Synthesis, X-ray Single-Crystal Analysis, and Anticancer Activity Evaluation of New Alkylsulfanyl-Pyridazino[4,5-b]indole Compounds as Multitarget Inhibitors of EGFR and Its Downstream PI3K-AKT Pathway
Abstract
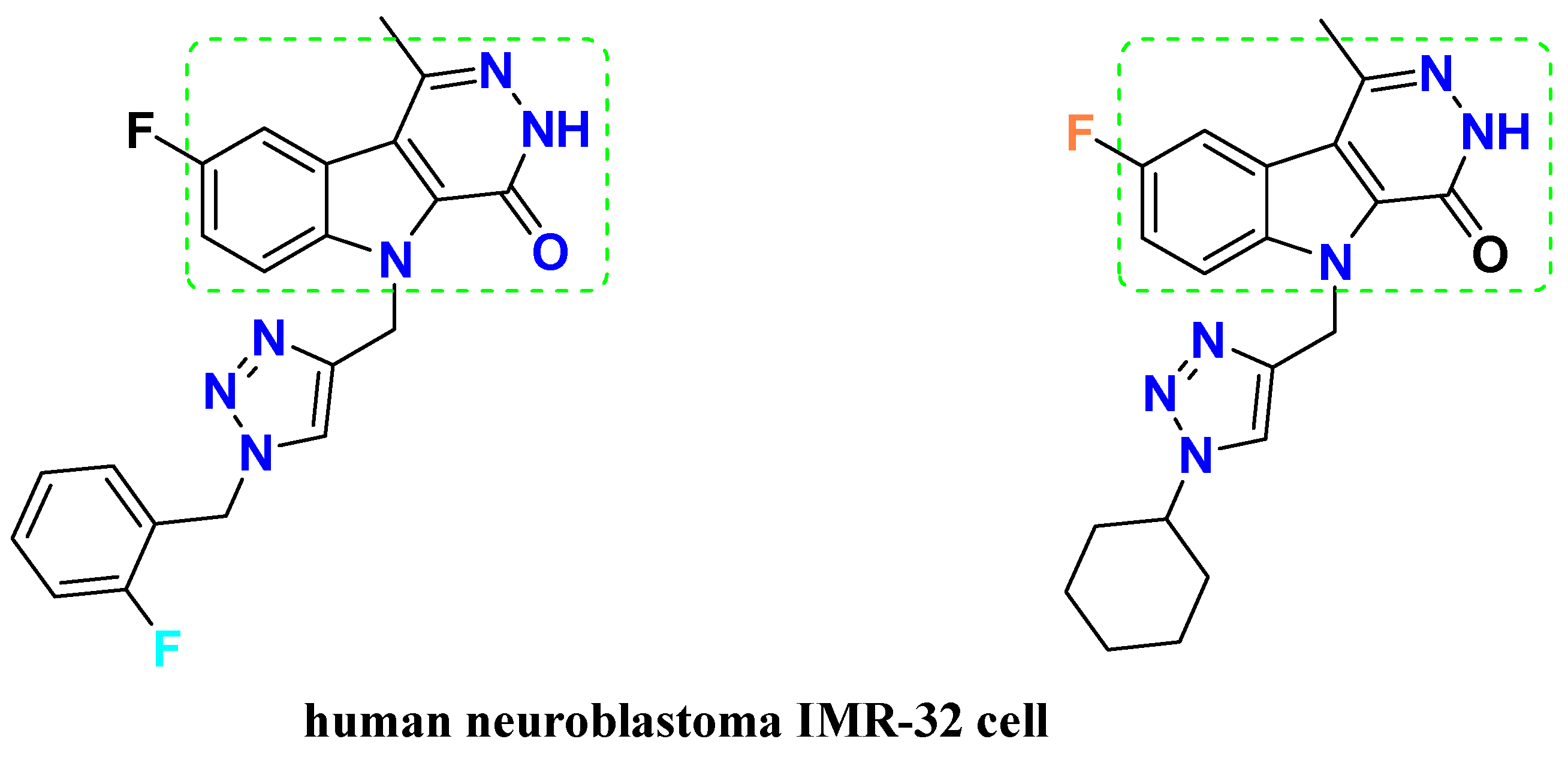
:1. Introduction
2. Materials and Methods
2.1. General Procedure
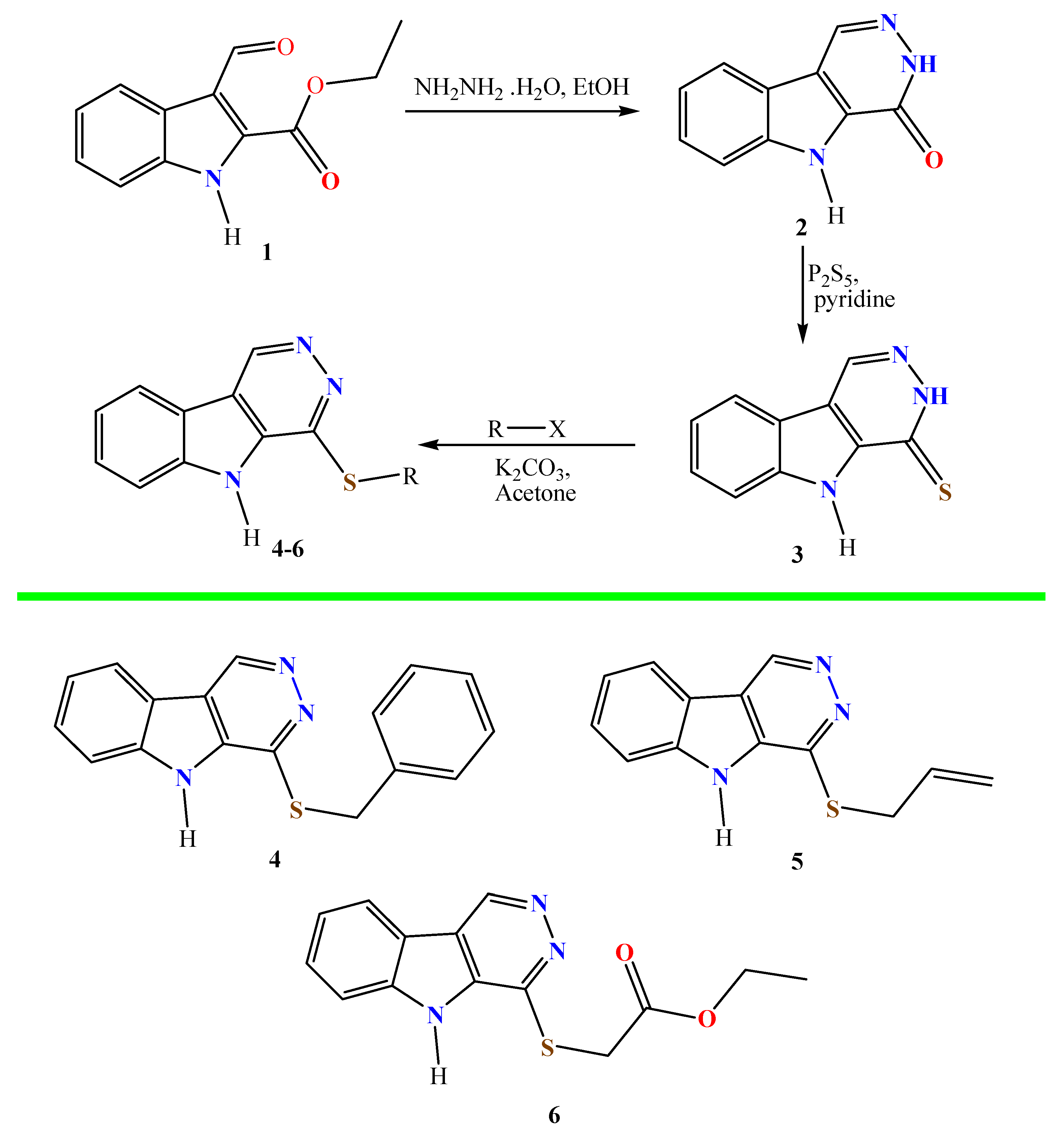
2.2. Synthesis
2.2.1. Synthesis of 3,5-Dihydro-4H-Pyridazino[4,5-b]indol-4-One (2)
2.2.2. Synthesis of 3,5-Dihydro-4H-Pyridazino[4,5-b]indole-4-Thione (3)
2.2.3. Alkylation of Pyridazino[4,5-b]indole-4-Thione (3)
- 4-(Benzylsulfanyl)-5H-Pyridazino[4,5-b]indole (4)
- 4-(Allylsulfanyl)-5H-Pyridazino[4,5-b]indole (5)
- Ethyl 2-((5H-Pyridazino[4,5-b]indol-4-yl)sulfanyl)acetate (6)
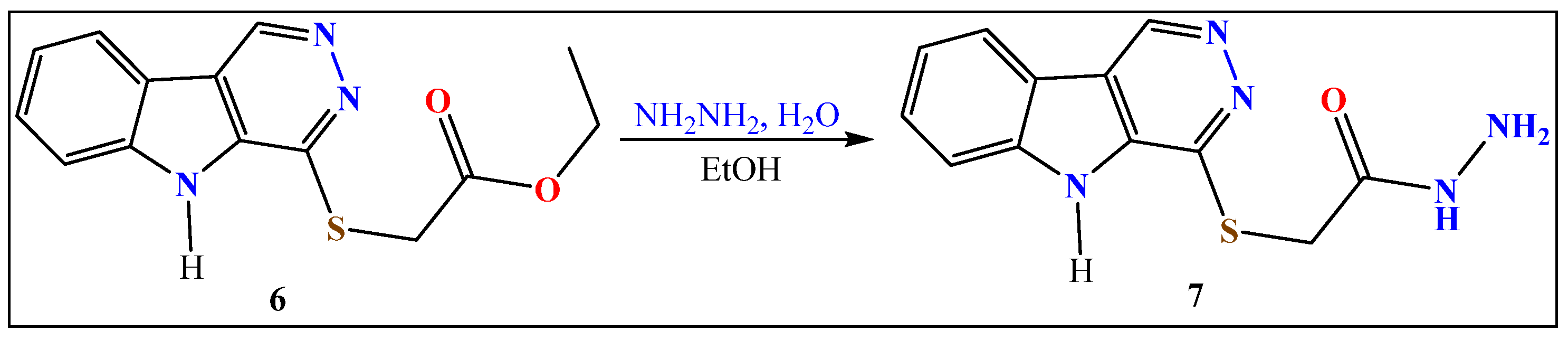
2.2.4. Hydrazinolysis of Ester 6
- 2-((5H-Pyridazino[4,5-b]indol-4-yl)thio)acetohydrazide (7)
2.3. Biology
2.3.1. Cytotoxicity
2.3.2. Enzymatic Targeting
2.3.3. Apoptosis Investigation
2.4. Molecular Docking
3. Results and Discussion
3.1. Chemistry
3.2. X-ray Single-Crystal Analysis and Structural Determinations
3.3. Biology
3.3.1. Cytotoxic Activity
3.3.2. Enzymatic Targeting
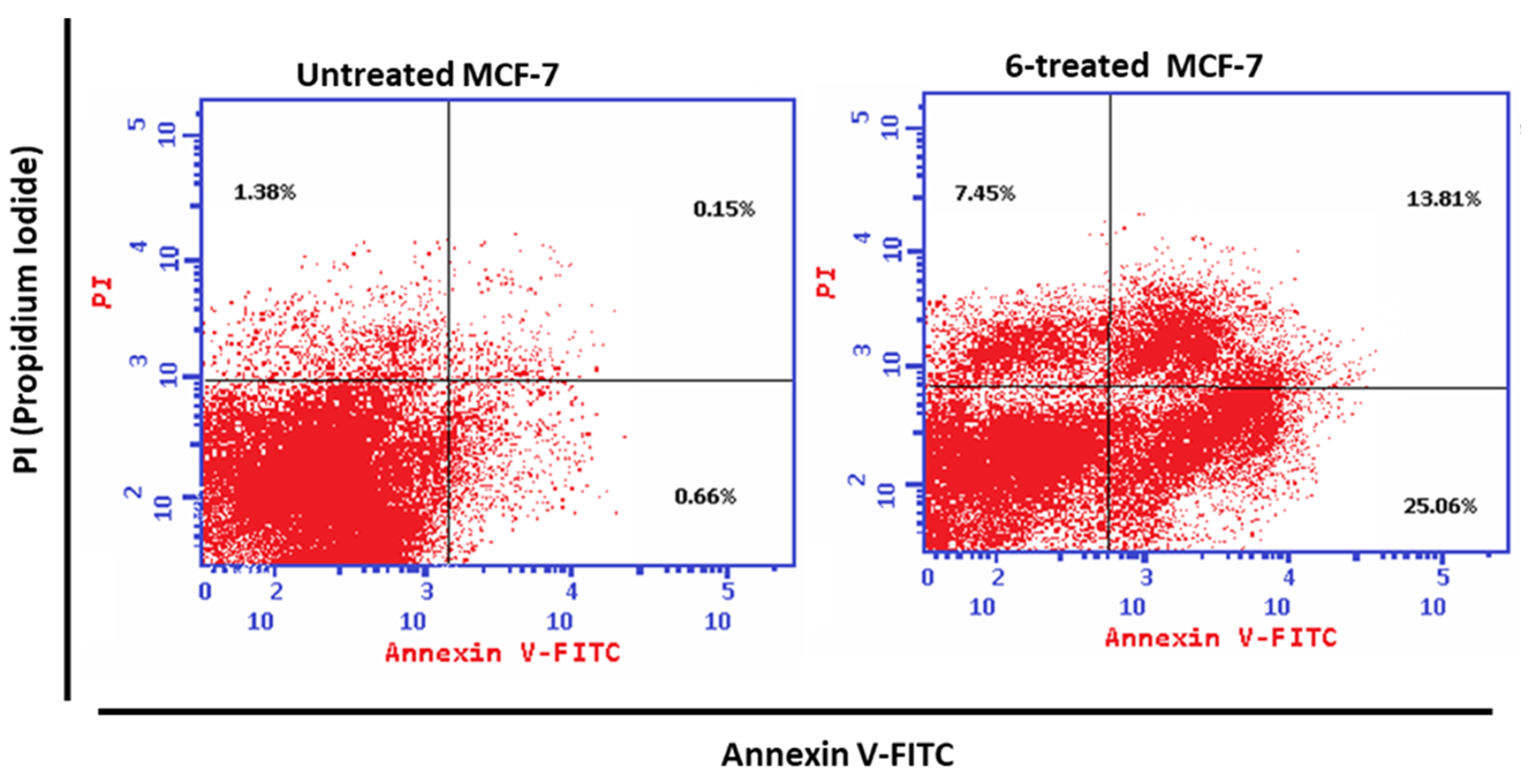
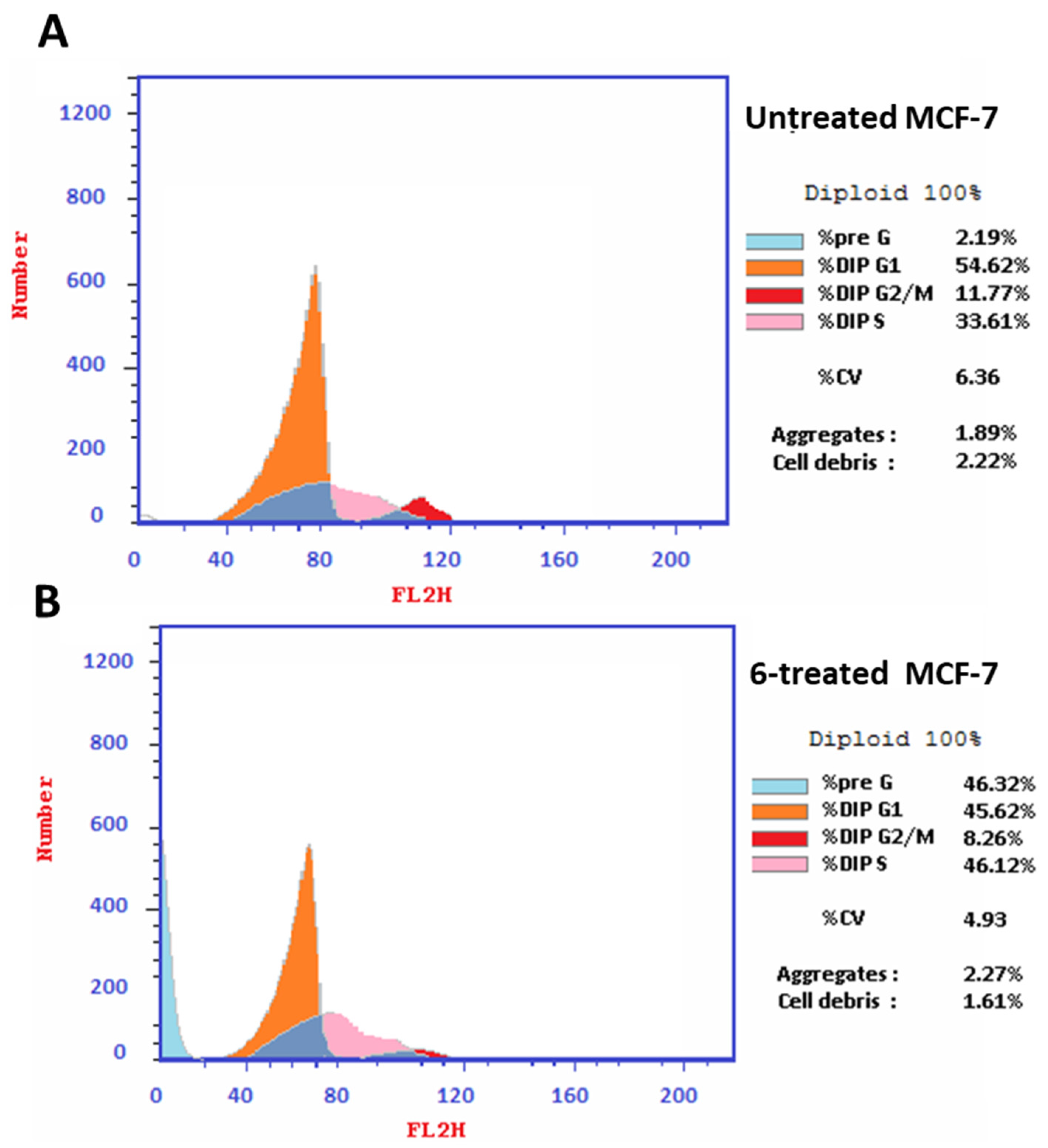
3.3.3. Apoptotic Investigation
- Annexin V/PI Staining
- Cell Cycle Analysis
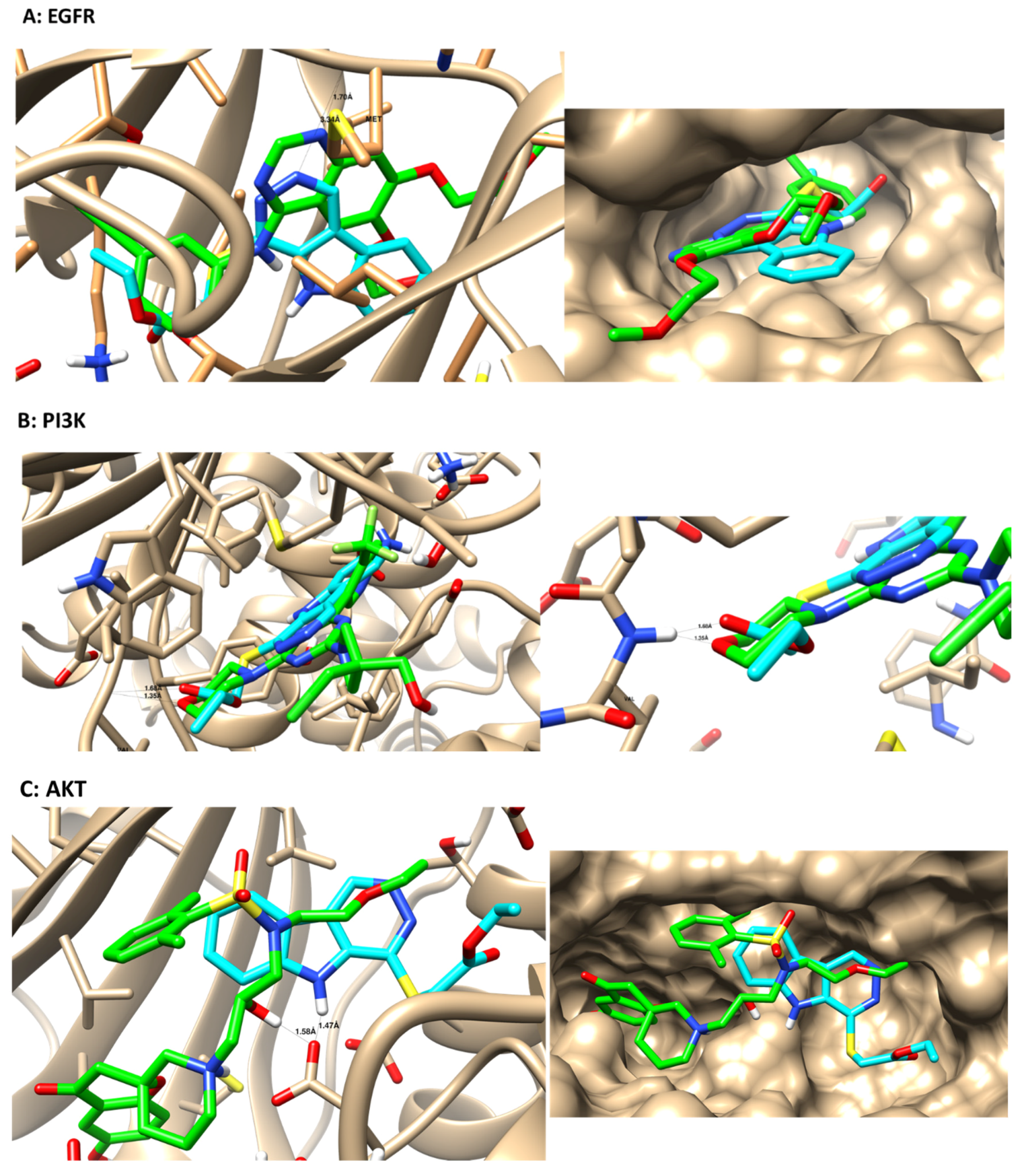
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Thiabat, M.G.; Saqallah, F.G.; Gazzalii, A.M.; Mohtar, N.; Yap, B.K.; Choong, Y.S.; Wahab, H.A. Heterocyclic Substitutions Greatly Improve Affinity and Stability of Folic Acid towards FRα. an In Silico Insight. Molecules 2021, 26, 1079. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel, A.; Bénéteau, R.; Chabanne, M.; Lozach, O.; Le Guevel, R.; Ravache, M.; Bénédetti, H.; Meijer, L.; Logé, C.; Robert, J.M. Synthesis of new pyridazino[4,5-b]indol-4-ones and pyridazin-3(2H)-one analogs as DYRK1A inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, P.V.; Fernandes, A.R. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar]
- Sravanthi, T.; Manju, S. Indoles—A promising scaffold for drug development. Eur. J. Pharm. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef]
- Panathur, N.; Gokhale, N.; Dalimba, U.; Venkat Koushik, P.; Yogeeswari, P.; Sriram, D. Synthesis of novel 5-[(1,2,3-triazol-4-yl)methyl]-1-methyl-3H-pyridazino[4,5-b]indol-4-one derivatives by click reaction and exploration of their anticancer activity. Med. Chem. Res. 2016, 25, 135–148. [Google Scholar] [CrossRef]
- Monge, A.; Aldana, I.; Alvarez, T.; Losa, M.J.; Font, M.; Cenarruzabeitia, E.; Lasheras, B.; Frechilla, D.; Castiella, E.; Fernandez-Alvarez, E. 1-Hydrazino-4-(3,5-dimethyl-1-pyrazolyl)-5H-pyridazino[4,5-b]indole. A new antihypertensive agent. Eur. J. Med. Chem. 1991, 26, 655–658. [Google Scholar] [CrossRef]
- Font, M.; Monge, A.; Cuartero, A.; Elorriaga, A.; Martínez-Irujo, J.J.; Alberdi, E.; Santiago, E.; Prieto, I.; Lasarte, J.J.; Sarobe, P.; et al. Indoles and pyridazino[4,5-b]indoles as nonnucleoside analog inhibitors of HIV-1 reverse transcriptase. Eur. J. Med. Chem. 1995, 30, 963–971. [Google Scholar] [CrossRef]
- Bruel, A.; Logé, C.; De Tauzia, M.-L.; Ravache, M.; Le Guevel, R.; Guillouzo, C.; Lohier, J.-F.; Santos, J.S.O.; Lozach, O.; Meijer, L.; et al. Synthesis and biological evaluation of new 5-benzylated 4-oxo-3,4-dihydro-5H-pyridazino[4,5-b]indoles as PI3Kα inhibitors. Eur. J. Med. Chem. 2012, 57, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Monge, A.; Aldana, I.; Alvarez, T.; Font, M.; Santiago, E.; Latre, J.A.; Bermejillo, M.J.; Lopez-Unzu, M.J.; Fernandez-Alvarez, E. New 5H-pyridazino[4,5-b]indole derivatives. Synthesis and studies as inhibitors of blood platelet aggregation and inotropics. J. Med. Chem. 1991, 34, 3023. [Google Scholar] [CrossRef] [PubMed]
- Nantka-Namirski, P.; Ozdowska, Z. 2-Carbethoxyindole derivatives. I. Synthesis of 8-alkoxy- and 8,9-benzo-3H-pyridazino(4,5-b) indol-4-ones. Acta Pol. Pharm. 1972, 29, 9–15. [Google Scholar] [PubMed]
- Evanno, Y.; Dubois, L.; Sevrin, M.; Marguet, F.; Froissant, J.; Bartsch, R.; Gille, C. 4-oxo-3,5-dihydro-4H-pyridazino[4,5-b]indole-1acetamide derivatives (CA 2298522 A1). Chem. Abstr. 1999, 130, 68385. [Google Scholar]
- Radini, I.; El-Kashef, H.; Haider, N.; Farghaly, A.-R. Synthesis and functionalization of some new pyridazino[4,5-b]indole derivatives. ARKIVOC 2016, 101, 117. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, A.; Drozdowska, D. Recent Design and Structure-Activity Relationship Studies on the Modifications of DHFR Inhibitors as Anticancer Agents. Curr. Med. Chem. 2021, 28, 910–939. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR inhibitors: Reading the past for discovering novel anticancer agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jendele, L.; Krivak, R.; Skoda, P.; Novotny, M.; Hoksza, D. PrankWeb: A web server for ligand binding site prediction and visualization. Nucleic Acids Res. 2019, 47, W345–W349. [Google Scholar] [CrossRef] [Green Version]
- Vin, V.; Leducq, N.; Bono, F.; Herbert, J.M. Binding characteristics of SSR180575, a potent and selective peripheral benzodiazepine ligand. Biochem. Biophys. Res. Commun. 2003, 310, 785–790. [Google Scholar] [CrossRef]
- Avan, I.; Güven, A.; Güven, K. Synthesis and antimicrobial investigation of some 5H-pyridazino[4,5-b]indoles. Turk. J. Chem. 2013, 37, 271–291. [Google Scholar]
- Vasir, J.K.; Labhasetwar, V. Targeted Drug Delivery in Cancer Therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef]
- Salama, E.E. Synthesis of new 2-amino-1,3,4-oxadiazole derivatives with anti-salmonella typhi activity evaluation. BMC Chem. 2020, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Zaryouh, H.; De Pauw, I.; Baysal, H.; Peeters, M.; Vermorken, J.B.; Lardon, F.; Wouters, A. Recent insights in the PI3K/Akt pathway as a promising therapeutic target in combination with EGFR-targeting agents to treat head and neck squamous cell carcinoma. Med. Res. Rev. 2022, 42, 112–155. [Google Scholar] [CrossRef]
- Fedele, M.; Cerchia, L.; Chiappetta, G. The epithelial-to-mesenchymal transition in breast cancer: Focus on basal-like carcinomas. Cancers 2017, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.Y.; Shanmugam, M.K.; Sethi, G.; Bishayee, A. Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anti-Cancer Drugs 2016, 27, 147–155. [Google Scholar] [CrossRef]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.; Hartman, M.J. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kumar, S.; Narasimhan, B. Estrogen alpha receptor antagonists for the treatment of breast cancer: A review. Chem. Cent. J. 2018, 12, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ming, B.; Gong, G.H.; Wang, D.; Bao, G.L.; Yu, L.J. Current research on anti-breast cancer synthetic compounds. RSC Adv. 2018, 8, 4386–4416. [Google Scholar]
- Mostafa, A.S.; Gomaa, R.M.; Elmorsy, M.A. Design and synthesis of 2-phenyl benzimidazole derivatives as VEGFR-2 inhibitors with anti-breast cancer activity. Chem. Biol. Drug Des. 2019, 93, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Branowska, D.; Lawecka, J.; Sobiczewski, M.; Karczmarzyk, Z.; Wysocki, W.; Wolińska, E.; Olender, E.; Miroslaw, B.; Perzyna, A.; Bielawska, A.; et al. Synthesis of unsymmetrical disulfanes bearing 1,2,4-triazine scaffold and their in vitro screening towards anti-breast cancer activity. Mon. Chem.-Chem. Mon. 2018, 149, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ElZahabi, H.S.A.; Nafie, M.S.; Osman, D.; Elghazawy, N.H.; Soliman, D.H.; EL-Helby, A.A.H.; Arafa, R.K. Design, Synthesis and Evaluation of New Quinazolin-4-One Derivatives as Apoptotic Enhancers and Autophagy Inhibitors with Potent Antitumor Activity. Eur. J. Med. Chem. 2021, 222, 113609. [Google Scholar] [CrossRef] [PubMed]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.S.A.G.; Barakat, A.; Boraei, A.T.A. Discovery of New Apoptosis-Inducing Agents for Breast Cancer Based on Ethyl 2-Amino-4,5,6,7-Tetra Hydrobenzo[b]Thiophene-3-Carboxylate: Synthesis, In Vitro, and In Vivo Activity Evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; Soliman, S.M.; Haukka, M.; Salama, E.E.; Sopaih, M.; Barakat, A.; Sarhan, A.A.M. Straightforward green synthesis of indeno-furan carboxylates from ninhydrin and β-ketoesters: X-ray crystal structure, Hirshfeld and DFT investigations. J. Mol. Struct. 2022, 12255, 132433. [Google Scholar] [CrossRef]
- Sarhan, A.A.M.; Boraei, A.T.A.; Barakat, A.; Nafie, M.S. Discovery of Hydrazide-Based Pyridazino[4,5-b]Indole Scaffold as a New Phosphoinositide 3-Kinase (PI3K) Inhibitor for Breast Cancer Therapy. RSC Adv. 2020, 10, 19534–19541. [Google Scholar] [CrossRef]
- Nafie, M.S.; Amer, A.M.; Mohamed, A.K.; Tantawy, E.S. Discovery of Novel Pyrazolo[3,4-b]Pyridine Scaffold-Based Derivatives as Potential PIM-1 Kinase Inhibitors in Breast Cancer MCF-7 Cells. Bioorg. Med. Chem. 2020, 28, 115828. [Google Scholar] [CrossRef]
- Nafie, M.S.; Arafa, K.; Sedky, N.K.; Alakhdar, A.A.; Arafa, R.K. Triaryl Dicationic DNA Minor-Groove Binders with Antioxidant Activity Display Cytotoxicity and Induce Apoptosis in Breast Cancer. Chem.-Biol. Interact. 2020, 324, 109087. [Google Scholar] [CrossRef] [PubMed]
- Nafie, M.S.; Khodair, A.I.; Hassan, H.A.Y.; El-Fadeal, N.M.A.; Bogari, H.A.; Elhady, S.S.; Ahmed, S.A. Evaluation of 2-Thioxoimadazolidin-4-One Derivatives as Potent Anti-Cancer Agents through Apoptosis Induction and Antioxidant Activation: In Vitro and In Vivo Approaches. Molecules 2022, 27, 83. [Google Scholar] [CrossRef] [PubMed]
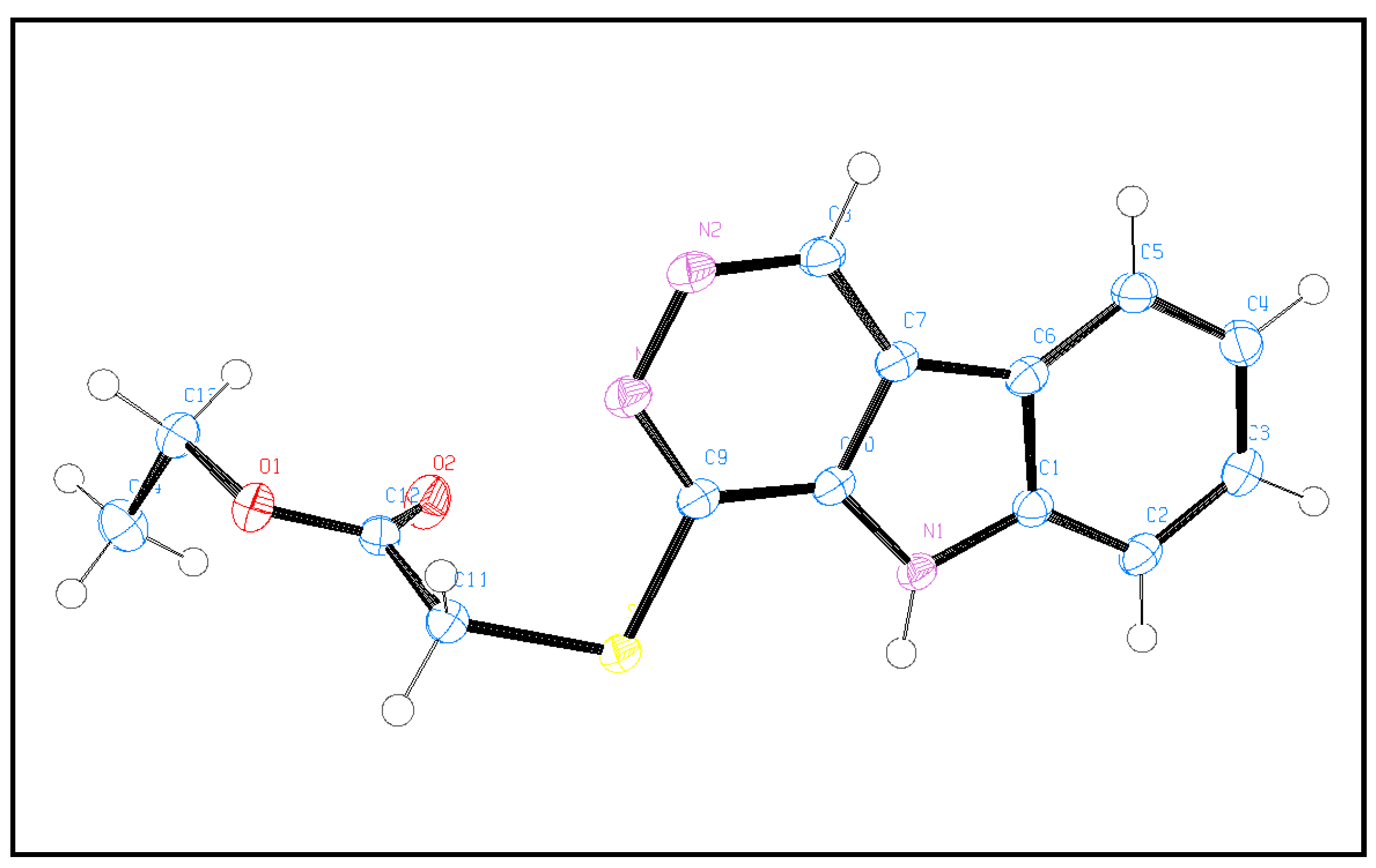
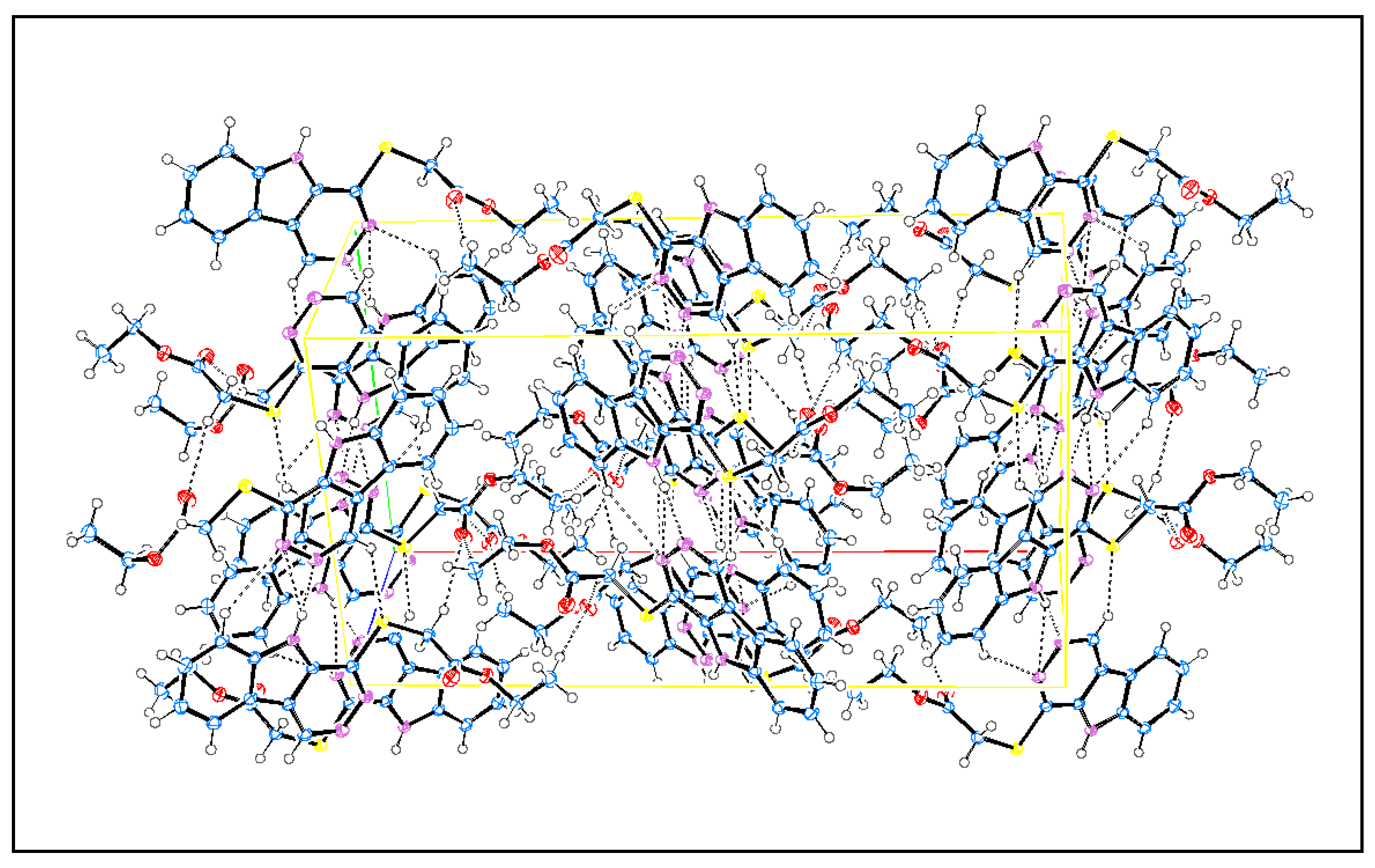
| Compound | 6 |
| CCDC | 2133074 |
| Empirical formula | C14H13N3O2S |
| FW | 287.33 |
| Temp (K) | 120 (2) |
| λ (Å) | 1.54184 |
| Crystal system | Orthorhombic |
| Space group | Pccn |
| a (Å) | 23.7290 (2) |
| b (Å) | 13.06630 (10) |
| c (Å) | 8.45260 (10) |
| V (Å3) | 2620.73 (4) |
| Z | 8 |
| ρcalc (Mg/m3) | 1.456 |
| μ(Mo Kα) (mm−1) | 2.248 |
| No. reflns. | 31606 |
| Unique reflns. | 2768 |
| Completeness to θ = 67.684° | 100.0% |
| GOOF (F2) | 1.038 |
| Rint | 0.0335 |
| R1 a (I ≥ 2σ) | 0.0284 |
| wR2 b (I ≥ 2σ) | 0.0764 |
| Compound | IC50 ± SD *,# (µM)/MCF7 | IC50 ± SD *,# (µM)/MCF-10A |
|---|---|---|
| (1) | 19.7 ± 2.31 | - |
| (2) | 37.5 ± 1.95 | - |
| (3) | 94.3 ± 4.9 | - |
| (4) | 20.4 ± 1.06 | - |
| (6) | 12.0 ± 0.63 | 75.13 ± 1.87 |
| (7) | 39.9 ± 2.07 | - |
| Staurosporin | 8.32 ± 0.43 | 21.43 ± 1.06 |
| Compound | EGFR (pg/mL) * | PI3K (ng/mL) * | AKT (ng/mL) * |
|---|---|---|---|
| Control | 730 ± 62.2 | 6.638 ± 0.28 | 45.39 ± 3.74 |
| Treated with 6 | 191.3 ± 7.3 | 3.28 ± 0.09 | 14.06 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, E.E.; Althobaiti, I.O.; Haukka, M.; Boraei, A.T.A. Synthesis, X-ray Single-Crystal Analysis, and Anticancer Activity Evaluation of New Alkylsulfanyl-Pyridazino[4,5-b]indole Compounds as Multitarget Inhibitors of EGFR and Its Downstream PI3K-AKT Pathway. Crystals 2022, 12, 353. https://doi.org/10.3390/cryst12030353
Salama EE, Althobaiti IO, Haukka M, Boraei ATA. Synthesis, X-ray Single-Crystal Analysis, and Anticancer Activity Evaluation of New Alkylsulfanyl-Pyridazino[4,5-b]indole Compounds as Multitarget Inhibitors of EGFR and Its Downstream PI3K-AKT Pathway. Crystals. 2022; 12(3):353. https://doi.org/10.3390/cryst12030353
Chicago/Turabian StyleSalama, Eid E., Ibrahim O. Althobaiti, Matti Haukka, and Ahmed T. A. Boraei. 2022. "Synthesis, X-ray Single-Crystal Analysis, and Anticancer Activity Evaluation of New Alkylsulfanyl-Pyridazino[4,5-b]indole Compounds as Multitarget Inhibitors of EGFR and Its Downstream PI3K-AKT Pathway" Crystals 12, no. 3: 353. https://doi.org/10.3390/cryst12030353
APA StyleSalama, E. E., Althobaiti, I. O., Haukka, M., & Boraei, A. T. A. (2022). Synthesis, X-ray Single-Crystal Analysis, and Anticancer Activity Evaluation of New Alkylsulfanyl-Pyridazino[4,5-b]indole Compounds as Multitarget Inhibitors of EGFR and Its Downstream PI3K-AKT Pathway. Crystals, 12(3), 353. https://doi.org/10.3390/cryst12030353







