Effect of Different Ca2+ and Zr4+ Contents on Microstructure and Electrical Properties of (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
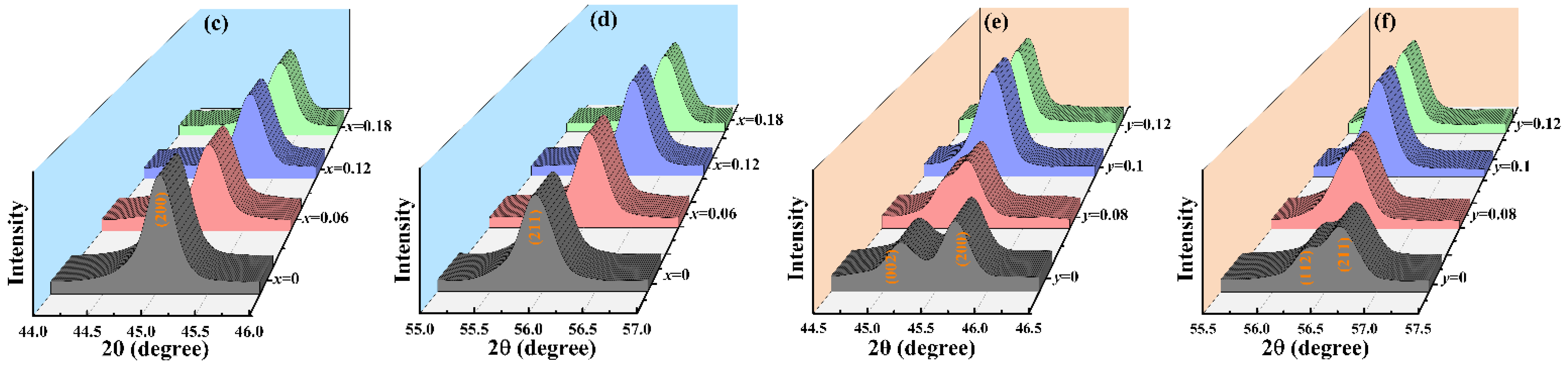
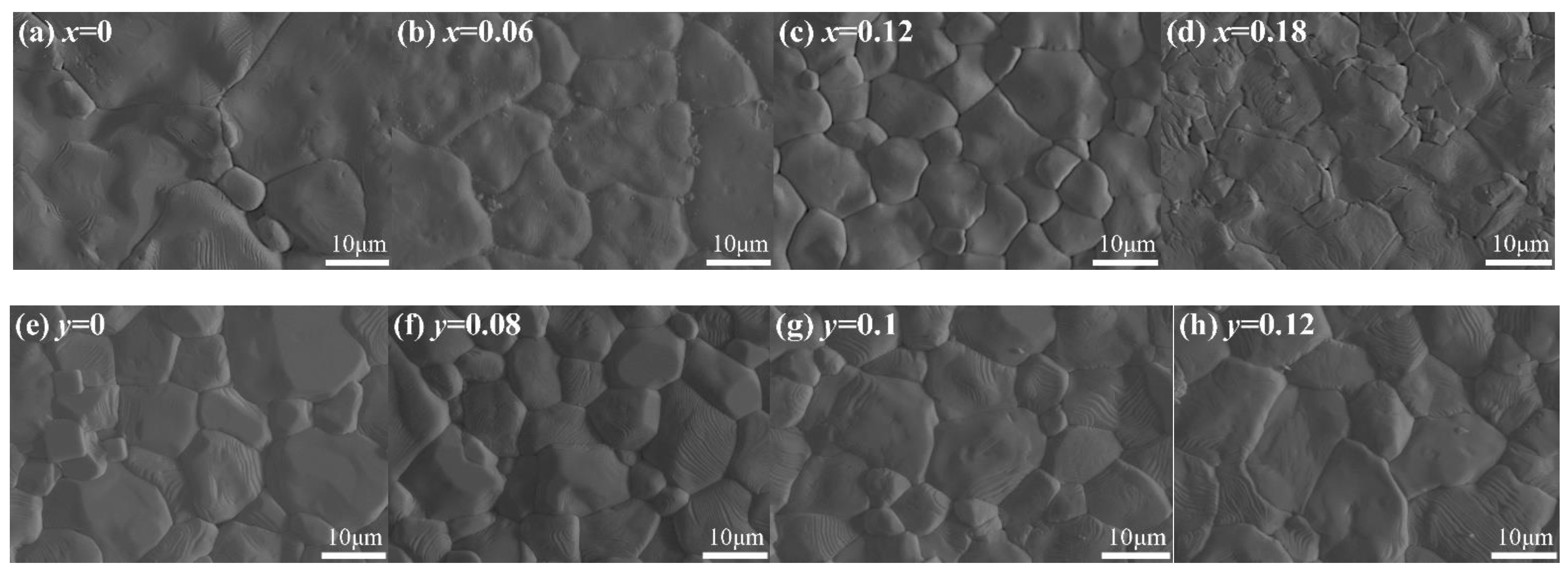
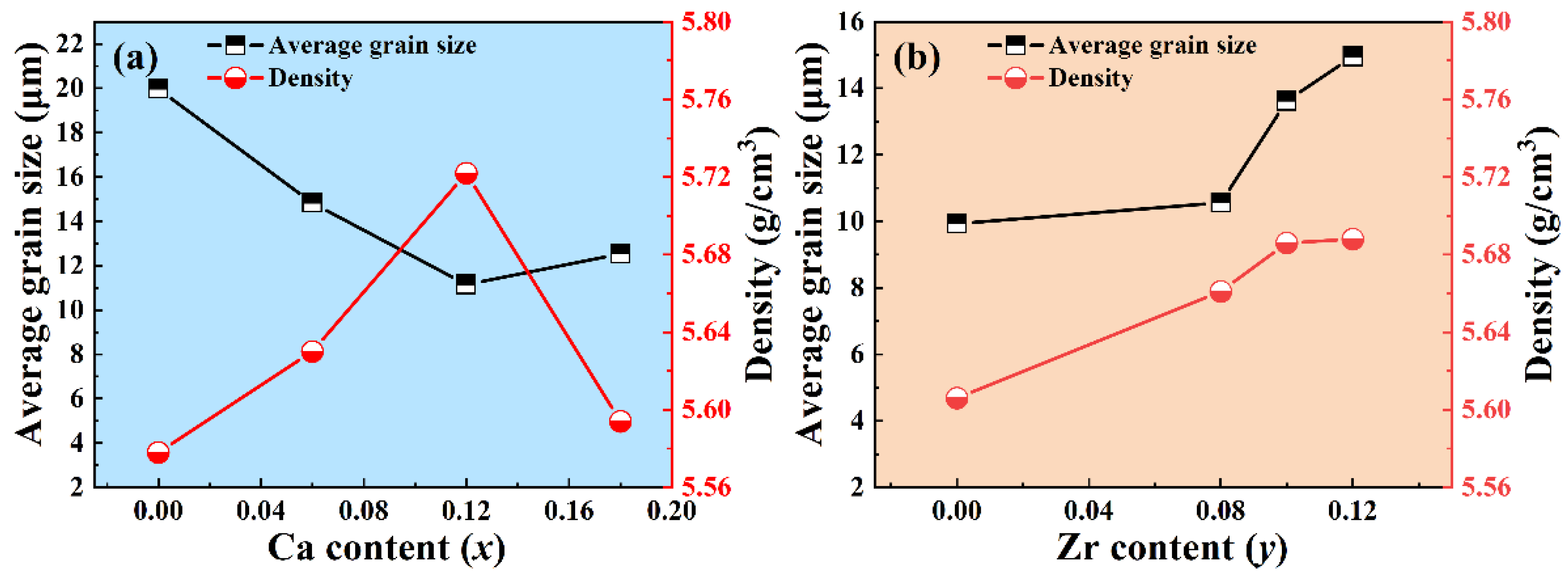
3.1. Crystal Phase and Structure
3.2. Dielectric Properties
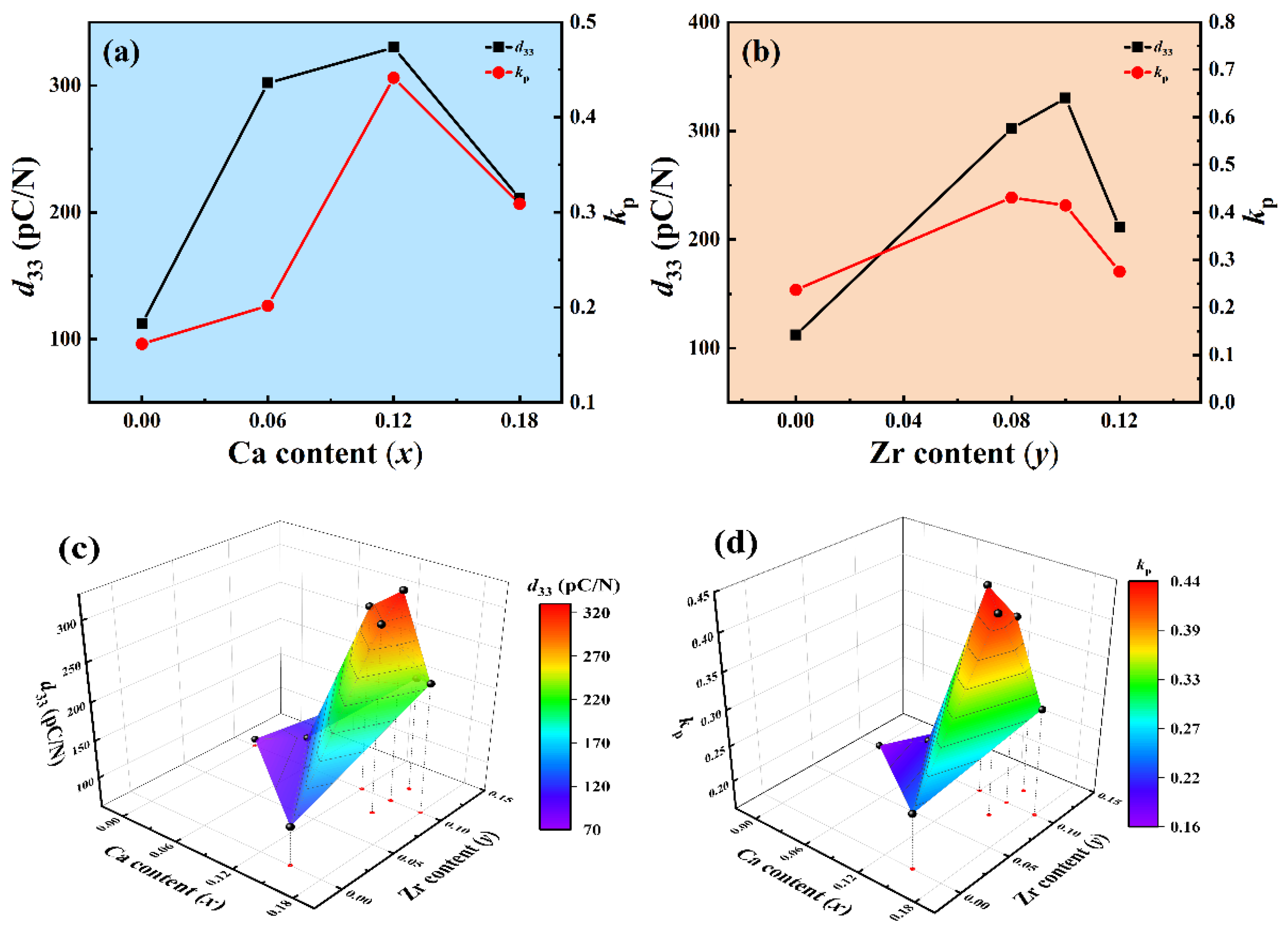
3.3. Piezoelectric Properties
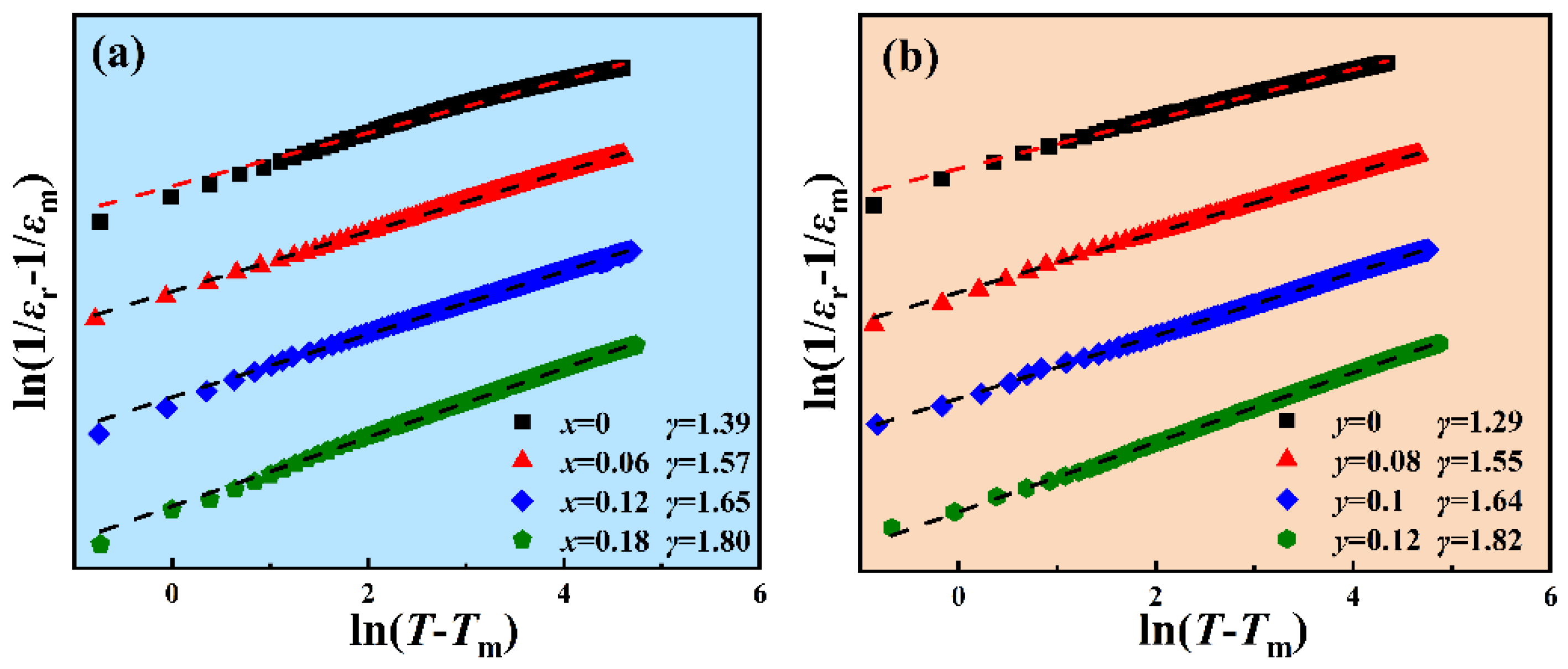
3.4. Ferroelectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.X.; Yang, Q.; Luo, M.D.; Liu, R.Y. Effect of the physical parameters of longitudinally polarized PZT tubes on PZT sensors. J. Phys. D Appl. Phys. 2020, 53, 275501. [Google Scholar] [CrossRef]
- Sabri, M.F.M.; Ono, T.; Said, S.M.; Kawai, Y.; Esashi, M. Fabrication and Characterization of Microstacked PZT Actuator for MEMS Applications. J. Microelectromech. Syst. 2015, 24, 80–90. [Google Scholar] [CrossRef]
- Liu, W.F.; Cheng, L.; Li, S.T. Prospective of (BaCa)(ZrTi)O3 Lead-free Piezoelectric Ceramics. Crystals 2019, 9, 179. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Thong, H.C.; Zhu, Z.X.; Nie, J.K.; Liu, Y.X.; Xu, Z.; Soon, P.S.; Gong, W.; Wang, K. Hardening effect in lead-free piezoelectric ceramics. J. Mater. Res. 2021, 36, 996–1014. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, K.; Zhu, F.Y.; Cheng, L.Q.; Yao, F.Z. (K, Na) NbO3-Based Lead-Free Piezoceramics: Fundamental Aspects, Processing Technologies, and Remaining Challenges. J. Am. Ceram. Soc. 2013, 96, 3677–3696. [Google Scholar] [CrossRef]
- Shalini, K.; Giridharan, N.V. Structural, dielectric and magnetic properties of K0.5Na0.5NbO3 and K0.5 Na0.5Nb0.975Co0.025O3 lead free ceramics. Ferroelectrics 2017, 518, 52–58. [Google Scholar] [CrossRef]
- Dong, N.N.; Gao, X.Y.; Xia, F.Q.; Liu, H.X.; Hao, H.; Zhang, S.J. Dielectric and Piezoelectric Properties of Textured Lead-Free Na0.5Bi0.5TiO3-Based Ceramics. Crystals 2019, 9, 206. [Google Scholar] [CrossRef]
- Trelcat, J.F.; d’Astorg, S.; Courtois, C.; Champagne, P.; Rguiti, M.; Leriche, A. Influence of hydrothermal synthesis conditions on BNT-based piezoceramics. J. Eur. Ceram. Soc. 2011, 31, 1997–2004. [Google Scholar] [CrossRef]
- Vila-Fungueirino, J.M.; Gomez, A.; Antoja-Lleonart, J.; Gazquez, J.; Magen, C.; Noheda, B.; Carretero-Genevrier, A. Direct and converse piezoelectric responses at the nanoscale from epitaxial BiFeO3 thin films grown by polymer assisted deposition. Nanoscale 2018, 10, 20155–20161. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Q.; Li, X.L.; Wang, S.; Liu, H.J.; Shi, L.; Luo, Q.Y.; Song, W.L.; Chen, D. Fabrication and Photoelectrochemical Performances of BiFeO3 Thin Film Photoelectrodes: Effect of Annealing Temperature. Int. J. Electrochem. Sci. 2021, 16, 210721. [Google Scholar] [CrossRef]
- Zhu, K.J.; Qiu, J.H.; Kajiyoshi, K.; Takai, M.; Yanagisawa, K. Effect of washing of barium titanate powders synthesized by hydrothermal method on their sinterability and piezoelectric properties. Ceram. Int. 2009, 35, 1947–1951. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.C.; Chang, Y.F.; Li, F.; Yang, B.; Sun, Y.; Wu, J.; Zhang, S.T.; Wang, R.X.; Cao, W.W. Exceptionally High Piezoelectric Coefficient and Low Strain Hysteresis in Grain-Oriented (Ba, Ca)(Ti, Zr)O3 through Integrating Crystallographic Texture and Domain Engineering. ACS Appl. Mater. Interfaces 2017, 9, 29863–29871. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.F.; Ren, X.B. Large Piezoelectric Effect in Pb-Free Ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Li, X.L.; Dai, Y.J.; Ji, H.M.; Su, D. Highly textured Ba0.85Ca0.15Ti0.90Zr0.10O3 ceramics prepared by reactive template grain growth process. Mater. Lett. 2016, 165, 131–134. [Google Scholar] [CrossRef]
- Sun, M.Z.; Du, J.; Chen, C.; Fu, P.; Li, P.; Hao, J.G.; Yue, Z.X.; Li, W. Enhanced piezoelectric properties in M (M = Co or Zn)-doped Ba0.99Ca0.01Ti0.98Zr0.02O3 ceramics. Ceram. Int. 2020, 46, 17351–17360. [Google Scholar] [CrossRef]
- Nie, X.; Yan, S.G.; Guo, S.B.; Cao, F.; Yao, C.H.; Mao, C.L.; Dong, X.L.; Wang, G.S. Influence of Ca2+ concentration on structure and electrical properties of (Ba1-xCax)(Zr0.2Ti0.8)O3 ceramics. Mater. Res. Express 2018, 5, 036301. [Google Scholar] [CrossRef]
- Chen, X.F.; Peng, Z.H.; Chao, X.L.; Yang, Z.P. Structure, electrical properties and reaction mechanism of (Ba0.85Ca0.15) (Zr0.1Ti0.9)O3 ceramics synthesized by the molten salt method. Ceram. Int. 2017, 43, 11920–11928. [Google Scholar] [CrossRef]
- Ji, W.W.; Fang, B.J.; Lu, X.Y.; Zhang, S.; Yuan, N.Y.; Ding, J.N. Tailoring structure and performance of BCZT ceramics prepared via hydrothermal method. Phys. B 2019, 567, 65–78. [Google Scholar] [CrossRef]
- Bai, Y.; Matousek, A.; Tofel, P.; Bijalwan, V.; Nan, B.; Hughes, H.; Button, T.W. (Ba,Ca)(Zr,Ti)O3 lead-free piezoelectric ceramics—The critical role of processing on properties. J. Eur. Ceram. Soc. 2015, 35, 3445–3456. [Google Scholar] [CrossRef]
- Zhou, M.X.; Liang, R.H.; Zhou, Z.Y.; Xu, C.H.; Nie, X.; Dong, X.L. Enhanced Curie temperature and piezoelectric properties of (Ba0.85Ca0.15) (Zr0.10Ti0.90)O3 lead-free ceramics after the addition of LiTaO3. Mater. Res. Bull. 2018, 106, 213–219. [Google Scholar] [CrossRef]
- Kaddoussi, H.; Lahmar, A.; Gagou, Y.; Manoun, B.; Chotard, J.N.; Dellis, J.L.; Kutnjak, Z.; Khemakhem, H.; Elouadi, B.; El Marssi, M. Sequence of structural transitions and electrocaloric properties in (Ba1-xCax)(Zr0.1Ti0.9)O3 ceramics. J. Alloy. Compd. 2017, 713, 164–179. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, L.L.; Chao, X.L.; Liu, Z.H.; Yang, Z.P. Phase Transition Behavior and Large Piezoelectricity Near the Morphotropic Phase Boundary of Lead-Free (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 Ceramics. J. Am. Ceram. Soc. 2013, 96, 496–502. [Google Scholar] [CrossRef]
- Guo, H.Z.; Voas, B.K.; Zhang, S.J.; Zhou, C.; Ren, X.B.; Beckman, S.P.; Tan, X.L. Polarization alignment, phase transition, and piezoelectricity development in polycrystalline 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3. Phys. Rev. B 2014, 90, 014103. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, Q.W.; Zhou, C.; Gao, R.L.; Zhang, S.L.; Li, Z.D.; Xu, R.C.; Chen, G.; Deng, X.L.; Wang, Z.H.; et al. Synergistic effect of grain size and phase boundary on energy storage performance and electric properties of BCZT ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 9167–9175. [Google Scholar] [CrossRef]
- ANSI/IEEE Std 176-1987; IEEE Standard on Piezoelectricity. IEEE: Piscataway, NJ, USA, 1988.
- Bai, W.F.; Chen, D.Q.; Zhang, J.J.; Zhong, J.S.; Ding, M.Y.; Shen, B.; Zhai, J.W.; Ji, Z.G. Phase transition behavior and enhanced electromechanical properties in (Ba0.85Ca0.15)(ZrxTi1-x)O3 lead-free piezoceramics. Ceram. Int. 2016, 42, 3598–3608. [Google Scholar] [CrossRef]
- Tian, Y.S.; Li, S.Y.; Gong, Y.S.; Yu, Y.S.; Tang, Y.T.; Liu, P.; Jing, Q.S. Diversified electrical properties of (1-x)Ba0.90Ca0.10Ti0.95Zr0.05O 3-xRuO2 ceramics with defect electron complexes. Mater. Chem. Phys. 2018, 204, 163–170. [Google Scholar] [CrossRef]
- Wu, J.G.; Xiao, D.Q.; Wu, W.J.; Chen, Q.; Zhu, J.G.; Yang, Z.C.; Wang, J. Composition and poling condition-induced electrical behavior of (Ba0.85Ca0.15)(Ti1-xZrx)O3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 2012, 32, 891–898. [Google Scholar] [CrossRef]



| Composition | x = 0 | x = 0.06 | x = 0.12 | x = 0.18 | y = 0 | y = 0.08 | y = 0.1 | y = 0.12 |
|---|---|---|---|---|---|---|---|---|
| d33 (pC/N) | 70 | 102 | 300 | 231 | 122 | 302 | 330 | 211 |
| kp | 0.16 | 0.20 | 0.44 | 0.31 | 0.24 | 0.43 | 0.41 | 0.27 |
| εr | 2398 | 1744 | 2632 | 3928 | 928 | 2942 | 4069 | 3832 |
| tanδ | 9.8% | 3.9% | 1.1% | 1.2% | 9.7% | 1.1% | 1.5% | 1.6% |
| Tc (°C) | 87.1 | 86.6 | 78.5 | 73.1 | 119.3 | 91 | 80.5 | 66.05 |
| Pr (μC/cm2) | - | 4.5 | 6.3 | 3.6 | 3.5 | 5.7 | 4.8 | 5.1 |
| Ec (kV/cm) | - | 0.9 | 2.3 | 3.1 | 3.3 | 4.6 | 3.1 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Qiu, L.; Yang, C.; Chen, Y.; Zhu, K.; Wang, L. Effect of Different Ca2+ and Zr4+ Contents on Microstructure and Electrical Properties of (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics. Crystals 2022, 12, 896. https://doi.org/10.3390/cryst12070896
Du J, Qiu L, Yang C, Chen Y, Zhu K, Wang L. Effect of Different Ca2+ and Zr4+ Contents on Microstructure and Electrical Properties of (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics. Crystals. 2022; 12(7):896. https://doi.org/10.3390/cryst12070896
Chicago/Turabian StyleDu, Jianzhou, Long Qiu, Cong Yang, Yuansheng Chen, Kongjun Zhu, and Luming Wang. 2022. "Effect of Different Ca2+ and Zr4+ Contents on Microstructure and Electrical Properties of (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics" Crystals 12, no. 7: 896. https://doi.org/10.3390/cryst12070896
APA StyleDu, J., Qiu, L., Yang, C., Chen, Y., Zhu, K., & Wang, L. (2022). Effect of Different Ca2+ and Zr4+ Contents on Microstructure and Electrical Properties of (Ba,Ca)(Zr,Ti)O3 Lead-Free Piezoelectric Ceramics. Crystals, 12(7), 896. https://doi.org/10.3390/cryst12070896









