1. Introduction
The development of rapid, point of care and timely diagnostic technologies for cancerous cells is a promising research field. The unwanted growth of cells anywhere in the human body may become the root cause of malignancy. In 2018 around 19 million new cancer cases and 10 million deaths due to cancer have been reported as per the study by the international society which monitors cancer. Most of these demises were due to untimely diagnosis of cancer [
1,
2]. Therefore, it is an essential requirement to develop near patient, cost effective and accurate testing facilities. Recently photonic technology based biosensors have emerged as a potential candidate for label free, rapid and accurate detection of malignant tissues which causes cancer in the human body. These biosensors are capable of detecting minute changes in the refractive index of the sample under consideration [
3,
4,
5,
6,
7,
8]. The sensing mechanism of exhaled breath to detect the presence of toluene traces in the human body has been studied [
9] with the help of a 1D photonic crystal (PhC). The results of this study may be helpful for the diagnosis of lung cancer. Actually, organs of the human body are made up of cells of a specific refractive index. For example, the refractive index variation of cells of different parts of the human brain is between 1.3 to 1.42 due to changes in the concentration of protein levels inside cells [
10]. The malignant cells which are responsible for cancerous brain tumors have a large amount of water content than normal cells [
11]. This excess water results in higher dielectric constant and electrical conductivity values of cancerous cells and hence, the refractive index of cancerous cells is more than normal cells. The brain is one of the most sophisticated and important organs controlling all the real and virtual functions of our body and it is very difficult as well as costly to examine cancerous brain tumors with the help of existing conventional cancer diagnostic technologies [
12,
13,
14,
15]. A lot of studies have been carried out for rapid, accurate and cost effective detection of cancer cells, as well as other diseases in the human body. For example, Nejad et al. reported a metamaterial based tunable and supersensitive biosensor composed of split ring resonators which could detect various cancer cells in the infrared wavelength region. The sensitivity of this structure varies between 633 nm/RIU to 658 nm/RIU [
11]. A surface plasmon resonance based PhC fiber biosensor was studied by Yasli for early stage detection of cancer cells belonging to various parts of the body except for the brain [
14]. Recently Panda et al. studied a graphene based 1D PhC biosensor capable of real time examination of cancers cells. This design could achieve a sensitivity of 290 nm/RIU only [
8]. Auguie et al. investigated Tamm plasmon (TP) resonance dependent biosensing applications of 1D PhC based on theoretical simulations which were verified experimentally [
16]. In addition, TP resonance based gas sensing applications of 1D PhCs have been theoretically examined by Zaky et al. [
17]. They obtained ultra high sensitivity of value 5018 nm/RIU. In addition, they studied the hemoglobin detection strategy employing surface plasmon resonance with high quality factor and figure of merit. A novel way of detecting tuberculosis by using 1D DPhC is studied by Arafa et al. [
18]. By keeping all the technological aspects involved in the timely detection of cancerous brain tumors by employing several photonic biosensors, in this study we have proposed a photonic biosensor capable of detecting cancerous brain tumors with extremely high sensitivity.
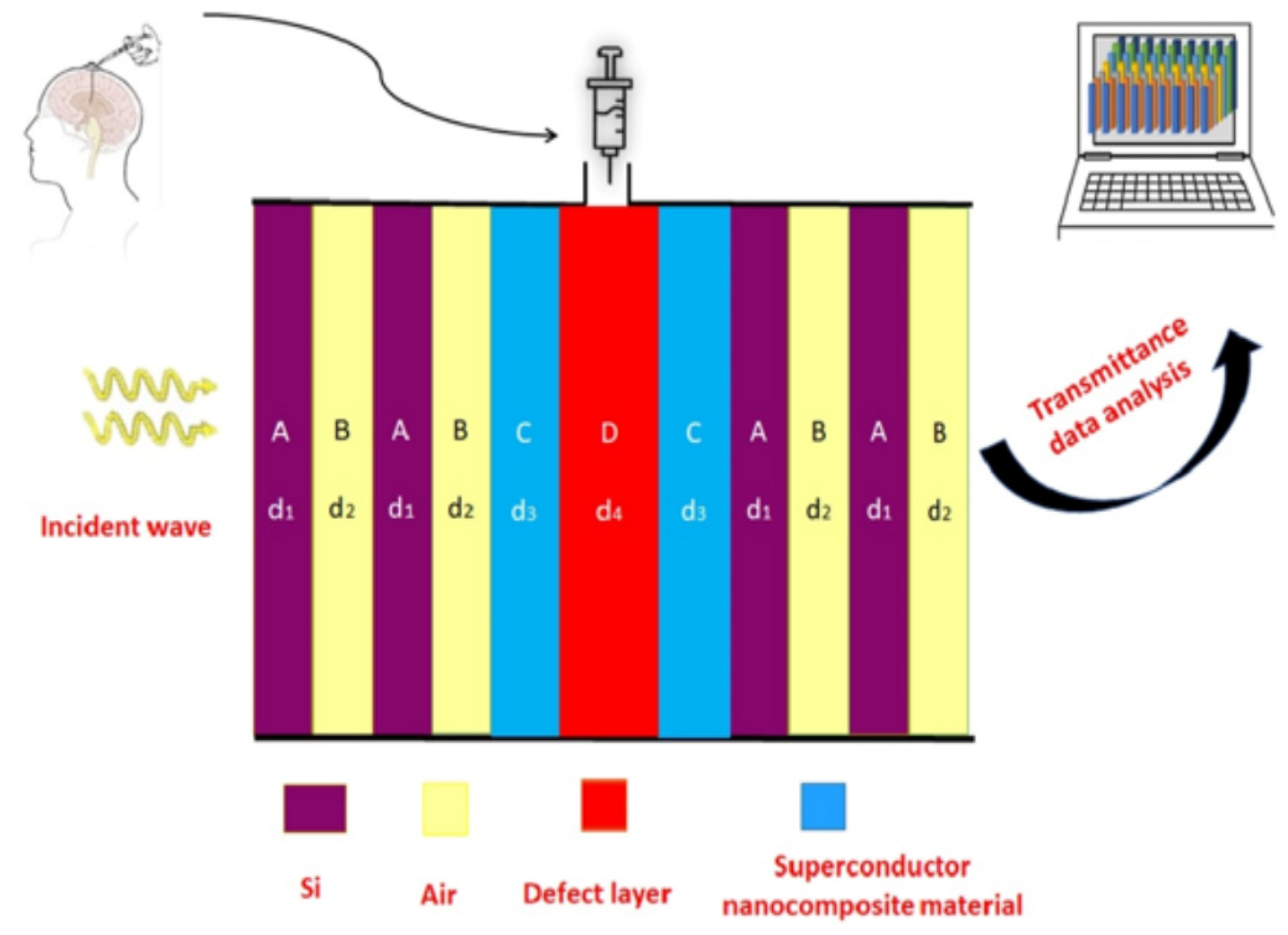
In the present piece of research work, we have proposed a simple 1D DPhC design for the timely detection of various malignant tissues and cells of the human brain. The purpose of this study is to detect malignant brain tissues so that their rapid growth may be prevented to save the life of the sufferer. The proposed design utilizes the conventional 1D DPhC associated with a nanocomposite superconducting material coated cavity of air. The different samples under investigation are infiltrated into the cavity one by one to obtain the desired results. To the best of our knowledge, a 1D DPhC whose cavity is coated with nanocomposite material has been rarely used for the accurate detection of brain tumors. The normal and cancerous cells are infiltrated into the cavity using refractive index miniaturization sensing applications to obtain enhanced sensitivity from the design. The transmission spectra have been computed by using the transfer matrix method (TMM).
3. Theoretical Formulation of the Problem
The symbols n1, n2 and d1, d2 represented the refractive index (RI) and thickness of layers A and B, respectively, of 1D PhC (AB)N used in the structure (AB)NCDC(AB)N. The refractive index and thickness of nanocomposite superconducting layers C of material YBa2 Cu3O7 are denoted by n3 and d3, respectively. The air cavity D of thickness d4 is to be used to examine various brain tissue samples. The period thickness of 1D PhC (AB)N is given dd = d1 + d2. The refractive index of various samples under investigation is being denoted by n4.
The two-fluid model describes the refractive index of high temperature superconductor
YBa2 Cu3O7 as [
19,
20,
21]
where
c,
ω and
λL are representing velocity of incident light in free space, angular frequency of the incident light and the temperature-dependent London penetration depth, respectively. The expression of temperature-dependent London penetration depth is given as [
21,
22]
Here is the London penetration depth at T = 0 K and Tc is the critical temperature of the superconductor material.
The refractive index n
3 of nanocomposite superconductor material is defined with the help of the Maxwell-Garnett formula as [
23,
24]
where
is the permittivity of the dielectric material SiO
2,
() is the permittivity of high temperature superconductor material
YBa2Cu3O7 and
is representing the volume fraction of SiO
2 material embedded into
YBa2Cu3O7. The effective permittivity of the nanocomposite layer is denoted as
εeff.
The transfer matrix representing the
jth (=A, B, C and D) layer of the proposed design at an angle
θ is given [
25,
26,
27]
The value of
pj of
jth layer is
and
for TE and TM polarized light waves, respectively. Here,
with
dj,
nj and
θj are representing the thickness, refractive index and ray angle of light inside the
jth layer of the structure. The free space impedance and wavelength are represented by
z0 and
λ0, respectively. The total transfer matrix of the whole structure can be obtained by using Equation (4) as
Here, M11, M12, M21 and M22 are representing elements of total transfer matrix MT.
The coefficient of transmission
t is given by
For TE and TM polarized light waves the values of
η0 and
ηs representing incident and exit medium, respectively are given as
and
The transmittance (
T) of the design (
AB)
NCDC(
AB)
N can be obtained as [
28,
29,
30]
4. Results and Discussions
The present research work highlights the bio-sensing capabilities of the proposed 1D DPhC based on the interaction of light and the structure. The proposed structure (
AB)
NCDC(
AB)
N is made up of two 1D binary PCs (
AB)
N of a similar kind. The dielectric material Si in association with air has been chosen to represent the
A and
B layers of both 1D binary PCs (
AB)
N, respectively. The refractive index of Si and air layers of the design has been fixed to
n1 = 3.3 and
n2 = 1, respectively. On the other hand, the thickness of the Si and air layers of the structure have been optimized to
d1 = 0.35 μm and
d2 = 0.275 μm, respectively, to obtain a wider PBG which is one of the essential requirements of designing any 1D photonic biosensors. Next, we are introducing the details of the design of the cavity region which will be used to examine different analytes in this manuscript. The cavity region of the design is composed of two identical thin buffer layers of thickness
d3 which are associated together through an air cavity of a thickness of
d4. The two identical buffer layers are made up of the nanocomposite high temperature superconducting material YBa
2Cu
3O
7 in which the dielectric material SiO
2 is embedded to obtain an engineered nanocomposite superconducting material. The thickness of both the nanocomposite buffer layers has been set to 0.09 μm. The refractive index of the nanocomposite buffer layer is dependent upon wavelength and governed by Equation (3). We have chosen the thickness of the cavity region such that
d4 =
dd =
d1 +
d2, where
dd is the length of the period of the 1D PhC (
AB)
N. We have fixed the period
N of both the 1D PhCs (
AB)
N used in this design as 2 to minimize the length of the design. The London penetration depth (
λ0) at temperature T = 0 K of the nanocomposite high superconductor layers is set to 200 nm. The numeric values of critical temperature (
Tc) and volume fraction (
η) of the nanocomposite layer are set to 92 K and 0.8, respectively. The permittivity of the S
iO2 material layer is taken as 2.1025 [
4]. In this simulation work, we have chosen air as a surrounding medium and substrate of the design. The purpose of selecting nanocomposite buffer layers to design this structure is due to the unique applications of nanocomposite materials which enable the development of a variety of biophotonic sensing applications on the basis of refractive index sensing mechanisms of various fluids provided by metal or dielectric nanoparticles. Moreover, the inclusion of nanocomposite buffer layers also increases external parameters, such as T of superconducting layers by which we can control the bio-sensing performance of the design externally. In this simulation work, we have studied the externally tunable bio-sensing performance of the proposed design dependent upon the angle of incidence (
θ) and ambient temperature (
T) of the nanocomposite superconducting material layer.
In recent years, the refractive index analysis of various kinds of body fluids containing normal and abnormal cells has become important for improving the operation of plasmonics and photonics-based bio-sensing applications. Photonic bio-sensing has emerged as a powerful tool in detection, monitoring and drug designing to accelerate the diagnostic technologies involved in rapid disease detection due to economic, accurate and highly sensitive results. This research work has been given attention to investigating the various brain lesion samples to find any kind of malignancy inside the human brain with the help of 1D DPhC. Actually damaged brain tissues which are localized inside the brain may result in brain tumors and are classified as brain lesions. The unwanted growth of these brain lesions may become the root cause for the development of malignant brain tumors and are fatal for the patient if not diagnosed on time. The lumbar puncture or spinal tap are the two common procedures to collect samples by injecting a thin and long needle through the skull or spinal cord of the affected person. The human brain floats in a colorless fluid called Cerebrospinal fluid (CSF). This fluid ensures all the requirements to various parts of the human brain as well as the spinal cord are met, and also filters the waste from those places. The patient having symptoms of autoimmune diseases, inflammation in the spinal cord and/or brain tissues or leukemia may be due to some viral or bacterial infections inside the brain so CSF investigation may become essential as per the advice of a doctor. Since it is a well-established fact that the refractive index of CSF of a healthy person is 1.3333 in the infrared region of the electromagnetic spectrum, we have taken this value of the refractive index as an internal reference to carry out the findings of the proposed work [
31].
The threshold value of the refractive index of CSF containing various brain lesions which determines the malignancy in different samples containing brain lesions is 1.395. This threshold value is very helpful in identifying the malignancy in the examination of different samples containing various brain lesions. If the refractive index of the sample under investigation is less or equal to 1.395 the sample is nonmalignant otherwise the sample is said to be malignant due to excess water content inside malignant cells. Actually, our design relocates the position of defect mode side the PBG of the structure depending upon the change in the refractive index of different brain tissues in the samples under investigation. Thus, by measuring the separation between positions of modified defect mode with respect to defect mode associated with the CSF sample, one can find malignancy in the sample. The refractive index values of different samples containing normal and damaged brain tissues used in this study have been mentioned by Biswas, T. [
31]. The change in the level of proteins inside brain tissues is responsible for the corresponding change in the RI of various brain tissues.
In order to carry out investigations pertaining to this research, we have infiltrated the cavity region of our 1D DPhC structure (
AB)
2CDC(
AB)
2 with different samples containing brain tissues as per the information given by Biswas, T. [
31], separately to identify the nature of the malignancy in these samples. For achieving the objective of the proposed work we have studied the transmission spectra of 1D DPhC structure (
AB)
2CDC(
AB)
2 dependent on two external factors, first, change in incident angle and the second change in the ambient temperature of nanocomposite superconducting material layer. The research has been conducted in the infrared region of the electromagnetic spectrum which extends from 3.2 µm to 4.7 µm.
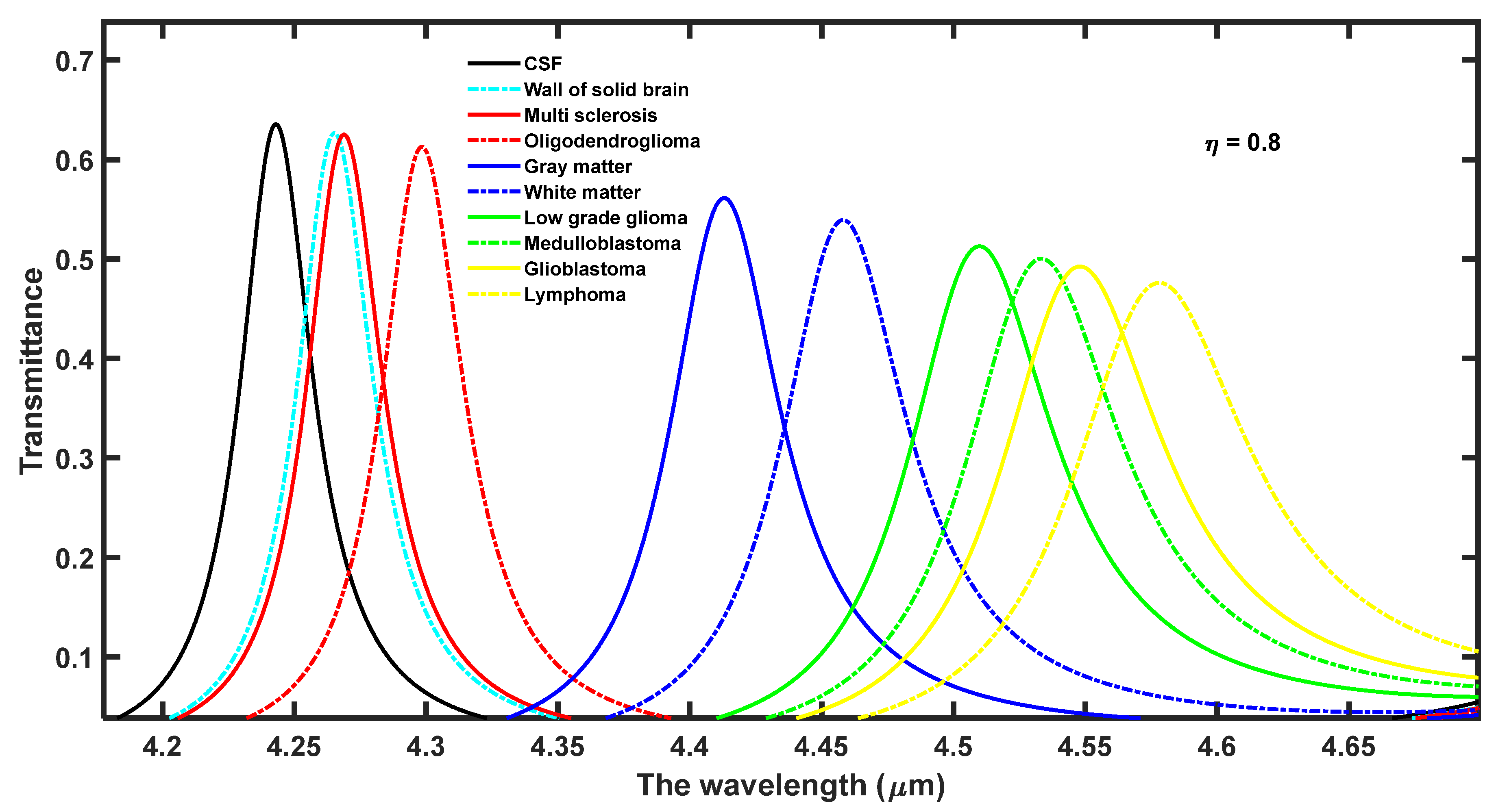
Figure 2 depicts the transmission spectra of proposed biosensor (
AB)
2CDC(
AB)
2 at a normal incidence with
d4 = 15
dd and
η = 0.8. It shows ten distinguishable defect mode peaks centered at different positions inside PBG which extends from 4.15 µm to 4.7 µm corresponding to ten separate samples containing different brain tissues as per the details given by Biswas, T. [
31]. The intensity of these peaks gradually varies between 65% to 45% corresponding to samples containing brain tissues separately from CSF to lymphoma, respectively. The intensity of these defect modes is sufficient to be detected by spectrometer through optical fiber into a computer with the help of software. The larger refractive index contrast between silicon and air layers of the 1D DPhC results in a wider PBG width of 0.6 µm. The change in the analyte into the cavity region from CSF to Lymphoma as given by Biswas, T. [
31] one by one results in the movement of defect mode inside PBG towards a higher wavelength with a gradual decrease in the intensity of the respective defect mode as shown in
Figure 2. This shifting of defect mode inside PBG is measured with reference to the position of defect mode corresponding to the CSF sample. The standing wave formulation inside the laser cavity can be used to justify the movement of defect mode inside PBG as given in Equation (9). The change in the refractive index of the analyte under investigation alters the resonant condition which governs laser radiation from the cavity by fixing the optical path difference inside the laser cavity.
In order to represent the optical path difference between standing waves inside the laser cavity, an integer, central wavelength of defect mode, effective refractive index of the cavity and geometrical path difference we have used notations Δ, p, λ, Z and neff, respectively.
We have evaluated the performance of the proposed design capable of sensing various samples containing different brain tissues with the help of one of the most popular parameters known as sensitivity (
S) of the structure. It determines the minute shift in the central wavelength of defect mode due to the corresponding change in the refractive index of various analytes involved in the investigation. Basically, it helps to estimate the minute sensing capabilities of the biosensors which is defined as [
28,
29,
30,
31,
32,
33]
Here, the change in the refractive index of the sample under investigation and the resulting shift in the position of the central wavelength of the defect mode inside the PBG are represented by
δn and
δλ, respectively. We have used the CSF sample as a reference to calculate the
S of the proposed design. This calculation has been implemented by loading the cavity of the design one by one with different samples containing various brain tissues. The central wavelength of defect mode of wavelength (
λd) and sensitivity (
S) of the structure with of cavity thickness
d4 = 15
dd and volume fraction of a nanocomposite layer
η = 0.8 at
θ = 0° is being summarized in
Table 1 corresponding to different samples containing brain tissues one by one.
From the data mentioned in
Table 1, it can be observed that the
S of the structure varies between the maximum value of 2.80434 µm/RIU to the minimum value of 2.66136 µm/RIU when the cavity is loaded separately with samples containing multiple sclerosis to lymphoma brain tissues, respectively. Now we extend this study further to improve the sensitivity of the design by tuning the external parameters of the design, such as angle of incidence and temperature of nanocomposite high superconductor layer keeping all other internal parameters constant as discussed above.
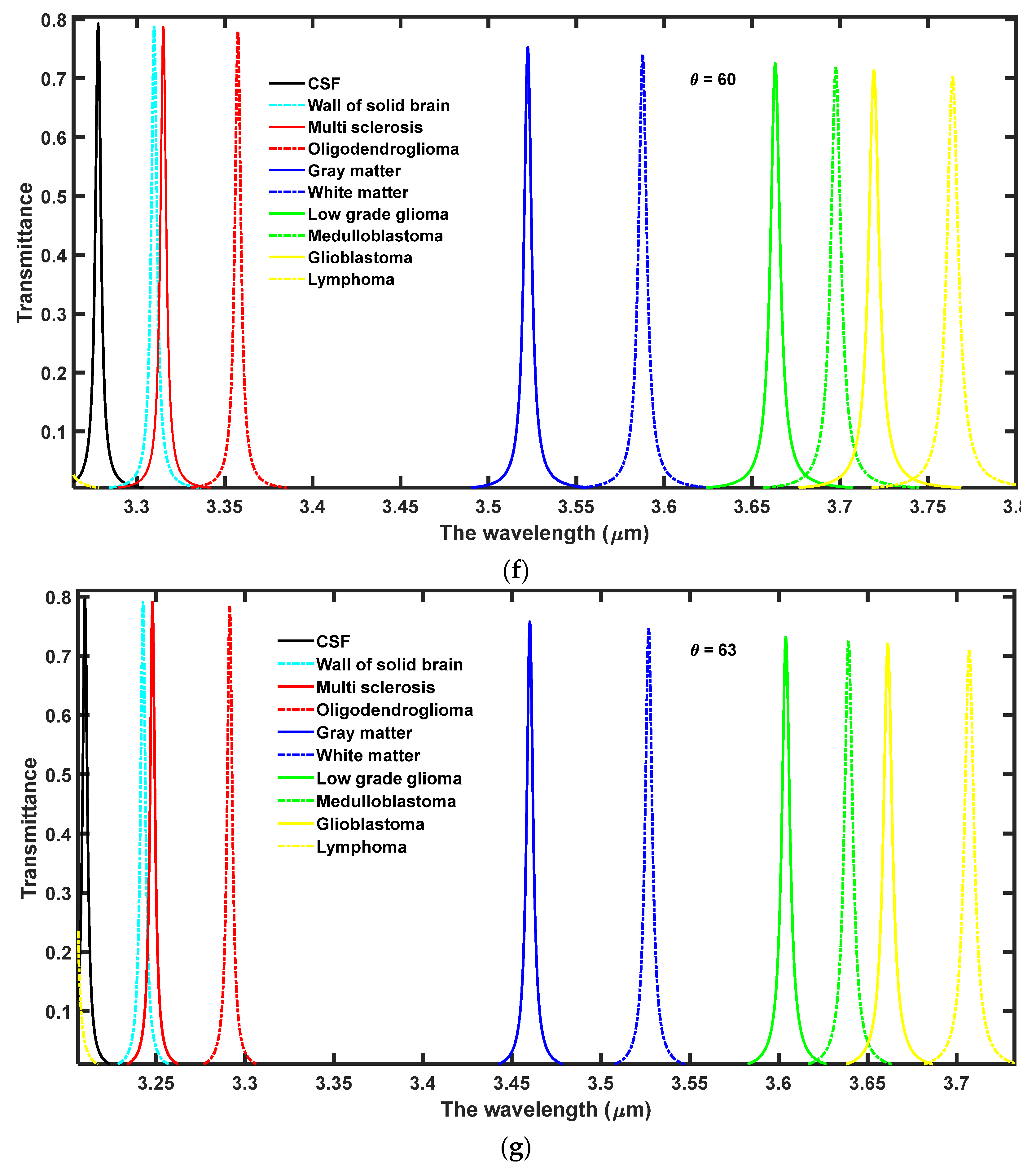
4.1. The Effect of Increasing the Angle of Incidence on the Performance of the Design
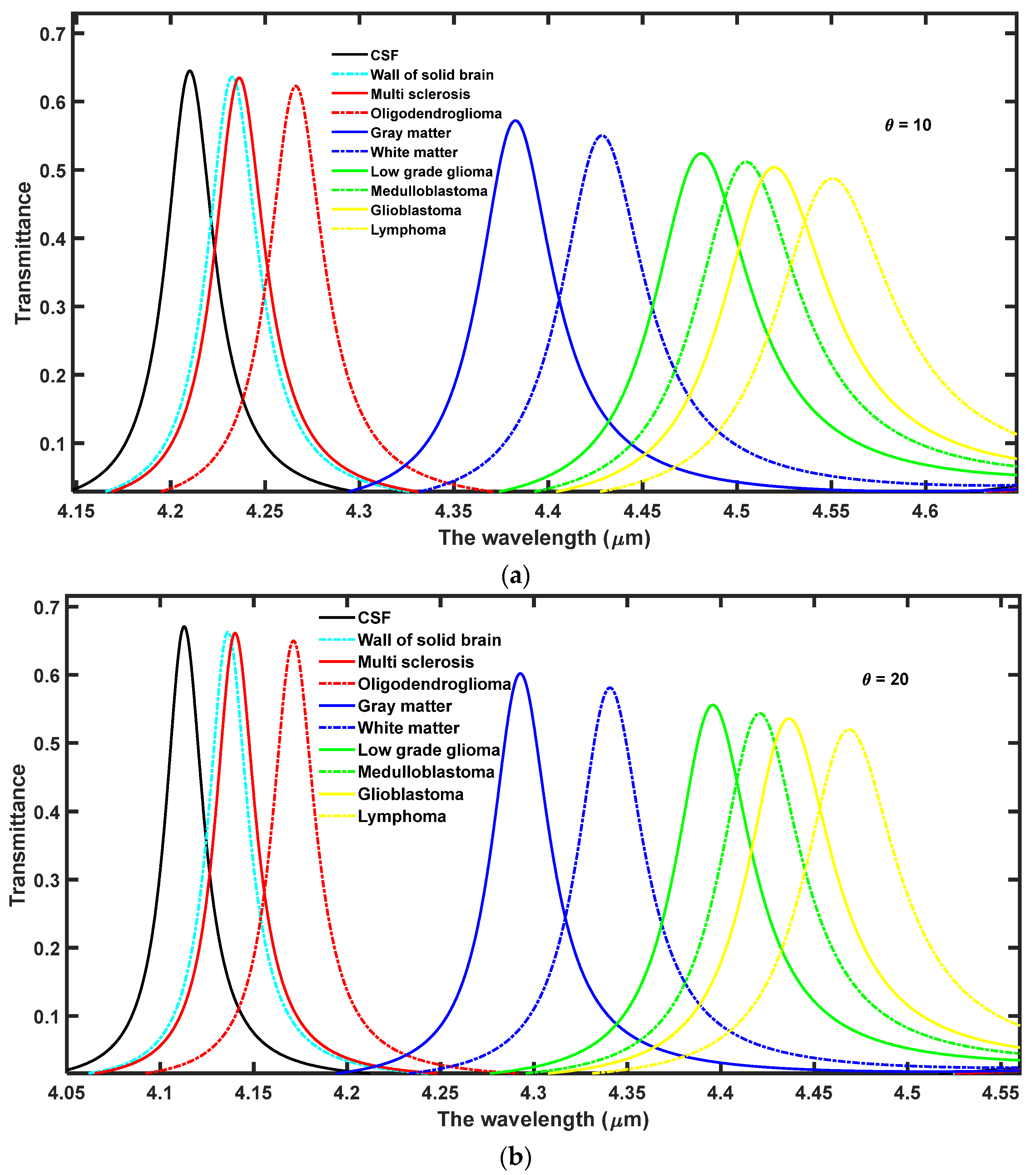
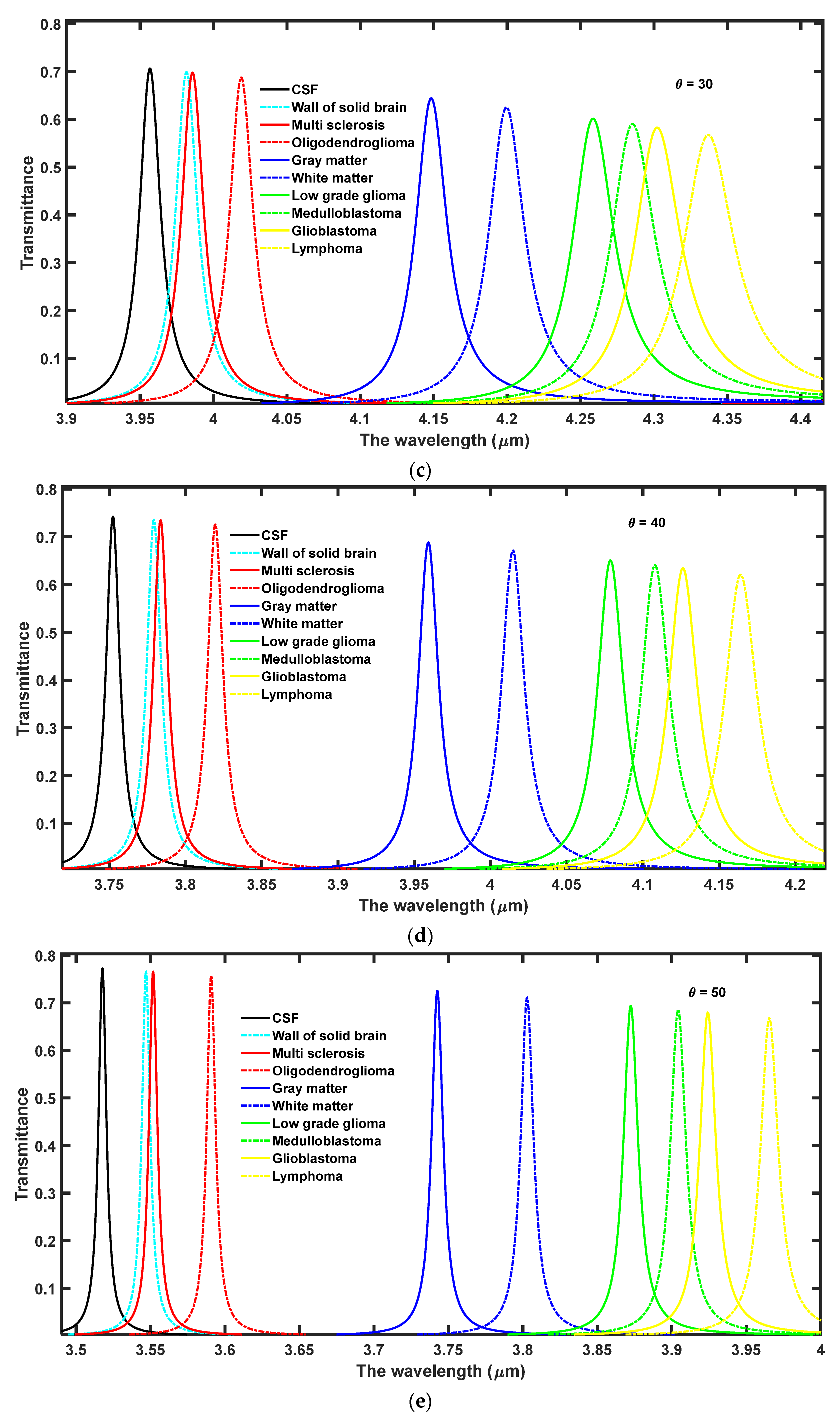
In this section, the effect of increasing the angle of incidence from 0° to 63° has been observed on the performance of the proposed 1D DPhC (
AB)
2CDC(
AB)
2. For this purpose the transmission spectra of the proposed design corresponding to different incident angles at
θ = 10°,
θ = 20°,
θ = 30°,
θ = 40°,
θ = 50°,
θ = 60° and
θ = 63° have been plotted in
Figure 3a–g, respectively. Here, the ambient temperature of the nanocomposite layer has been fixed to
T = 4.5 K.
As evident from
Figure 3 the increase in the angle of incidence from 0° to 63° results in the blue shift of defect modes associated with each sample. Moreover, this increase in incident angle also reduces the full-width half maximum (FWHM) of each resonant mode inside PBG which in turn improves the intensity of each defect mode by fulfilling the law of conservation of energy. Actually due to an increase in the angle of incidence the energy associated with each defect mode remains constant inside PBG. The optimum value of angle of incidence has been found to be θ = 63° at which the sensitivity of the proposed design varies between the maximum value of 4.13924 µm/RIU to the minimum value of 3.9523 µm/RIU corresponding to samples containing a wall of the solid brain to lymphoma brain tissues, respectively. Thus the increase in the incident angle results in a significant improvement in the sensitivity of the proposed structure in contrast to the results of
Figure 2 at
θ = 0°. The increase in the angle of incident improves the interaction between the sample under investigation and light. This interaction improves the resonance between the standing waves inside the cavity region which results in the reduction of FWHM with an enhanced intensity of defect mode associated with each sample containing different brain tissues. Thus, by increasing the incident angle one can improve the performance of the proposed design composed of 1D DPhCs. The angle dependent sensitivity and central wavelength of defect modes associated with samples containing various brain tissues separately are summarized in
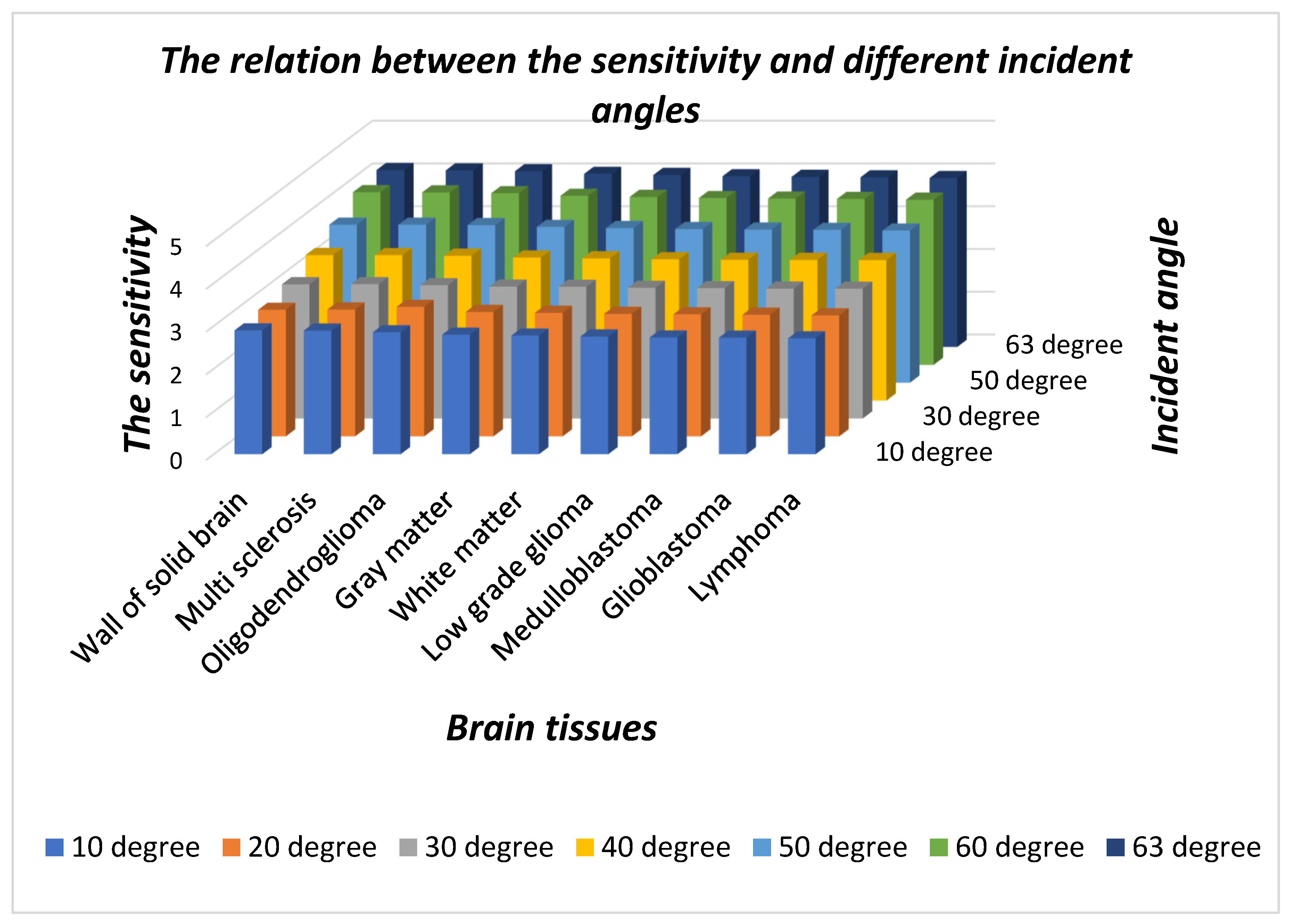
Table 2. The pictorial representation in three-dimensional bar graph of the data presented in
Table 2 is shown in
Figure 4 below.
Figure 4 shows the sensitivity of the proposed design dependent upon different incident angles under the influence of nine samples of different brain tissues with respect to the sample containing nonmalignant CSF brain tissue. It is evident from the figure that as the incident angle increases it improves the sensitivity of the design because of improved interaction between light and the sample under investigation. The sensitivity of the structure loaded with different samples is almost stagnant at a particular incident angle. Thus, for designing any high performance photonic biosensor, we should focus our attention to obtain the optimum value of incident angle which corresponds to maximum sensitivity. In this study, we have limited the optimum value of incident angle to 63° which corresponds to sensitivity variation between the maximum value of 4.13924 µm/RIU to the minimum value of 3.9523 µm/RIU corresponding to a wall of the solid brain to lymphoma brain tissue samples, respectively. Besides this, the minimum size of the structure has also been achieved by fixing the period number to 2 which makes the fabrication of the design easier and cheaper. This design utilizes only two material layers in addition to the air layer. In all the above mentioned findings, the maximum 80% intensity of defect mode has been achieved.
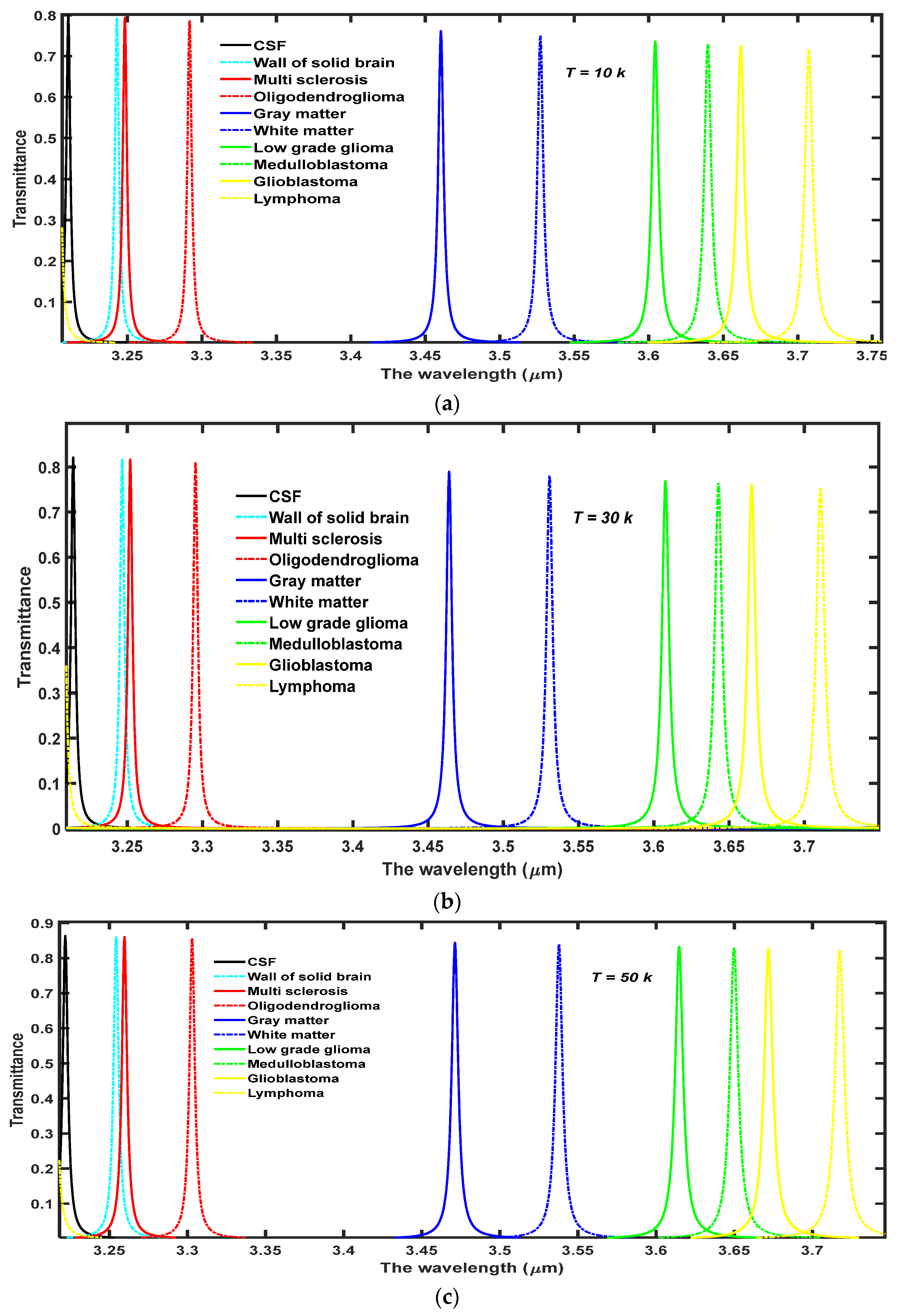
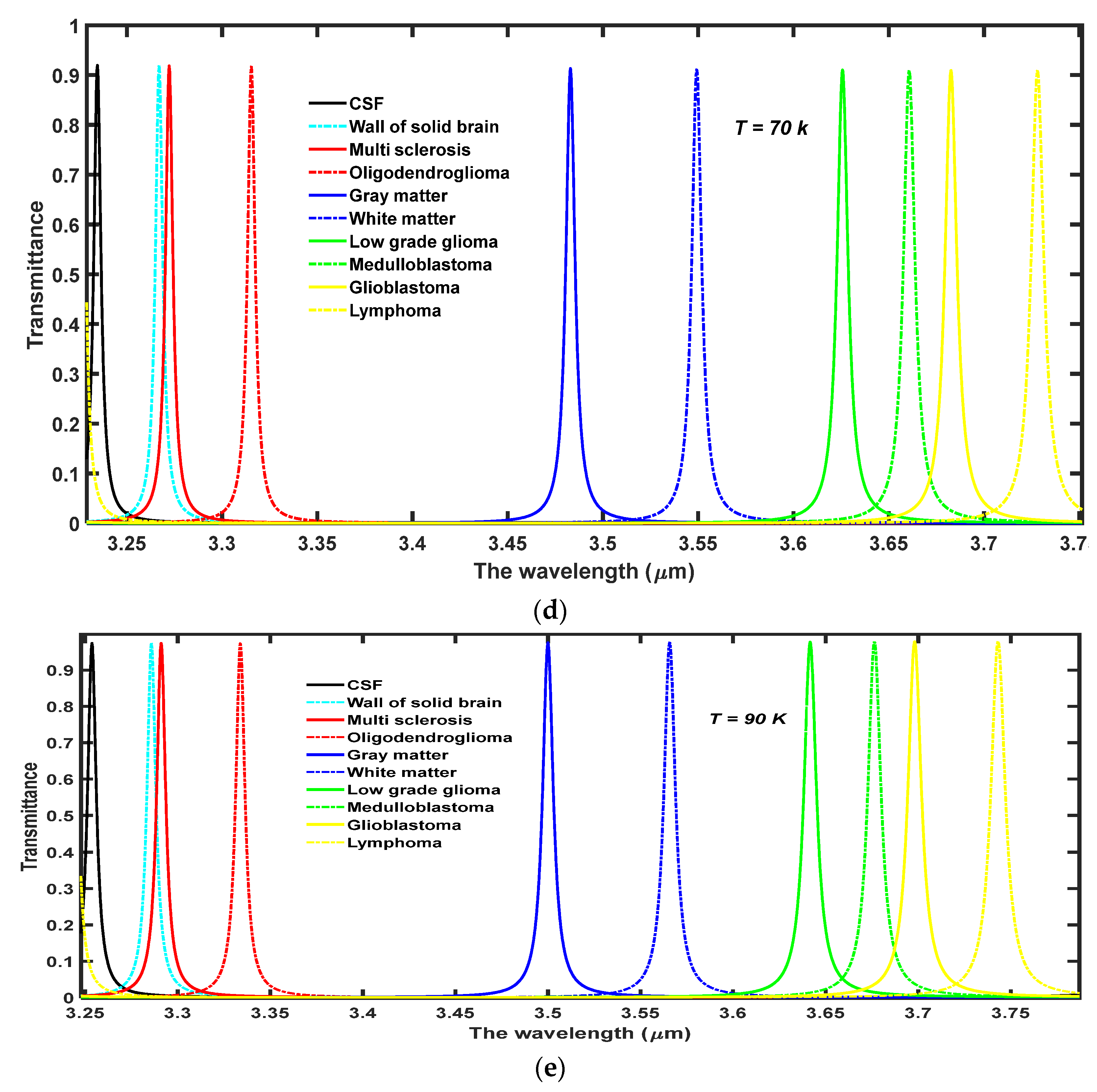
4.2. The Effect of Increasing the Temperature of Nanocomposite Buffer Layer on the Performance of the Design
Finally, we have studied the effects of increasing the temperature of nanocomposite buffer layers made up of the high temperature superconductor YBa
2Cu
3O
7, in which the dielectric material SiO
2 is embedded. The performance of our proposed structure has a cavity thickness of
d4 = 15
dd, and a volume fraction of SiO
2 material embedded into the nanocomposite buffer layers
and at
. We wish to improve the intensity of defect modes inside PBG by increasing the ambient temperature
T of the buffer layers. For this purpose, we have studied the effect of increasing the ambient temperature of superconducting material layers to
T = 10 K, 30 K, 50 K, 70 K, and 90 K, on the transmission spectra of the proposed design loaded with different samples containing brain tissues extracted from various parts of the brain, as shown in
Figure 5a–e, respectively. This range of temperature has been selected keeping the critical temperature (
Tc = 92 K) of the superconducting material into account. Moreover, it is the experimentally observed fact by Marel et al. that YBa
2Cu3O
7 shows the critical reflectivity below
Tc in the infrared region of the electromagnetic spectrum which is in agreement with the two fluid model of superconductors [
31]. It has been observed that the increase in temperature results in the moderate shifting of defect modes towards higher wavelength side inside PBG. The increase in
T increases
λL, which is a function of
T as evident from Equation (2) which in turn also increases the refractive index of superconducting material. Due to the increment in the refractive index value of superconductor material the refractive index contrast between the layers of the structure reduces which leads to the shifting of PBG towards the higher wavelength side. This shifting of PBG also triggers the red shifting of defect modes which are located inside PBG and are associated with different samples as evident in
Figure 5. The increase in temperature also improves the transmission intensity of each defect mode associated with each sample which reaches the maximum (close to unity corresponding to all samples) at
T = 90 K though the FWHM of each defect mode remains stagnant. The numeric values of the central wavelength of defect mode (
λd), percentage transmittance of defect mode (Trans.) and sensitivity (
S) of the proposed structure (
AB)
2CDC(
AB)
2 are associated with different samples containing brain tissues of various parts of the brain have been listed in
Table 3 corresponding to different ambient temperatures of nanocomposite layers. Moreover, the increase in the temperature of the ambient medium of the superconducting buffer layers decreases the superconducting electrons (
ns) of the material, which in turn increases the number of normal electrons (
ne) of the superconducting material. The superconductor is transformed into normal material when the ambient temperature exceeds the critical temperature of the material due to the presence of a large number of
ne in comparison to
ns in the superconducting material. This effect has not been included in this study because the present structure will be transformed into conventional PhC and has already been discussed by many researchers working in this field.
The numeric data of
Table 3 have been visualized with the help of
Figure 6 and
Figure 7.
Figure 6 and
Figure 7 have been plotted to depict a 3D bar graph showing temperature dependent sensitivity of the proposed design and transmittance of defect mode associated with different fluid samples containing brain tissues respectively, as per the information given in
Table 3.
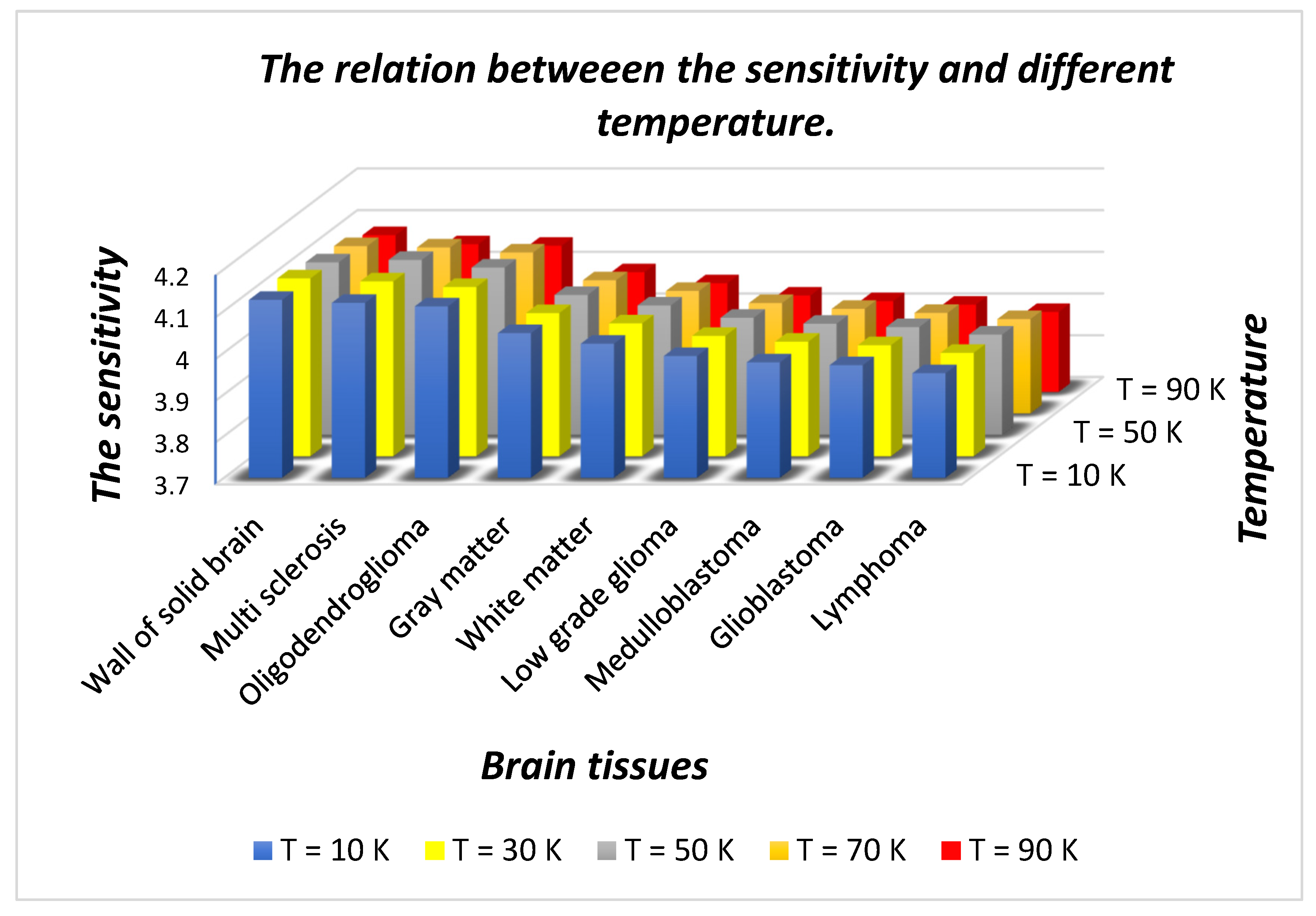
Figure 6 shows as T increases from 10 K to 90 K in steps of 20 K, the sensitivity of the design varies between a maximum value of 4.12658 µm/RIU to a minimum of 3.95071 µm/RIU corresponding to a sample containing a wall of the solid brain and lymphoma brain tissues, respectively, at
T = 10 K. Further increases in temperature gradually reduces the sensitivity corresponding to each sample under investigation. The sensitivity variation lowers down between a maximum of 4.07594 µm/RIU to a minimum of 3.89189 corresponding to separate samples containing walls of solid brain and lymphoma brain tissues, respectively, at
T = 90 K. This reduction in sensitivity is due to the increase in the number of n
e in contrast to the number of n
s with an increase in ambient temperature. It has been also noticed in
Figure 6 that the sensitivity of the structure varies between maximum to minimum values corresponding to samples containing walls of solid brain and lymphoma brain tissues, respectively, at all temperatures.
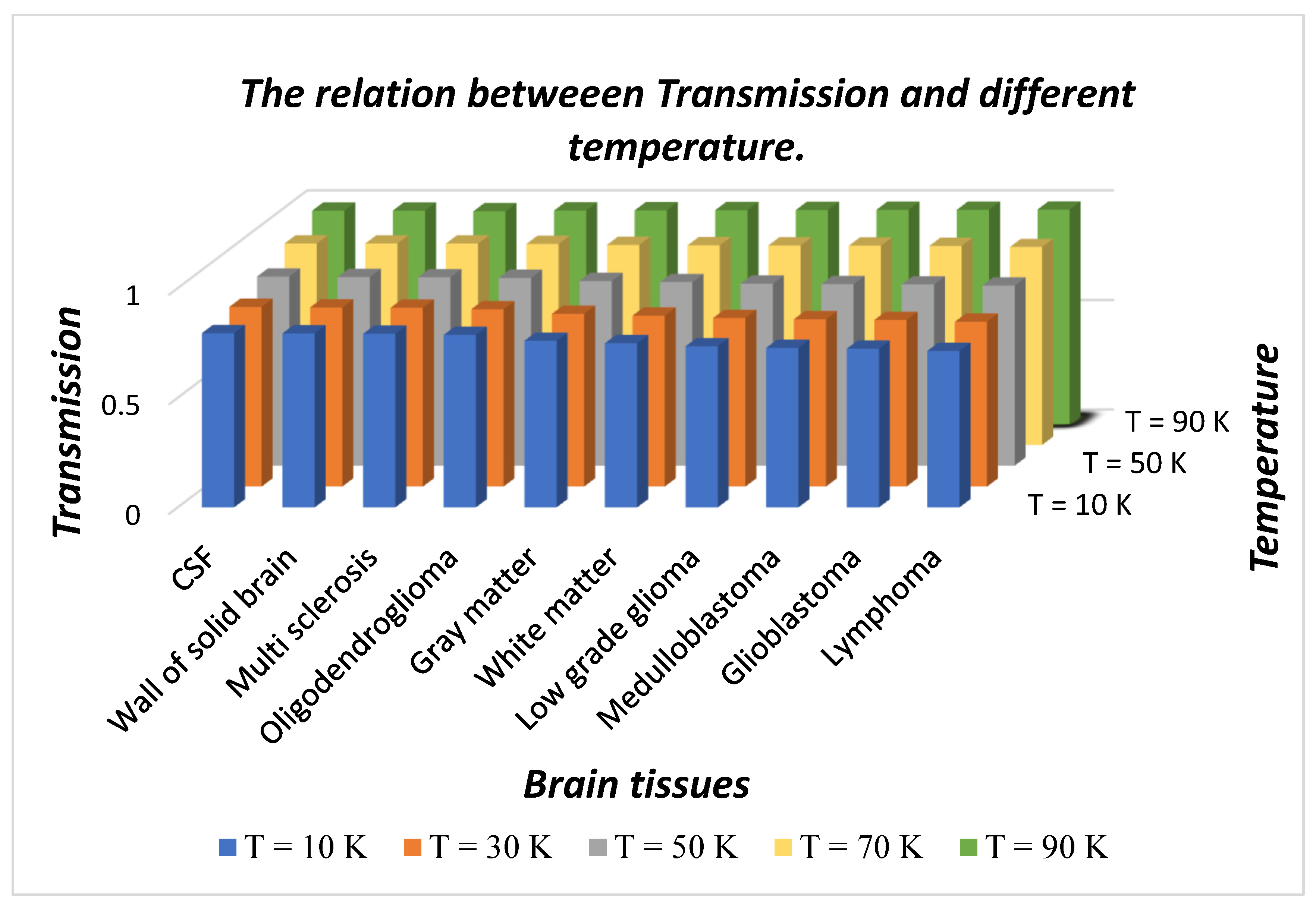
Besides this, we have also plotted a 3D bar graph in
Figure 7 which shows the dependence of transmission intensity of defect modes associated with the respective samples under investigation on the temperature of the ambient medium.
Figure 7 shows that transmission intensity of the defect modes varies between maximum to minimum values associated with CSF to lymphoma samples, respectively, at fixed temperatures. The increase in temperature of nanocomposite buffer layers improves the intensity of defect mode which originates due to the presence of various samples inside the cavity. The intensity reaches a maximum that is close to unity for all samples when the ambient temperature is kept at 90 K which is close to the critical value of the superconducting layer.
4.3. The Evaluation of Bio-Sensing Performance of the Proposed Design
In this section of the manuscript, we have analyzed the bio-sensing performance of the proposed design with the help of various parameters as listed in
Table 4. For the fulfillment of our goal we have calculated the numeric values of parameters
FWHM, quality factor (
Q), figure of merit (
FoM) and limit of detection (
LoD) associated with the design under optimum conditions which are already discussed in
Section 4.1 and
Section 4.2 in addition to the sensitivity of the design. The optimum conditions are very helpful to ensure the maximum performance of the design. We have used the standard definitions defined in references [
32,
33] for calculating the numeric values of parameters
FWHM,
Q,
FoM and
LoD associated with our structure. The various parameters evaluating the performance of the proposed design under optimum conditions with
d4 = 15
dd,
η = 0.8,
θ = 63° and
T = 50 K have been summarized in
Table 4. The sensitivity of the design varies between a maximum value of 4139.241 nm/RIU to a minimum value of 3952.305 nm/RIU corresponding to sample wall of the solid brain to lymphoma, respectively. The average value of the sensitivity of the design is 4038. Furthermore, our design also possesses reasonably high quality factor and figure of merit values of order 10
3 which makes our design suitable for sensitive detection of cancerous brain tumors. The order of limit of detection of our design is 10
−5 which is extremely low. The low value of the limit of detection is one of the desirable requirements for designing any biosensor. It signifies the smallest change in the refractive index of the sample under investigation which can be detected accurately.
Finally, the comparative study shows the outcomes of our proposed work in contrast to the findings of recently published work based on 1D DPhCs for the detection of different kinds of cancerous cells in our body. This comparison has been conducted on the basis of the parameters
S,
FoM and
Q as indicated in
Table 5. The analysis of the data presented in
Table 5 shows that the proposed design composed of SiO
2 embedded nanocomposite buffer layers of high temperature superconductor has better performance than the other configurations listed in the above table. Moreover, SiO
2 embedded nanocomposite superconducting buffer layers bring the opportunity to induce the tunability of the defect mode inside side PBG by changing the ambient temperature of nanocomposite material layers which in turn controls the performance of the design by means of improving the intensity of defect modes. The proposed design gives an opportunity to modulate defect mode and PBG both either left or right by changing θ or
T, respectively.