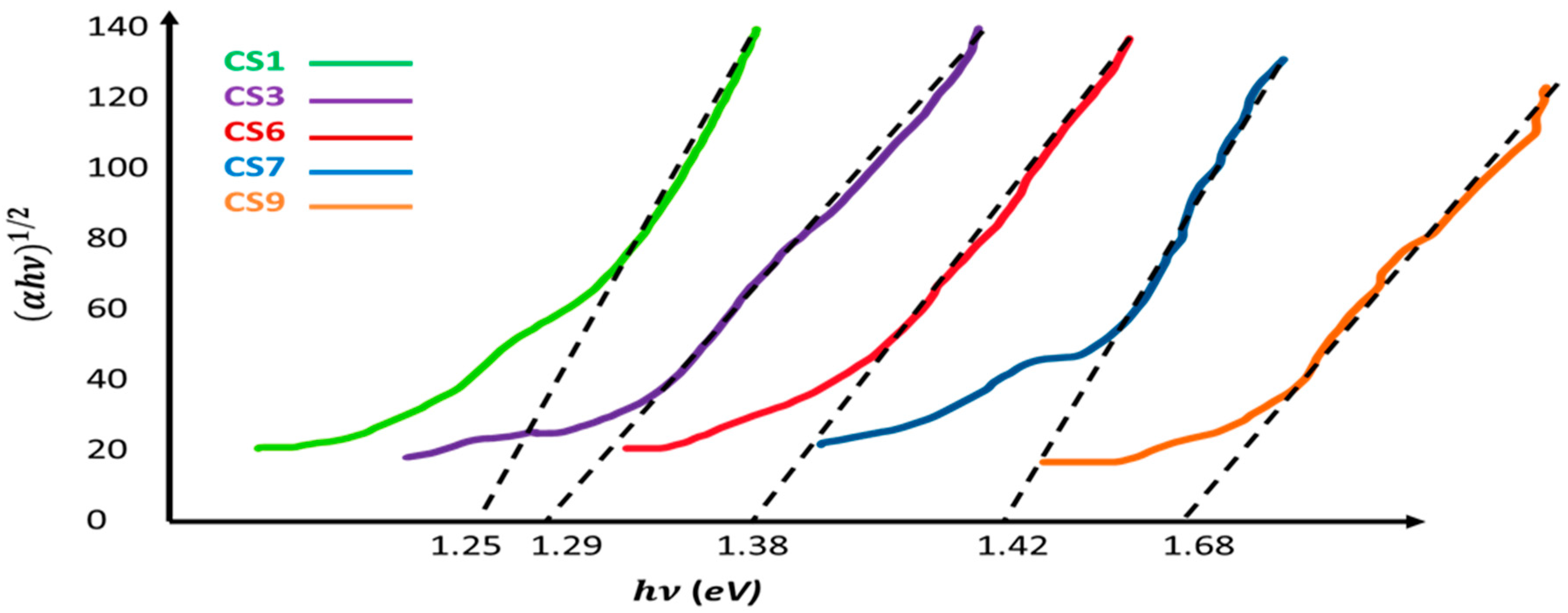
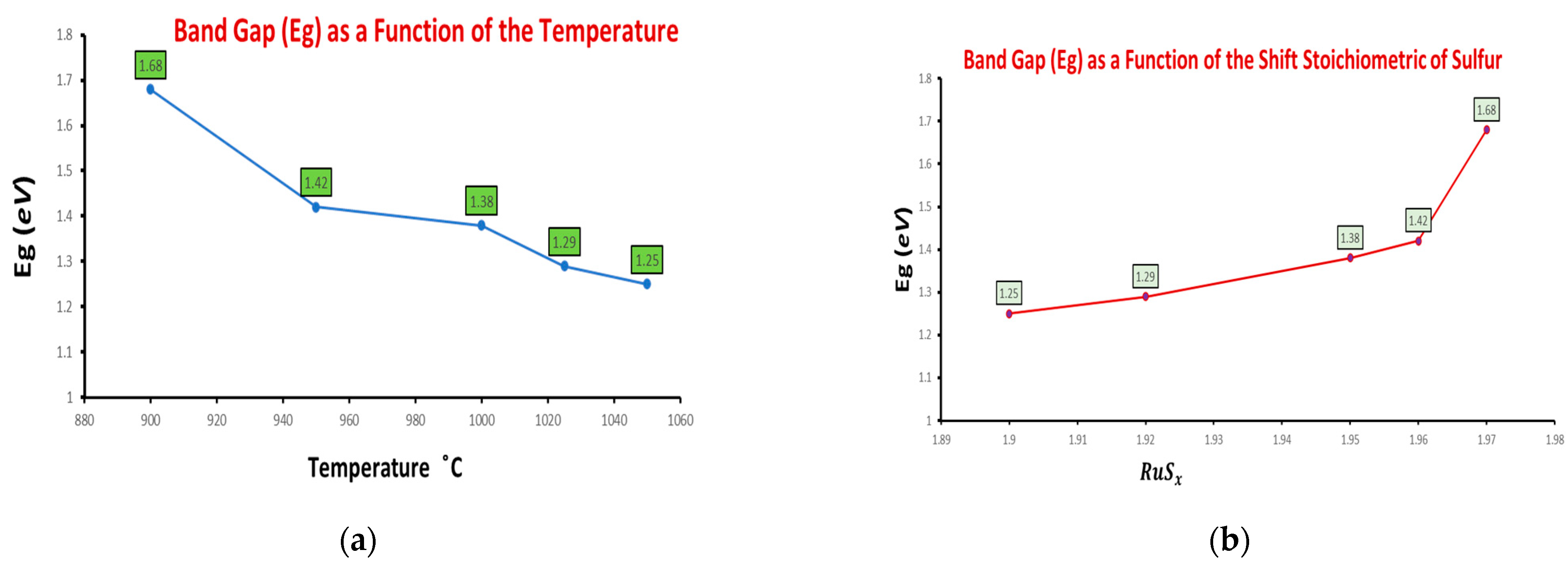
| CS1 | 1050 °C | 1 | 5.635 | RuS1.90 | 1.25 |
| CS3 | 1025 °C | 1 | 5.630 | RuS1.92 | 1.29 |
| CS5 | 1000 °C | 1 | 5.624 | RuS1.95 | 1.36 |
| CS6 | 1000 °C | 2 | 5.617 | RuS1.96 | 1.38 |
| CS7 | 950 °C | 1 | 5.611 | RuS1.96 | 1.42 |
| CS9 | 900 °C | 1 | 5.609 | RuS1.97 | 1.68 |
| Samples | Eg (eV) | ||
|---|---|---|---|
| CS1 | 2.118 | 1.25 | 1050 |
| CS3 | 2.097 | 1.29 | 1025 |
| CS5 | 2.094 | 1.36 | 1000 |
| CS7 | 2.041 | 1.42 | 950 |
| CS9 | 1.990 | 1.68 | 900 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sai, R.; Gorochov, O.; Alghamdi, E.A.; Ezzaouia, H. Crystal Growth of RuS2 Using a Chemical Vapor Transport Technique and Its Properties. Crystals 2022, 12, 994. https://doi.org/10.3390/cryst12070994
Sai R, Gorochov O, Alghamdi EA, Ezzaouia H. Crystal Growth of RuS2 Using a Chemical Vapor Transport Technique and Its Properties. Crystals. 2022; 12(7):994. https://doi.org/10.3390/cryst12070994
Chicago/Turabian StyleSai, Refka, Ouri Gorochov, Eman A. Alghamdi, and Hatem Ezzaouia. 2022. "Crystal Growth of RuS2 Using a Chemical Vapor Transport Technique and Its Properties" Crystals 12, no. 7: 994. https://doi.org/10.3390/cryst12070994
APA StyleSai, R., Gorochov, O., Alghamdi, E. A., & Ezzaouia, H. (2022). Crystal Growth of RuS2 Using a Chemical Vapor Transport Technique and Its Properties. Crystals, 12(7), 994. https://doi.org/10.3390/cryst12070994






