Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Nanoparticles
2.2. Coating Process
2.3. Microstructural Characterization
2.4. Corrosion Test
3. Results and Discussion
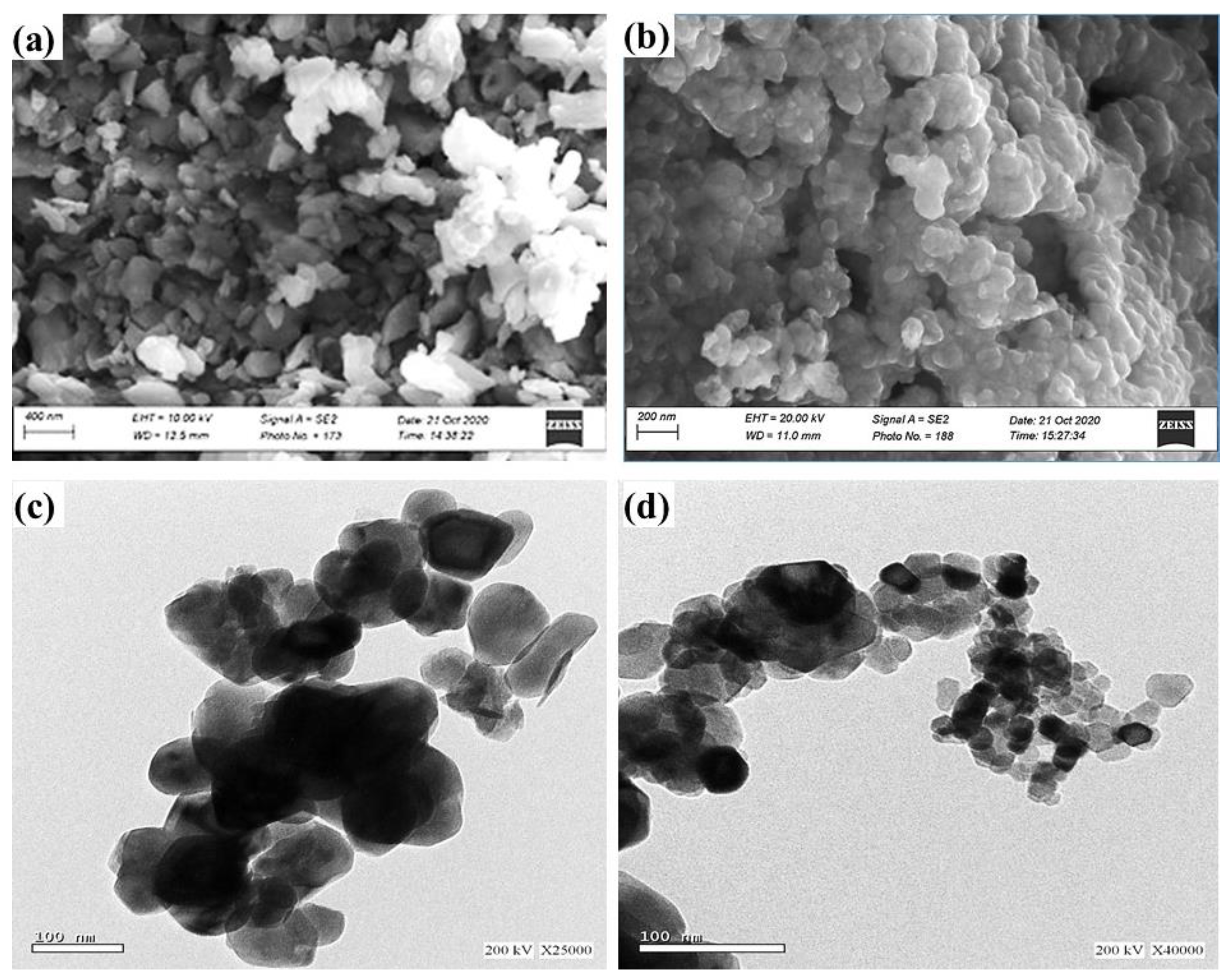
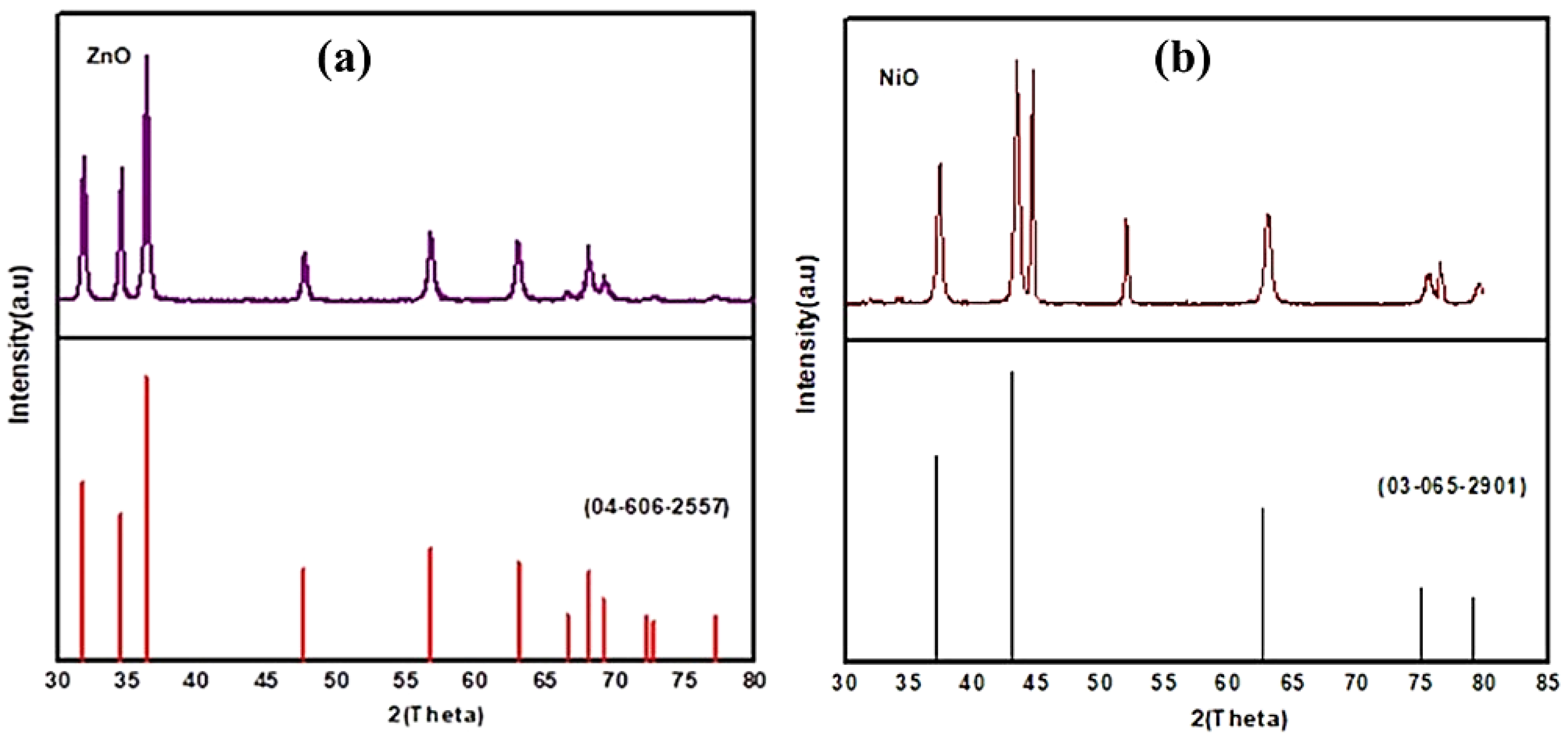
3.1. Characterization of the Starting Materials
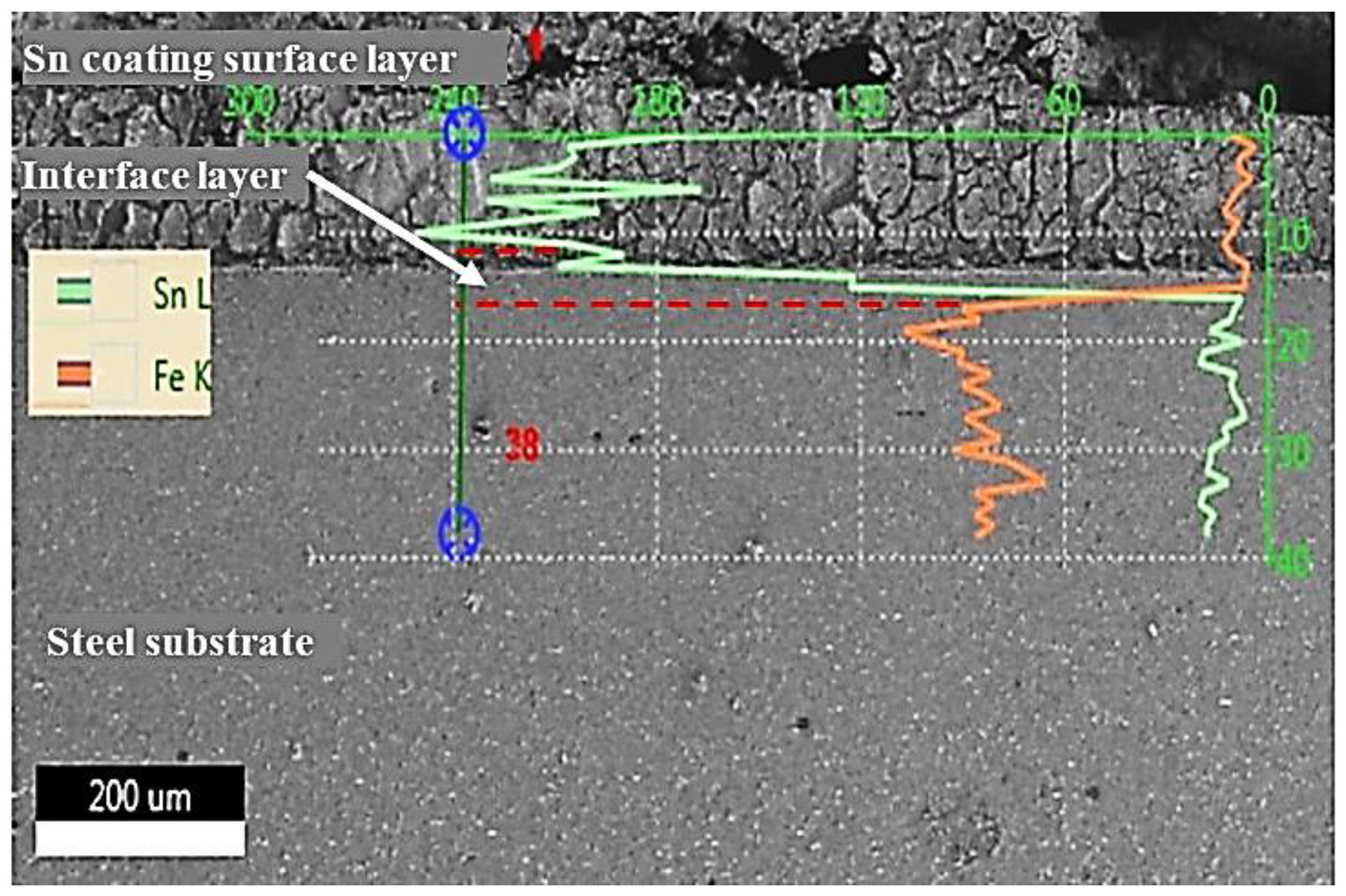
3.2. Microstructural Changes Accompanying Tinning Process
3.3. Corrosion Resistance Enhancement
3.3.1. Potentiodynamic Cyclic Polarization Measurements
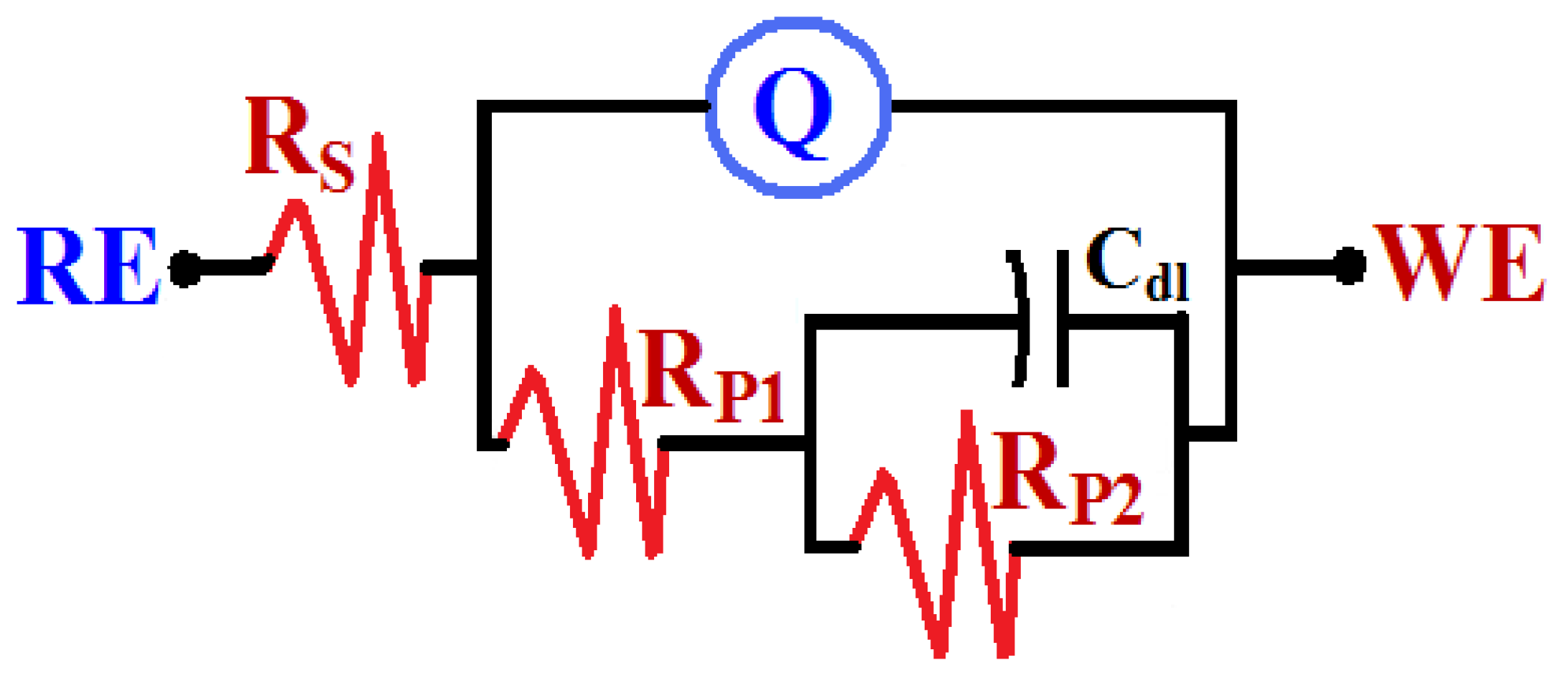
3.3.2. Electrochemical Impedance Spectroscopy Measurements
4. Conclusions
- The uniform coating surface with regular interface structure was achieved by individual ZnO and the combined addition of ZnO and NiO nanoparticles in the tin coating.
- The tin coating thickness increased with the addition of ZnO, NiO, and hybrid ZnO/NiO nanoparticles. The maximum coating thickness of 69.61 ± 2 µm was achieved for hybrid ZnO and NiO nanoparticles in the tin coating.
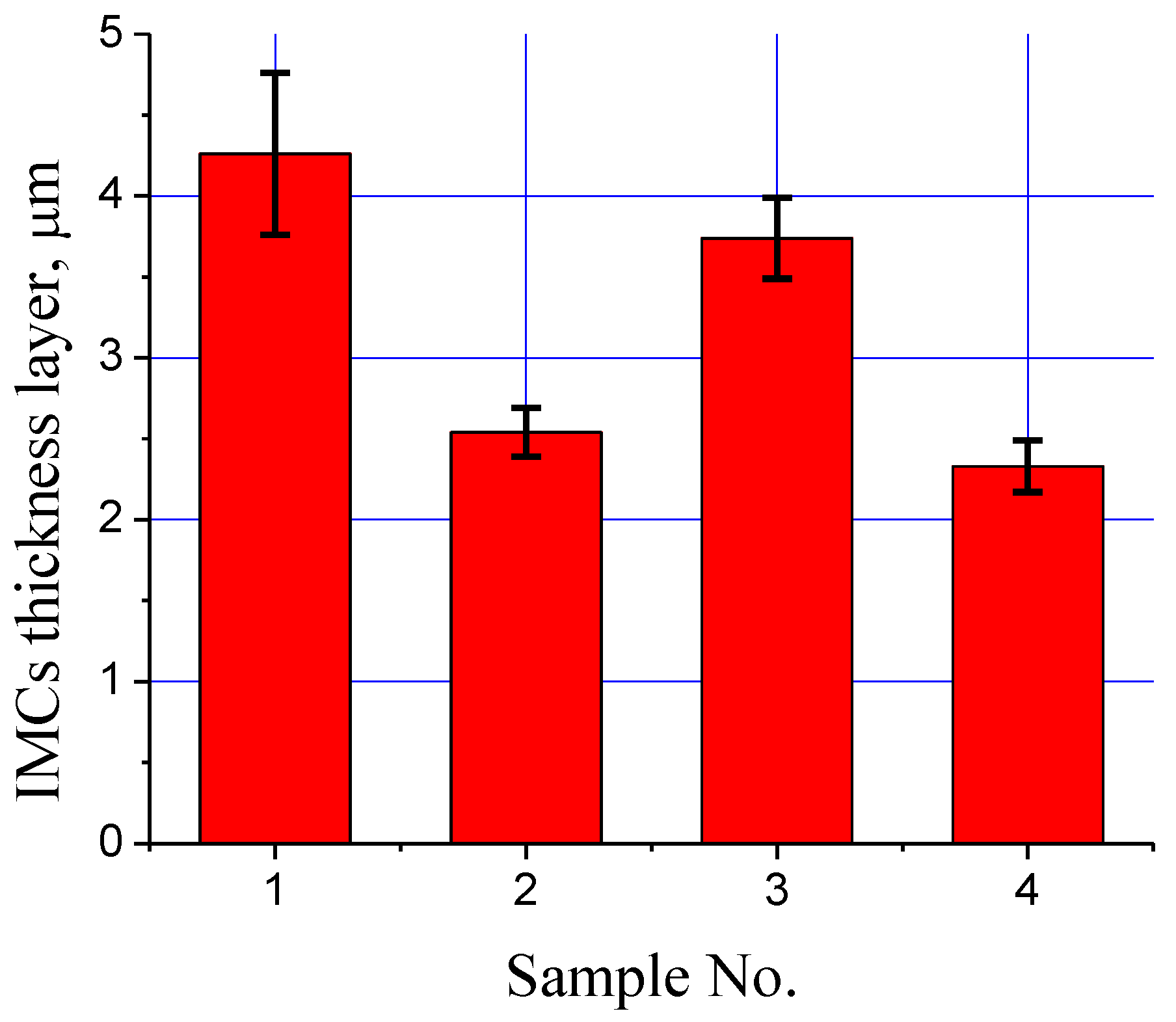
- Fe-tin intermetallic interfacial layer thickness decreased with the addition of ZnO, NiO, and hybrid ZnO/NiO nanoparticles. The minimum coating thickness of about 2.33 ± 0.16 µm was achieved for hybrid ZnO and NiO nanoparticles in the tin coating.
- Tin coatings which were formed by the addition of ZnO, NiO, and hybrid ZnO/NiO nanoparticles showed improvement in corrosion resistance in comparison with the pure tin coating.
- The best improvement in corrosion resistance was achieved by using hybrid ZnO/NiO nanoparticles in the tin coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chintada, V.B.; Koona, R.; Raju Bahubalendruni, M.V.A. State of Art Review on Nickel-Based Electroless Coatings and Materials. J. Bio- Tribo-Corros. 2021, 7, 134. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A. Surface coating methods. In Tribology Series Book; Elsevier Science: Amsterdam, The Netherlands, 1994; Volume 28, ISBN 9780080875927. [Google Scholar]
- Sharma, S.; Agarwala, P.; Garg, R.; Gopinath, P. A study on Ni-P and Ni-P-ZnO composite coatings developed by electroless technique. In Advanced Materials Research; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 2012; Volume 585, pp. 512–516. [Google Scholar]
- Vitry, V.; Kanta, A.F.; Delaunois, F. Application of nitriding to electroless nickel–boron coatings: Chemical and ctural effects; mechanical characterization; corrosion resistance. Mater. Des. 2012, 39, 269–278. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Krishnaveni, K.; Seshadri, S.K. Electroless Ni–P/Ni–B duplex coatings: Preparation and evaluation of microhardness, wear and corrosion resistance. Mater. Chem. Phys. 2003, 82, 771–779. [Google Scholar] [CrossRef]
- Zhang, W.X.; Jiang, Z.H.; Li, G.Y.; Jiang, Q.; Lian, J.S. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 254, 4949–4955. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Rajam, K.S. Electrochemical behaviour of low phosphorus electroless Ni–P–Si3N4 composite coatings. Mater. Chem. Phys. 2010, 120, 546–551. [Google Scholar] [CrossRef]
- Gines, M.J.L.; Benítez, G.J.; Egli, W.; Zubimendi, J.L.; Pérez, Z.T. Formation of an Fe-Sn Intermetallic Layer During the Reflow Process After Tin Plating. Plat. Surf. Finish. 2003, 90, 44–49. [Google Scholar]
- Wijaya, R.H.; Soegijono, B. Corrosion Resistance of Sn-Zn Coated on Low Carbon Steel Material in Wet Gas Pipeline. IOP Conf. Ser. Mater. Sci. Eng. 2019, 694, 012029. [Google Scholar] [CrossRef] [Green Version]
- Xiang, N.; Yin, T.; Tian, B.; Tang, S.; Chen, E. Evaluation on the Manufacturability of Solderable Sn Coatings Obtained by Employing Hot-Dipped Tinning Process. JOM Adv. Surf. Eng. 2019, 71, 4284. [Google Scholar] [CrossRef]
- Diao, H.; Wang, C.Q.; Wang, L. Bonding of Aluminum Alloy by Hot-Dipping Tin Coating. Adv. Mater. Res. 2008, 32, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Spitz, M.; Fleischanderl, M.; Sierlinger, R.; Reischauerb, M.; Perndorfer, F.; Fafilek, G. Surface lubrication influence on electrode degradation during resistance spot welding of hot dip galvanized steel sheets. J. Mater. Process. Technol. 2015, 216, 339–347. [Google Scholar] [CrossRef]
- Makhatha, M.E.; Fatoba, O.S.; Akinlabi, E.T. Effect of rapid solidification on the microstructure and surface analyses of laser deposited Al-Sn coatings on AISI 1015 steel. Int. J. Adv. Manuf. Technol. 2018, 94, 773–787. [Google Scholar] [CrossRef]
- Morita, J.; Yoshida, M. Chromium coating on partially tin precoated steel sheet. J. Appl. Electrochem. 1994, 24, 888–893. [Google Scholar] [CrossRef]
- Vitkin, A.I.; Surovtseva, V.S. New protective coating in the production of sheet metal. Metallurg 1972, 16, 286–290. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Y.; Li, Y.; Liu, Y.; Wang, Y. Effect of Fe2O3 nanoparticles size on the properties of Sn-1.0Age0.5Cu nano-composite solders and joints. J. Alloys Compd. 2016, 662, 272–282. [Google Scholar] [CrossRef]
- Nejad, M.M.; Habibolahzadeh, A.; Yousefpour, M. Effect of Nano_Oxide Addition on Corrosion Performance of Hot Dip Zinc Coating. Prot. Met. Phys. Chem. Surf. 2016, 52, 100–103. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Han, J.P.; Li, Y. Effect of tin on corrosion resistance and formability of tin bearing ferritic stainless steel. Mater. Res. Innov. 2014, 18, 9–11. [Google Scholar] [CrossRef]
- Awan, G.H.; Ahmed, F.; Ali, L.; Shuja, M.S.; Hasan, F. Effect of coating-thickness on the formability of hot dip aluminized steel. Pak. J. Engg. Appl. Sci. 2008, 2. Available online: https://www.researchgate.net/publication/241024789_Effect_of_Coating-thickness_on_the_Formability_of_Hot_Dip_Aluminized_Steel (accessed on 1 January 2022).
- Springer, H.; Kostka, A.; Payton, E.J.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between lowcarbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Dey, P.P.; Modak, P.; Ghosh, A.; Chakrabarti, D.; Banerjeem, P.S.; Ghosh, M. Investigation of phase evolution of Al–Si–Mg coating on hot dipped interstitial-free steel. Results Mater. 2020, 6, 100078. [Google Scholar] [CrossRef]
- Bahadur, A.; Mohanti, O.N. Aluminium diffusion coating on medium carbon steel. Mater. Trans. JIM 1995, 36, 1170–1175. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.D.; Datta, P.K. Relationship between pack chemistry and aluminide coating formation for low-temperature aluminisation of alloy steels. Acta Mater. 2006, 54, 4453–4463. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Hafez, K.M.; Subhani, T.; Abdel Halim, K.S. Development and Optimization of Tin/Flux Mixture for Direct Tinning and Interfacial Bonding in Aluminum/Steel Bimetallic Compound Casting. Materials 2020, 13, 5642. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Alghamdi, A.S.; Subhani, T.; Halim, K.S.A. Fabrication and Characterization of Sn-Based Babbitt Alloy Nanocomposite Reinforced with Al2O3 Nanoparticles/Carbon Steel Bimetallic Material. Materials 2020, 13, 2759. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.; Ayadi, B.; Rajhi, W.; Alghamdi, A.S. Influence of Tinning Material on Interfacial Microstructures and Mechanical Properties of Al12Sn4Si1Cu /Carbon Steel Bimetallic Castings for Bearing Applications. Key Eng. Mater. 2020, 835, 108–114. [Google Scholar] [CrossRef]
- Ramadan, M.; Hafez, K.M.; Alghamdi, A.S.; Ayadi, B.; Abdel Halim, K.S. Novel Approach for Using Ductile Iron as Substrate in Bimetallic Materials for Higher Interfacial Bonding Bearings. Int. J. Met. 2021, 1–14. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Halim, K.S.A.; Amin, A.; Alshammari, S.; Fathy, N.; Ramadan, M. Interfacial Microstructure and Corrosion Behaviour of Mild Steel Coated with Alumina Nanoparticles Doped Tin Composite via Direct Tinning Route. Coatings 2021, 11, 1318. [Google Scholar] [CrossRef]
- Fathy, N.; Ramadan, M. Influence of volume ratio of liquid to solid and low pouring temperature on interface structure of cast Babbitt-steel bimetal composite. AIP Conf. Proc. 2018, 1966, 20028. [Google Scholar]
- Narender, S.S.; Varma, V.S.; Sai Srikar, C.; Ruchitha, J.; Adarsh Varma, P.; Praveen, B.V.S. Nickel Oxide Nanoparticles: A Brief Review of Their Synthesis, Characterization, and Applications. Chem. Eng. Technol. 2022. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Balboul, B.A. Surface Active Rubidium Carbonate Obtained from the Thermal Decomposition Course of Rubidium Acetate. Egypt. J. Chem. 2012, 54, 455–467. [Google Scholar]
- Giri, P.K.; Singh, K.D.; Kesavamoorthy, R.; Panigrahi, B.K.; Nair, K.G.M. Correlating the microstructural and photoluminescence properties of ZnO nanoparticles prepared by ball milling. In 2007 International Workshop on Physics of Semiconductor Devices; IEEE: Piscataway, NJ, USA, 2007; pp. 905–908. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Sherif, E.-S.M.; Abdo, H.S.; Gad, E.S. Ethanedihydrazide as a Corrosion Inhibitor for Iron in 3.5% NaCl Solutions. ACS Omega 2021, 6, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.-S.M.; Ahmed, A.H.; Abdo, H.S.; DefAllah, M.N. Impediment of iron corrosion by N,N’-bis 2-hydroxynaphthylidene amino oxamide in 3.5% NaCl solution. Crystals 2021, 11, 1263. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Ahmed, A.H. Alleviation of Iron Corrosion in Chloride Solution by N,N′-bis 2-Methoxynaphthylidene amino oxamide as a Corrosion Inhibitor. Crystals 2021, 11, 1516. [Google Scholar] [CrossRef]
- Sherif, E.-S.M. Electrochemical investigations on the corrosion inhibition of aluminum by 3-amino-1,2,4-triazole-5-thiol in naturally aerated stagnant seawater. J. Ind. Eng. Chem. 2013, 19, 1884–1889. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Ragab, S.A.; Abdo, H.S. Role of Vanadium Additions on the Corrosion Mitigation of Ti-6Al-xV Alloy in Simulated Body Fluid. Metals 2020, 10, 903. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Abdo, H.S.; Alharthi, N.H. Beneficial Effects of Vanadium Additions on the Corrosion of Ti6AlxV Alloys in Chloride Solutions. Metals 2020, 10, 264. [Google Scholar] [CrossRef] [Green Version]
- Sherif, E.-S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Park, S.M. Inhibition of copper corrosion in 3.0% NaCl by N-phenyl-1,4-phenylenediamine. J. Electrochem. Soc. 2005, 152, 428–433. [Google Scholar] [CrossRef] [Green Version]





| Sample | Definitions |
|---|---|
| Sample 0 (S0) | Low carbon steel (LCS) |
| Sample 1 (S1) | LCS + Sn coating |
| Sample 2 (S2) | LCS + Sn coating + 0.25 ZnO nanoparticles |
| Sample 3 (S3) | LCS + Sn coating + 0.25 NiO nanoparticles |
| Sample 4 (S4) | LCS + Sn coating + (0.25 ZnO + 0.25 NiO) nanoparticles |
| Chemical Composition | C | Si | Mn | Cu | Cr | Ni | Al | Fe |
|---|---|---|---|---|---|---|---|---|
| Steel substrate | 0.08 | 0.01 | 0.33 | 0.03 | 0.02 | 0.05 | 0.05 | Bal. |
| Sample | βc (mV/dec) | ECorr (mV) | βa (mV/dec) | jCorr (μA/cm2) | RP (kΩ cm2) | RCorr (mmy−1) |
|---|---|---|---|---|---|---|
| Low carbon steel (LCS) | 125 | −774 | 180 | 17.5 | 1.83 | 0.2063 |
| LCS + Sn | 118 | −880 | 150 | 14.6 | 1.97 | 0.1721 |
| LCS + Sn + ZnO | 100 | −767 | 135 | 0.65 | 38.43 | 0.0077 |
| LCS + Sn + NiO | 92 | −755 | 122 | 0.55 | 41.46 | 0.0065 |
| LCS + Sn + ZnO + NiO | 83 | −745 | 120 | 0.45 | 47.40 | 0.0053 |
| Sample | RS/ Ω cm2 | Q | RP1/ Ω cm2 | Cdl/ µF cm−2 | RP2/ Ω cm2 | |
|---|---|---|---|---|---|---|
| YQ/F cm−2 | n | |||||
| Low carbon steel (LCS) | 2.799 | 0.004096 | 0.67 | 24.54 | 0.606 | 172.1 |
| LCS + Sn coating | 3.814 | 0.002401 | 0.80 | 29.97 | 0.355 | 255.8 |
| LCS + Sn + ZnO | 4.333 | 0.001905 | 0.75 | 31.29 | 0.250 | 376.9 |
| LCS + Sn + NiO | 5.503 | 0.001085 | 0.72 | 35.18 | 0.177 | 577.6 |
| LCS + Sn + ZnO + NiO | 6.645 | 0.000936 | 0.80 | 39.88 | 0.107 | 721.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Halim, K.S.; Ramadan, M.; Sherif, E.-S.M.; Hafez, K.M.; Subhani, T.; Fathy, N.; Alghamdi, A.S.; Khedr, M.H. Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating. Crystals 2022, 12, 332. https://doi.org/10.3390/cryst12030332
Abdel Halim KS, Ramadan M, Sherif E-SM, Hafez KM, Subhani T, Fathy N, Alghamdi AS, Khedr MH. Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating. Crystals. 2022; 12(3):332. https://doi.org/10.3390/cryst12030332
Chicago/Turabian StyleAbdel Halim, K. S., Mohamed Ramadan, El-Sayed M. Sherif, Khalid M. Hafez, Tayyab Subhani, Naglaa Fathy, Abdulaziz S. Alghamdi, and Mohamed H. Khedr. 2022. "Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating" Crystals 12, no. 3: 332. https://doi.org/10.3390/cryst12030332
APA StyleAbdel Halim, K. S., Ramadan, M., Sherif, E.-S. M., Hafez, K. M., Subhani, T., Fathy, N., Alghamdi, A. S., & Khedr, M. H. (2022). Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating. Crystals, 12(3), 332. https://doi.org/10.3390/cryst12030332









