Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses
2.3. Physical Measurements
2.4. Batch DCF− Sorption Studies
2.5. Preparation of the Column
2.6. Column Sorption Studies
3. Results and Discussion
3.1. Batch Sorption Studies
3.1.1. Sorption Kinetics
3.1.2. Sorption Thermodynamics
- (a)
- Langmuir
- (b)
- Freundlich
- c)
- Langmuir–Freundlich
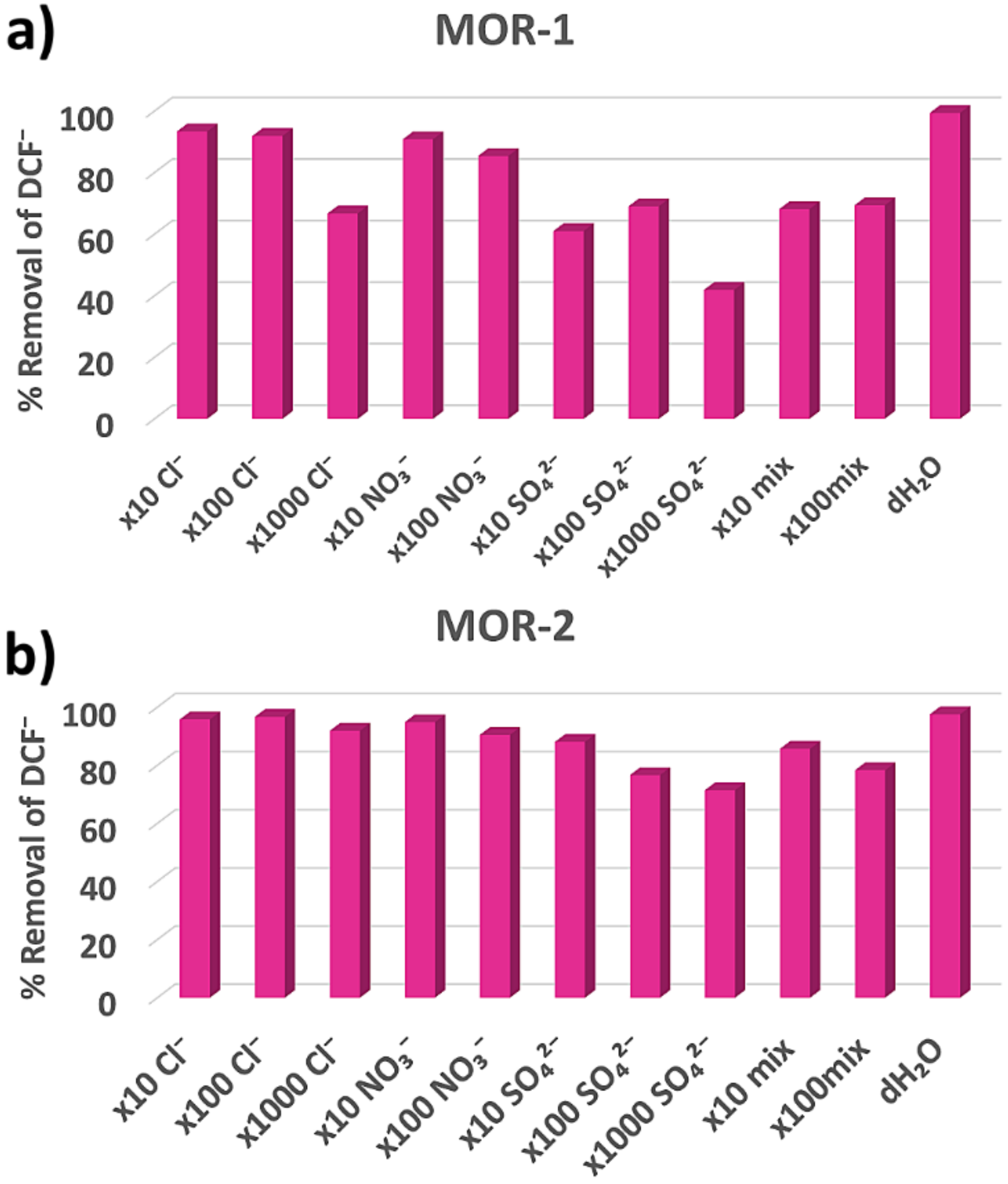
3.1.3. Selectivity Studies
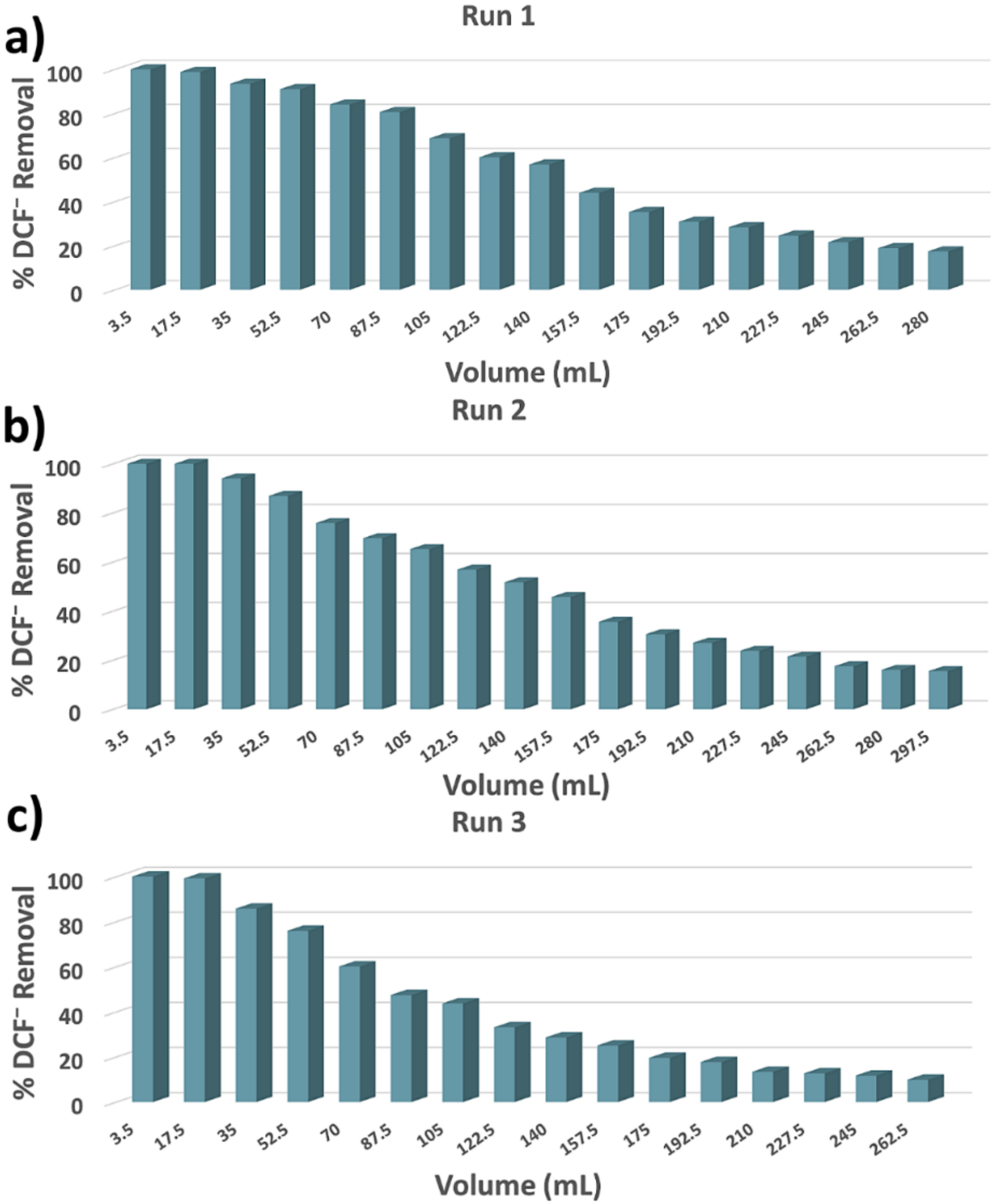
3.2. Column Sorption Study
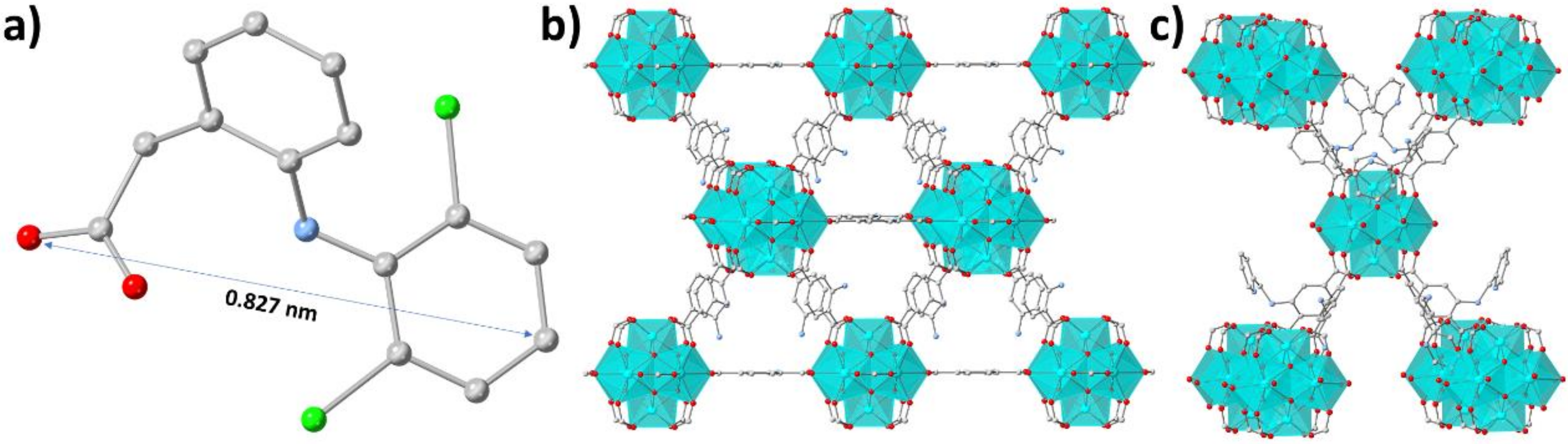
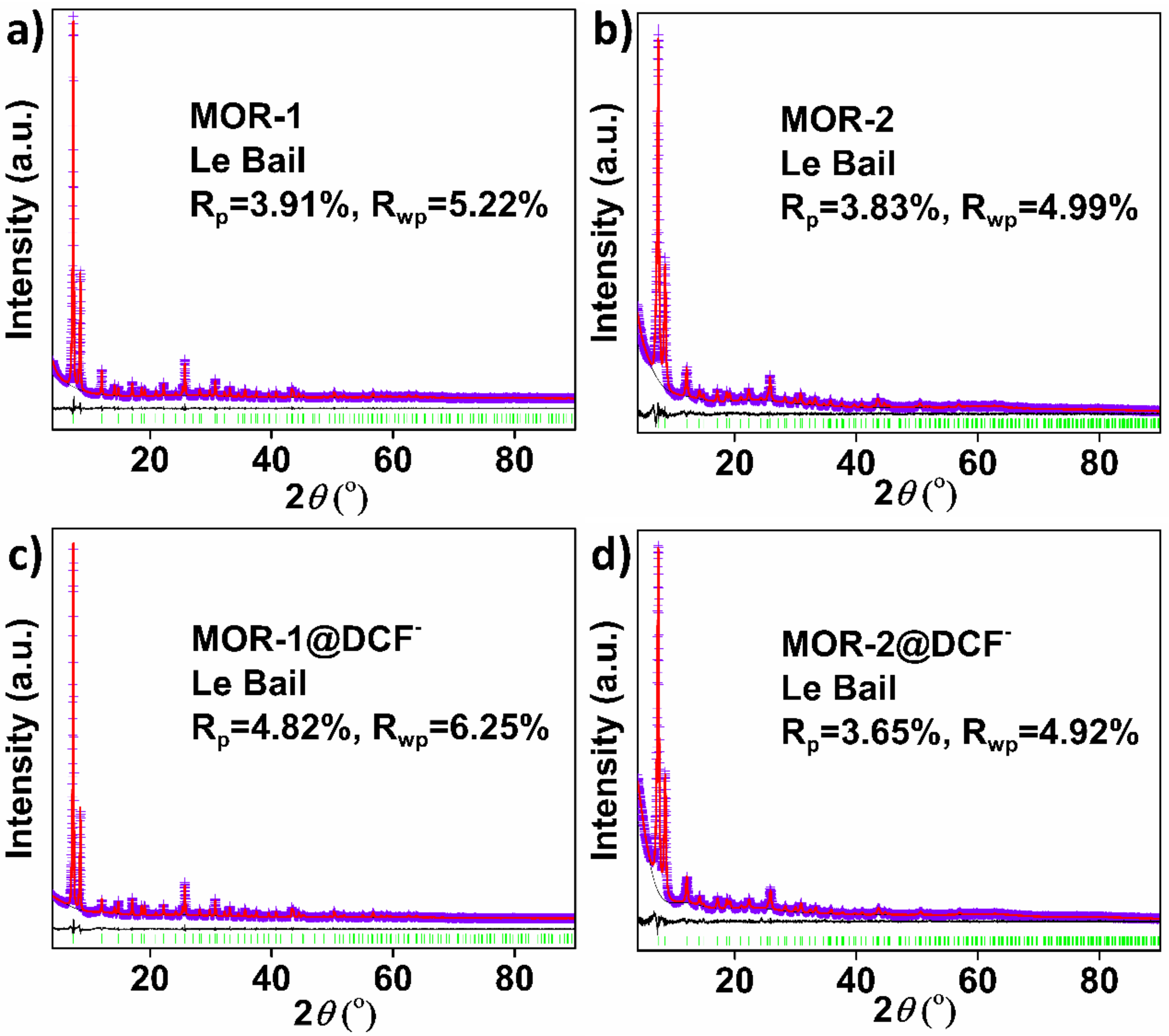
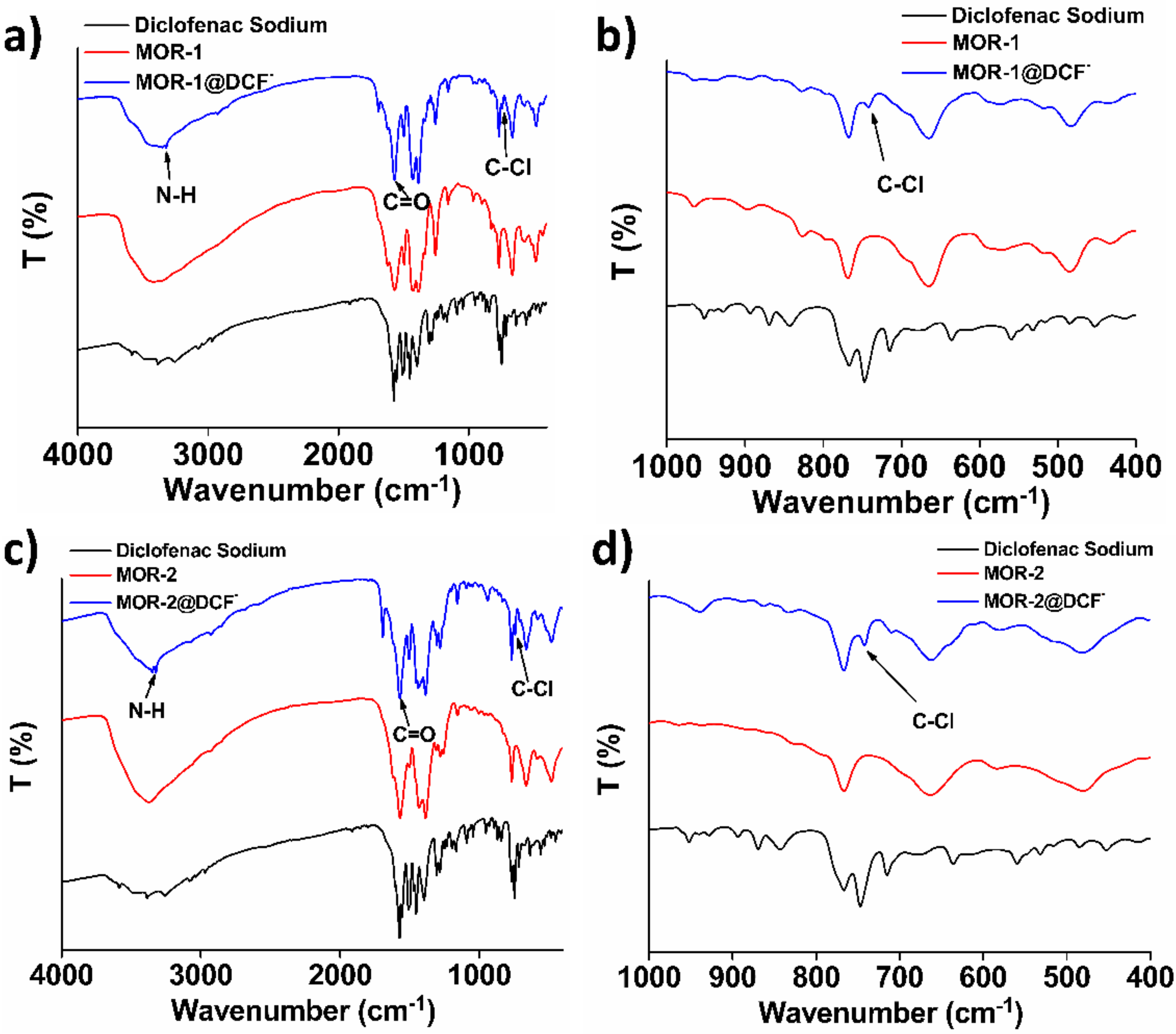
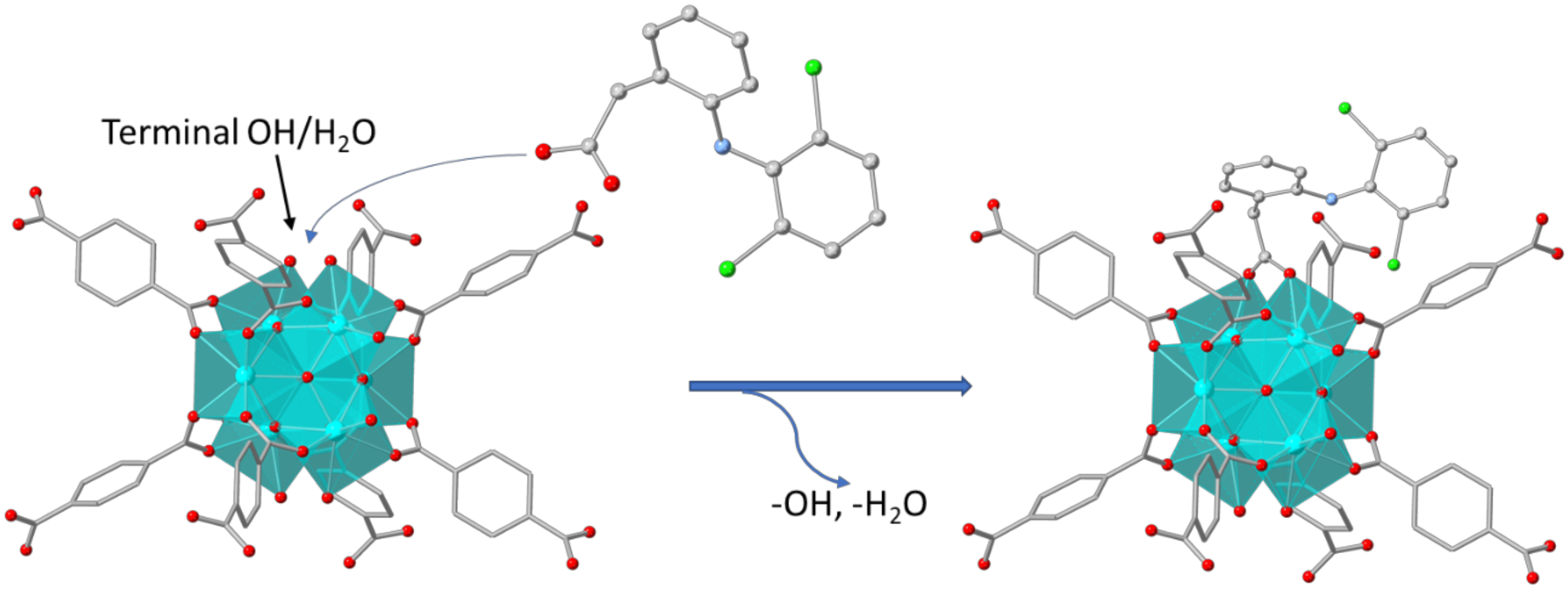
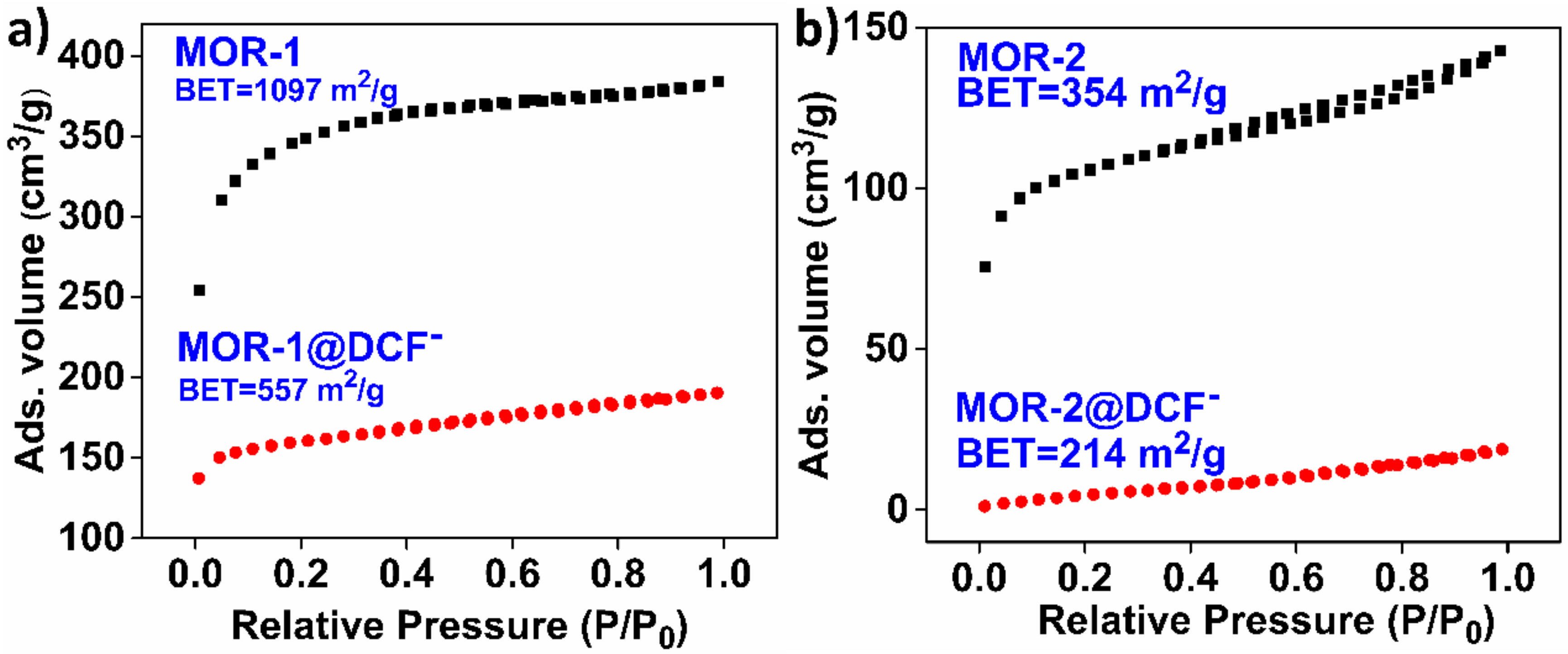
3.3. Characterization of the DSF−-Loaded MOFs Mechanism of the DCF− Sorption by MOR-1 and MOR-2
3.4. Comparison of MOR-1 and MOR-2 with Other MOF-Based Sorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 82, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P. The adverse effects of estrogenic pill driven after flexible fertility on environment in covid-19 situation. Eng. Sci. 2021, 14, 109–113. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Zembrzuska, J.; Bornikowska, J.; Wojciechowska, A.; Wojciechowska, I. Removal of naproxen from water by ionic liquid-modified polymer sorbents. Chem. Eng. Res. Des. 2017, 117, 698–705. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.M.; Xu, Z.C.; Guo, Q.W.; Hong, C.Y.; Bing, Y.X. Removal of naproxen and bezafibrate by activated sludge under aerobic conditions: Kinetics and effect of substrates. Biotechnol. Appl. Biochem. 2014, 61, 333–341. [Google Scholar] [CrossRef]
- Rivera-Jiménez, S.M.; Méndez-González, S.; Hernández-Maldonado, A. Metal (M = Co2+, Ni2+, and Cu2+) grafted mesoporous SBA-15: Effect of transition metal incorporation and pH conditions on the adsorption of Naproxen from water. Microporous Mesoporous Mater. 2010, 132, 470–479. [Google Scholar] [CrossRef]
- Boyd, G.R.; Reemtsma, H.; Grimm, D.A.; Mitra, S. Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci. Total Environ. 2003, 311, 135–149. [Google Scholar] [CrossRef]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Frimmel, F.H. Short-term tests with a pilot sewage plant and biofilm reactors for the biological degradation of the pharmaceutical compounds clofibric acid, ibuprofen, and diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Buser, H.R.; Poiger, T.; Müller, M.D. Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: Rapid photodegradation in a lake. Environ. Sci. Technol. 1998, 32, 3449–3456. [Google Scholar] [CrossRef]
- Boyd, G.R.; Zhang, S.; Grimm, D.A. Naproxen removal from water by chlorination and biofilm processes. Water Res. 2005, 39, 668–676. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Song, J.Y.; Jhung, S.H. Adsorption of pharmaceuticals and personal care products over metal-organic frameworks functionalized with hydroxyl groups: Quantitative analyses of H-bonding in adsorption. Chem. Eng. J. 2017, 322, 366–374. [Google Scholar] [CrossRef]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209–210, 151–157. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Sánchez-Polo, M.; Messoud, J.; Velo-Gala, I.; Ocampo-Pérez, R.; Rivera-Utrilla, J. Overall adsorption rate of metronidazole, dimetridazole and diatrizoate on activated carbons prepared from coffee residues and almond shells. J. Environ. Manag. 2016, 169, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Díaz, J.D.; Prados-Joya, G.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Ferro-García, M.A.; Medellín-Castillo, N.A. Kinetic study of the adsorption of nitroimidazole antibiotics on activated carbons in aqueous phase. J. Colloid Interface Sci. 2010, 345, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jiménez, S.M.; Hernández-Maldonado, A.J. Nickel (II) grafted MCM-41: A novel sorbent for the removal of Naproxen from water. Microporous Mesoporous Mater. 2008, 116, 246–252. [Google Scholar] [CrossRef]
- Domínguez, J.R.; González, T.; Palo, P.; Cuerda-Correa, E.M. Removal of common pharmaceuticals present in surface waters by Amberlite XAD-7 acrylic-ester-resin: Influence of pH and presence of other drugs. Desalination 2011, 269, 231–238. [Google Scholar] [CrossRef]
- Rahardjo, A.K.; Susanto, M.J.J.; Kurniawan, A.; Indraswati, N.; Ismadji, S. Modified Ponorogo bentonite for the removal of ampicillin from wastewater. J. Hazard. Mater. 2011, 190, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Liu, W.; Shen, X.; Han, Y.; Liu, Z.; Dai, W.; Dutta, A.; Kumar, A.; Liu, J. Selective adsorption and removal of drug contaminants by using an extremely stable Cu (II)-based 3D metal-organic framework. Chemosphere 2019, 215, 524–531. [Google Scholar] [CrossRef]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Ateia, M. Decorating graphene oxide with zeolitic imidazolate framework (ZIF-8) and pseudo-boehmite offers ultra-high adsorption capacity of diclofenac in hospital effluents. Chemosphere 2021, 271, 129610. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, J. Adsorptive removal of pharmaceutical pollutants by defective metal organic framework UiO-66: Insight into the contribution of defects. Chemosphere 2021, 281, 130997. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, N.; Li, K. MOF-808 and its hollow fibre adsorbents for efficient diclofenac removal. Chem. Eng. J. 2021, 417, 129216. [Google Scholar] [CrossRef]
- Yu, S.; Wan, J.; Chen, K. A facile synthesis of superparamagnetic Fe3O4 supraparticles@MIL-100(Fe) core-shell nanostructures: Preparation, characterization and biocompatibility. J. Colloid Interface Sci. 2016, 461, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, R.; Shuai, Q. Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: A review. J. Environ. Manag. 2021, 277, 111389. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Burton, F.L.; dan Stensel, H.D. Waste Water Engineering: Treatment and Reuse, 4th ed.; Metcalf & Eddy, Inc.: Boston, MA, USA, 2003. [Google Scholar]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Tsipis, A.C.; Lazarides, T.; Kanatzidis, M.G.; Manos, M.J. Selective capture of hexavalent chromium from an anion-exchange column of metal organic resin–alginic acid composite. Chem. Sci. 2016, 7, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Rapti, S.; Sarma, D.; Diamantis, S.A.; Skliri, E.; Armatas, G.S.; Tsipis, A.C.; Hassan, Y.S.; Alkordi, M.; Malliakas, C.D.; Kanatzidis, M.G.; et al. All in one porous material: Exceptional sorption and selective sensing of hexavalent chromium by using a Zr4+ MOF. J. Mater. Chem. A 2017, 5, 14707–14719. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galban-Malag, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Benhammou, A.; Yaacoubi, A.; Nibou, L.; Tanouti, B. Adsorption of metal ions onto Moroccan stevensite: Kinetic and isotherm studies. J. Colloid Interface Sci. 2005, 282, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.J.; Kanatzidis, M.G. Sequestration of heavy metals from water with layered metal sulfides. Chem. A Eur. J. 2009, 15, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.J.; Ding, N.; Kanatzidis, M.G. Layered metal sulfides: Exceptionally selective agents for radioactive strontium removal. Proc. Natl. Acad. Sci. USA 2008, 105, 3696–3699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapti, S.; Pournara, A.; Sarma, D.; Papadas, I.T.; Armatas, G.S.; Hassan, Y.S.; Alkordi, M.H.; Kanatzidis, M.G.; Manos, M.J. Rapid, green and inexpensive synthesis of high quality UiO-66 amino-functionalized materials with exceptional capability for removal of hexavalent chromium from industrial waste. Inorg. Chem. Front. 2016, 3, 635–644. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Wang, X.; Rasmussen, J.; Li, Z.; Zhu, J. Organokaolin for the uptake of pharmaceuticals diclofenac and chloramphenicol from water. Chem. Eng. J. 2017, 330, 1128–1136. [Google Scholar] [CrossRef]
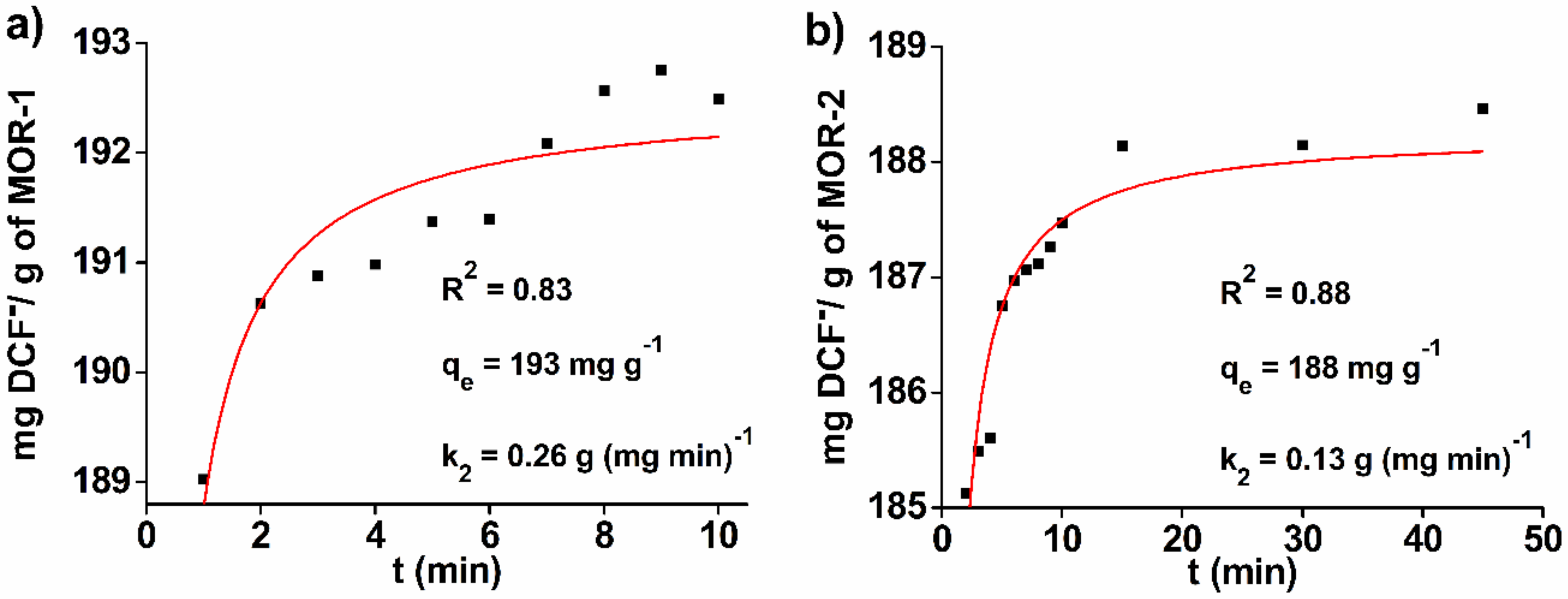
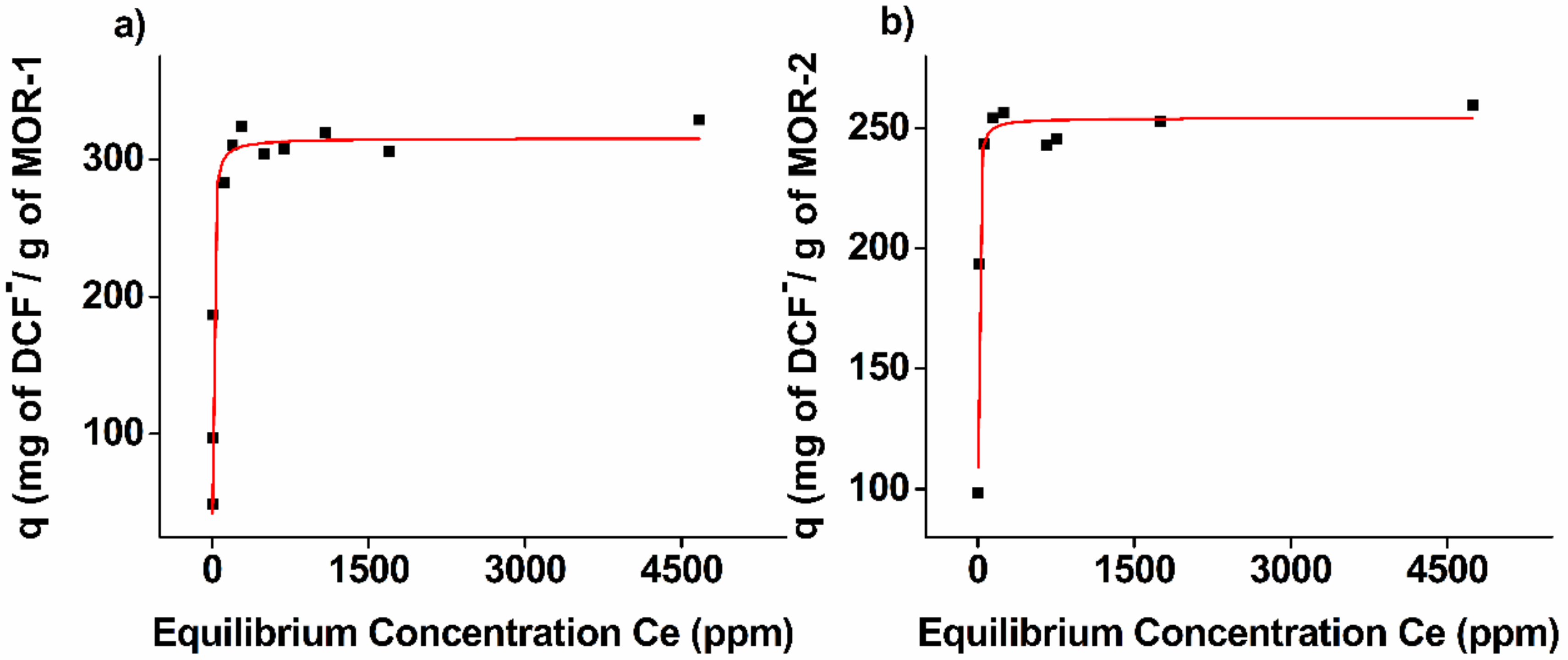

| Sorbent | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|
| KL(min −1) | qe1 (mg g−1) | R2 | k2 (g mg−1 min−1) | qe2 (mg g−1) | R2 | |
| MOR-1 | 4.26 | 192 | 0.5 | 0.26 | 192 | 0.83 |
| MOR-2 | 2.19 | 187 | 0.28 | 0.13 | 188 | 0.88 |
| Sorbent | Langmuir | Freundlich | LF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe (mg/g) | b (L/mg) | R2 | KF (L/g) | n | R2 | qe (mg/g) | b (L/mg) | n | R2 | |
| MOR-1 | 315 ± 4 | 0.18 ± 0.02 | 0.99 | 132 ±24 | 7.9 ± 1.8 | 0.76 | 318 ± 5 | 0.17 ± 0.02 | 1.12 ± 0.13 | 0.99 |
| MOR-2 | 254 ± 3 | 0.4 ± 0.05 | 0.97 | 157 ± 23 | 14 ± 4.8 | 0.57 | 252 ± 3 | 0.39 ± 0.03 | 0.73 ± 0.08 | 0.99 |
| MOF-Based Sorbent | Capacity mg/g | Equilibrium Time | Selectivity vs. | Reusability | Column Study | Ref. |
|---|---|---|---|---|---|---|
| Fe3O4@MIL-100(Fe) | 400 | 250 min | NA | NA | NA | [29] |
| [Cu(BTTA)]n•2DMF | 650 | 450 min | NA | Reusable | NA | [30] |
| Magnetic GO/ZIF-8/g-AlOOH-NC | 2594.3 | 50 min | NA | Reusable | NA | [31] |
| Defective UiO-66 | 321 | 120 min | Various pharmaceutical pollutants | NA | NA | [32] |
| MOF-808 | 833 | 5 h | NA | NA | NA | [33] |
| Fe3O4@MOF-100(Fe) | 377.36 | 24 h | NA | Reusable | NA | [34] |
| MOR-1 | 315 | 5 min | Cl−, NO3−, SO42− | Reusable | 3 runs | This work |
| MOR-2 | 254 | 5 min | Cl−, NO3−, SO42− | Reusable | NA | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions. Crystals 2022, 12, 424. https://doi.org/10.3390/cryst12030424
Pournara AD, Andreou EK, Armatas GS, Manos MJ. Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions. Crystals. 2022; 12(3):424. https://doi.org/10.3390/cryst12030424
Chicago/Turabian StylePournara, Anastasia D., Evangelos K. Andreou, Gerasimos S. Armatas, and Manolis J. Manos. 2022. "Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions" Crystals 12, no. 3: 424. https://doi.org/10.3390/cryst12030424
APA StylePournara, A. D., Andreou, E. K., Armatas, G. S., & Manos, M. J. (2022). Zirconium(IV) Metal Organic Frameworks with Highly Selective Sorption for Diclofenac under Batch and Continuous Flow Conditions. Crystals, 12(3), 424. https://doi.org/10.3390/cryst12030424







