Revisiting Alloy Design of Al-Base Alloys for Potential Orthotics and Prosthetics Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Age-Hardening Response of Al-Cu-Mg-Ag-Sn Alloys
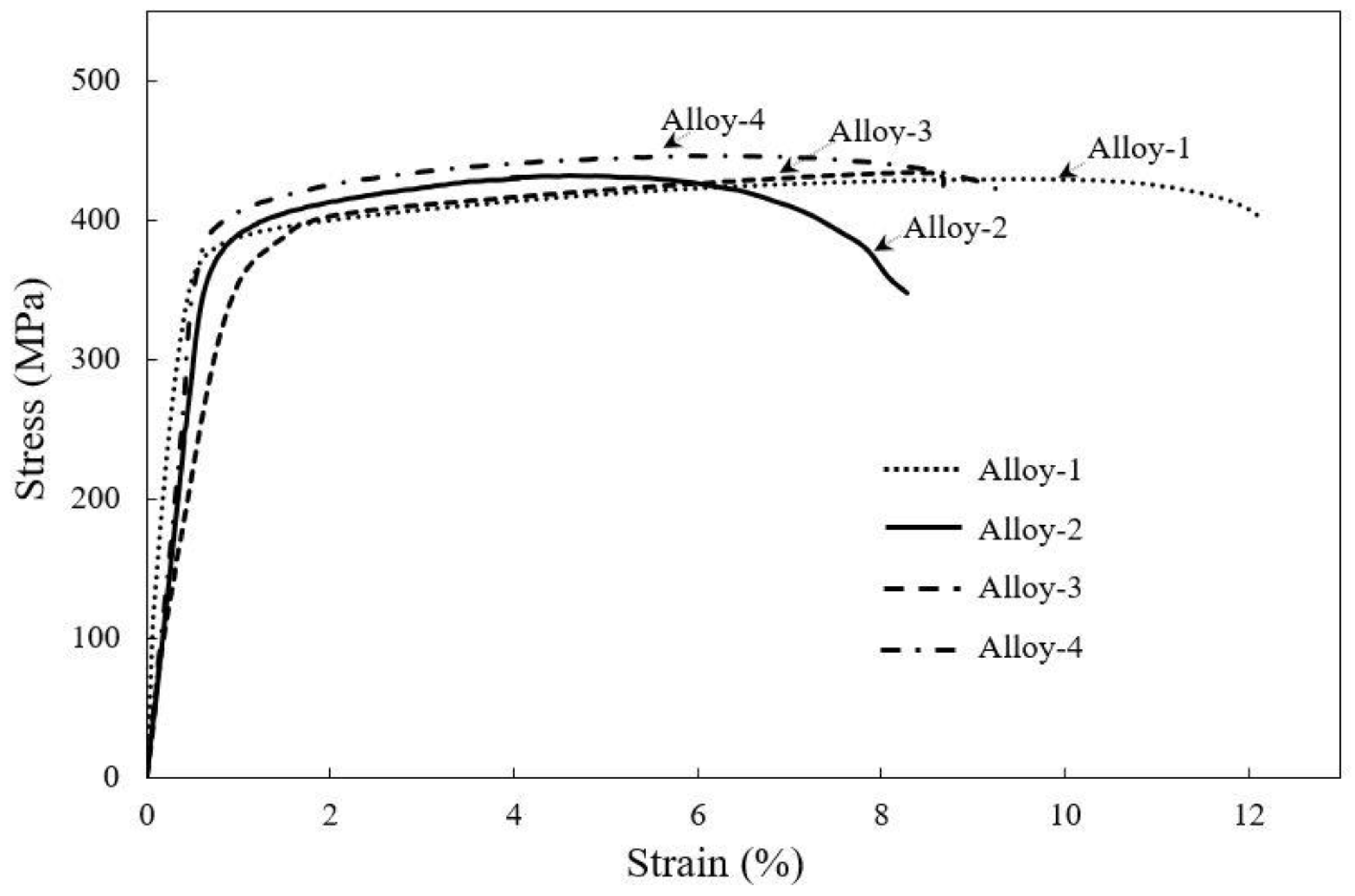
3.2. Comparison of Room Temperature Mechanical Properties of Peak-Aged Alloys
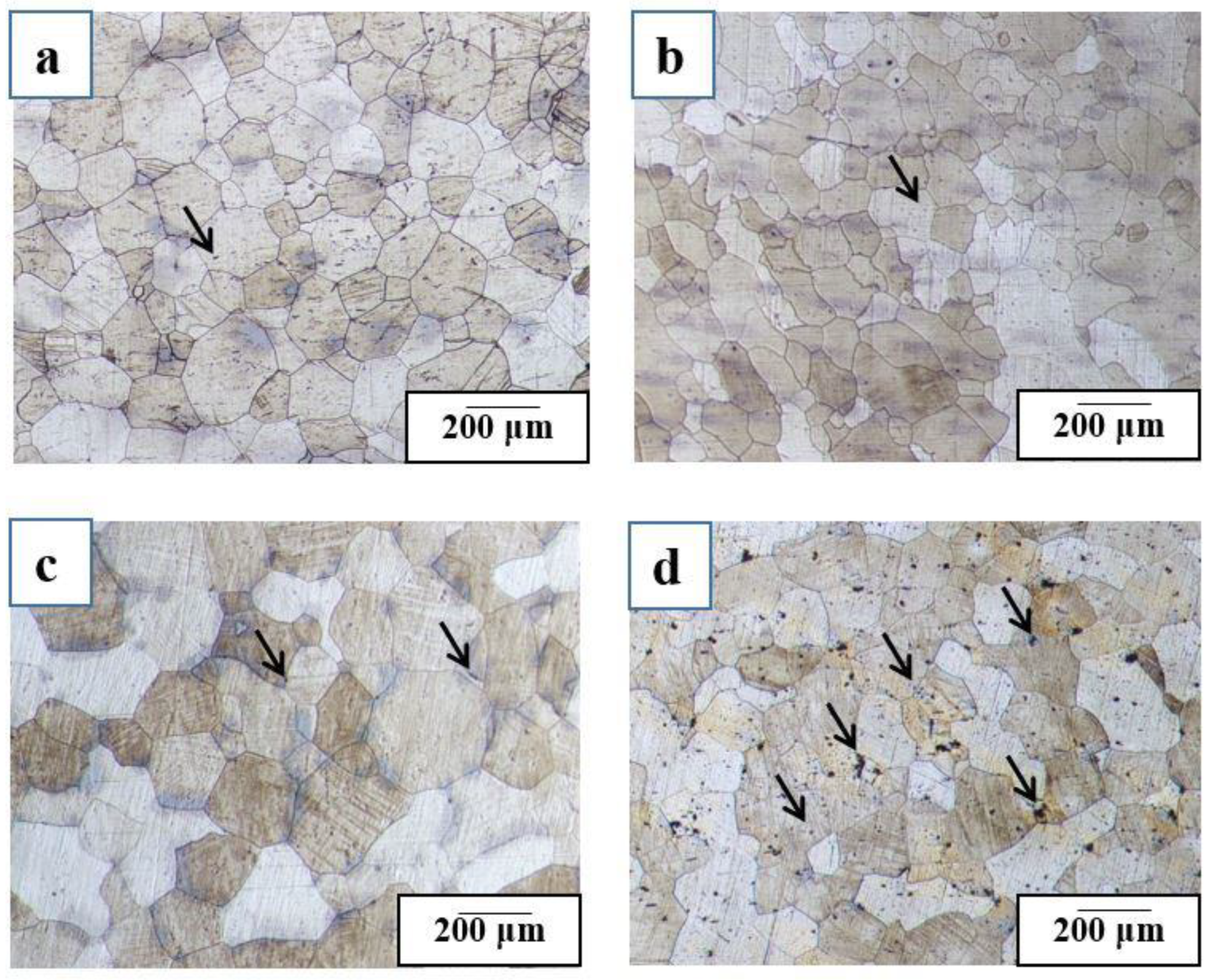
3.3. Comparison of the Microstructure of Al-Cu-Mg-Ag-Sn Alloys at Peak-Aged Condition
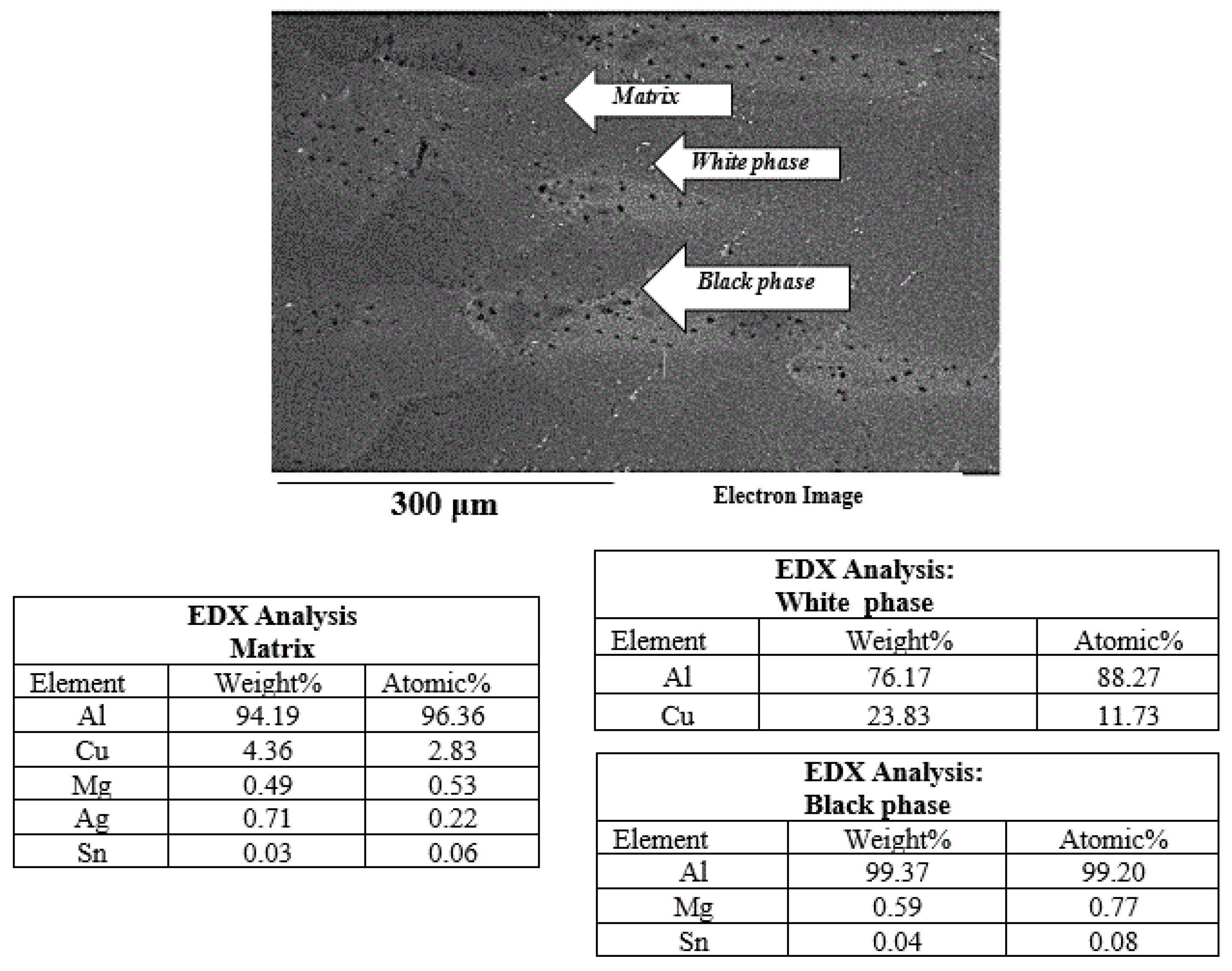
3.4. Identification of Precipitates Using EDS Microanalysis
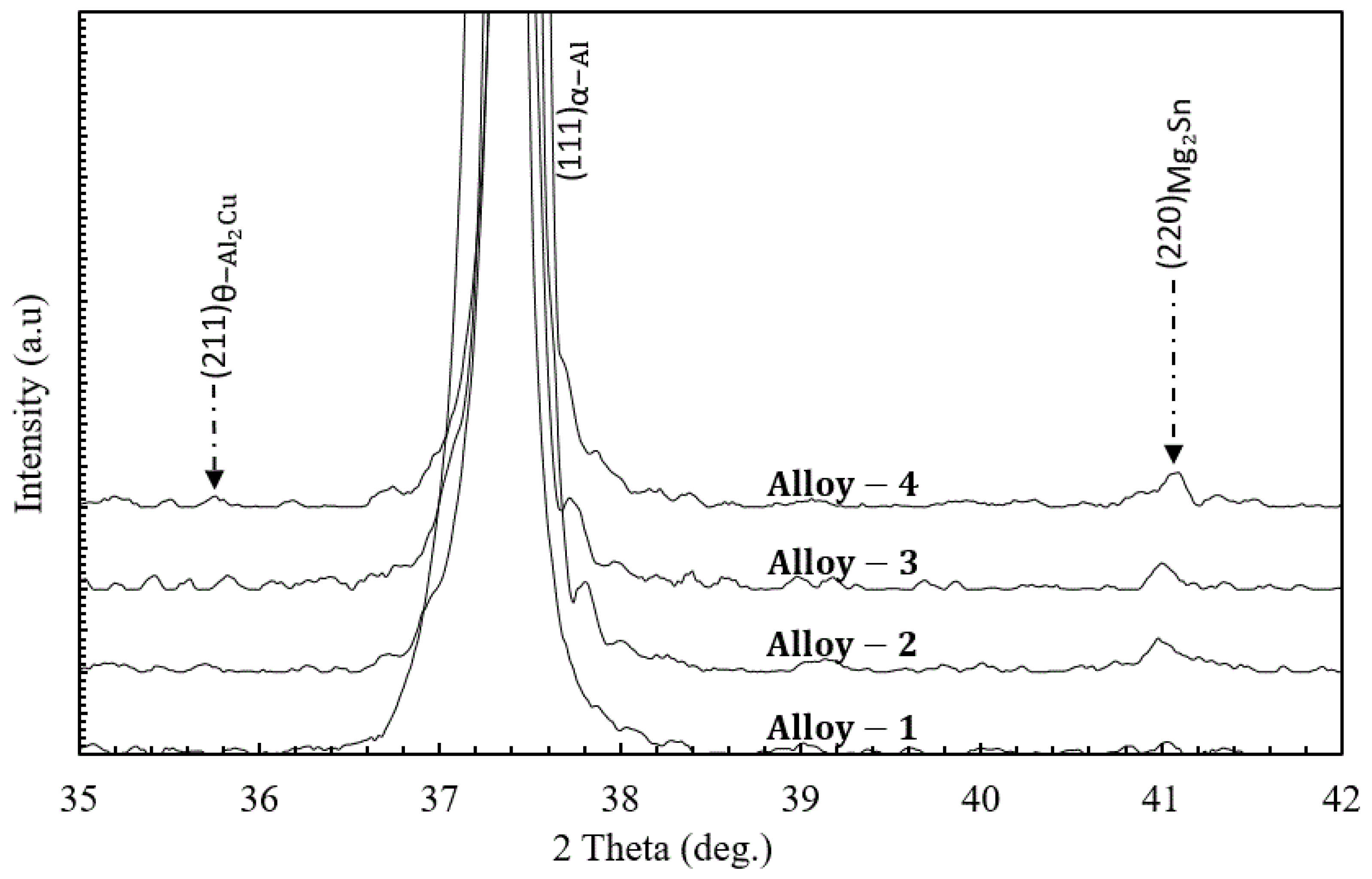
3.5. Comparison of Phase Constitution at Peak Aged Condition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronzino, J.D.; Peterson, D.R. Biomedical Engineering Fundamentals; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Cooper, R.A.; Ohnabe, H.; Hobson, D.A. An Introduction to Rehabilitation Engineering; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Thurston, A.J. Paré and prosthetics: The early history of artificial limbs. ANZ J. Surg. 2007, 77, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Cooper, R. Rehabilitation Engineering: A perspective on the past 40-years and thoughts for the future. Med. Eng. Phy. 2019, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- ASM Handbook Committee. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Russell Township, OH, USA, 1990. [Google Scholar]
- Spada, A.T. In Search of Lightweight Components: Automotive Cast Aluminum Conversion. Eng. Cast. Solut. 2002, 4, 28–31. [Google Scholar]
- ASM International. Properties and Selection of Aluminum Alloys; ASM International: Russell Township, OH, USA, 2019. [Google Scholar]
- Davis, J.R. Aluminum and Aluminum Alloys. Alloying: Understanding the Basics; ASM International: Russell Township, OH, USA, 1993; pp. 351–416. [Google Scholar]
- Liu, H.-Y.; Pearlman, J.; Cooper, R.; Hong, E.-K.; Wang, H.; Salatin, B.; Cooper, R.A. Evaluation of aluminum ultralight rigid wheelchairs versus other ultralight wheelchairs using ANSI/RESNA standards. J. Rehabil. Res. Dev. 2010, 47, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Gebrosky, B.; Pearlman, J.; Cooper, R. Comparison of High-Strength Aluminum Ultralight Wheelchairs Using ANSI/RESNA Testing Standards. Top Spinal Cord Inj. Rehabil. 2018, 24, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Hatch, J.E. Effects of alloying elements and impurities on properties. In Aluminum: Properties and Physical Metallurgy; ASM International: Russell Township, OH, USA, 1983; pp. 200–241. [Google Scholar]
- Erickson, R.B.; Murtha, S.J. Highly Formable Aluminum Alloy Rolled Sheet. U.S. Patent 5,582,660, 10 December 1996. [Google Scholar]
- Guinier, A. Structure of age-hardened aluminium-copper alloys. Nature 1938, 142, 569. [Google Scholar] [CrossRef]
- Totten, G.E.; MacKenzie, D.S. (Eds.) Handbook of Aluminum, Vol. 1: Physical Metallurgy and Process; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 114–168. [Google Scholar]
- Kanno, M.; Suzuki, H.; Kanoh, O. The precipitation of θ′ phase in an Al-4%Cu-0.06%In alloy. J. Jpn. Inst. Metals 1980, 44, 7. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.H.; Wang, S.B.; Liu, C.H.; Yang, S.S.; Wu, C.L. The effect of Si on precipitation in Al–Cu–Mg alloy with a high Cu/Mg ratio. Mater. Sci. Eng. A 2014, 606, 187–195. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J.; Gao, N. Overview of recent work on precipitation in Al-Cu-Mg alloys. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2007. [Google Scholar]
- Wang, S.C.; Starink, M.J. Two types of S phase precipitates in Al-Cu-Mg alloys. Acta Mater. 2007, 55, 933–941. [Google Scholar] [CrossRef]
- Starink, M.J.; Yan, J.L. Precipitation hardening in Al-Cu-Mg alloys: Analysis of precipitates, modelling of kinetics, strength predictions. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2006. [Google Scholar]
- Brook, G.B. Precipitation in Metals; Special Rept. No. 3; Fulmer Research Institute: Stoke Poges, UK, 1963. [Google Scholar]
- Liu, Z.R.; Chen, J.H.; Wang, S.B.; Yuan, D.W.; Yin, M.J.; Wu, C.L. The structure and the properties of S-phase in AlCuMg alloys. Acta Mater. 2011, 59, 7396–7405. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Soliman, M.S.; Alasmari, A.S.; Abbas, A.T.; Hashmi, F.H. Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature. Crystals 2021, 11, 1330. [Google Scholar] [CrossRef]
- Alshammari, T.T.; Alharbi, H.F.; Soliman, M.S.; Ijaz, M.F. Effects of Mg Content on the Microstructural and Mechanical Properties of Al-4Cu-xMg-0.3Ag Alloys. Crystals 2020, 10, 895. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Muhammad, J.A.; Soliman, M.S. Alloying Elements Effects on Electrical Conductivity and Mechanical Properties of Newly Fabricated Al Based Alloys Produced by Conventional Casting Process. Materials 2021, 14, 3971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Bai, S.; Cao, J.; Zhao, J.; Luo, L.; Li, J. Microstructure evolution and mechanical properties of the electron-beam welded joints of cast Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. A 2021, 801, 140363. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Campbell, J. Ductility, structural quality, and fracture toughnessof Al-Cu-Mg-Ag (A201) alloy castings. Mater. Sci. Technol. 2009, 25, 784–789. [Google Scholar] [CrossRef]
- Zamani, M.; Toschi, S.; Morri, A.; Ceschini, L.; Seifeddine, S. Optimisation of heat treatment of Al-Cu-(Mg-Ag) cast alloys. J. Therm. Anal. Calorim. 2020, 139, 3427–3440. [Google Scholar] [CrossRef]
- Li, J.F.; Ziqiao, Z.; Na, J.; Chengyu, T. Localized corrosion mechanism of 2×××-series Al alloy containing S(Al2CuMg) and θ (Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1. Mater. Chem. Phys. 2005, 91, 325–329. [Google Scholar] [CrossRef]
- AGupta, K.; Gaunt, P.; Chaturvedi, M.C. The crystallography and morphology of the S’-phase precipitate in an Al(CuMg) alloy. Philos. Mag. A 1987, 55, 375–387. [Google Scholar]
- So, H.; Won, S.; Oh, J.P.S.J.; Kang, L.; Kim, K. Mechanical properties and microstructural evolution in Al–Cu–Mg–Ag alloy with a CuxMgx/10 content. Mater. Sci. Eng. A 2021, 824, 141573. [Google Scholar] [CrossRef]
- Chester, R.J.; Polmear, I.J. The Metallurgy of Light Alloys; Institution of Metallurgists: London, UK, 1983. [Google Scholar]
- Taylor, J.A.; Parker, B.A.; Polmear, I.J. Precipitation in AI-Cu-Mg-Ag casting alloy. Met. Sci. 1978, 12, 478–482. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, W.L.; Ge, S.J.; Zhao, W.T.; Hanada, S.; Ma, C.L. Effects of Cu content and Cu/Mg ratio on the microstructure and mechanical properties of Al-Si-Cu-Mg alloys. J. Alloys Compd. 2015, 649, 291–296. [Google Scholar] [CrossRef]
- Ringer, S.P.; Yeung, W.; Muddle, B.C.; Polmear, I.J. Precipitate stability in Al-Cu-MgAg alloys aged at high temperatures. Acta Metall. Mater. 1994, 42, 1715–1725. [Google Scholar] [CrossRef]
- Silcock, J.M.; Heal, T.J.; Hardy, H.K. The structural aging characteristics of ternary aluminum-copper alloys with cadmium, indium, or tin. J. Inst. Met. 1955, 84, 13. [Google Scholar]
- Ringer, S.P.; Hono, K.; Sakurai, T. The effect of trace additions of Sn on precipitation in Al-Cu alloys—An atom probe field ion microscopy study Metall. Mater. Trans. A 1995, 26A, 11. [Google Scholar] [CrossRef]
- Ringer, S.P.; Hono, K.; Sakurai, T. Nucleation and growth of θ′ precipitation in Sn-modified Al-Cu alloys: APFIM/TEM observations. Appl. Surf. Sci. 1995, 87/88, 223. [Google Scholar] [CrossRef]
- Bourgeois, L.; Nie, J.F.; Muddle, B.C. Assisted nucleation of θ′ phase in Al–Cu–Sn: The modified crystallography of tin precipitates. Philos. Mag. 2005, 85, 3487–3509. [Google Scholar] [CrossRef]
- Bourgeois, L.; Dwyer, C.; Weyland, M.; Nie, J.F.; Muddle, B.C. The magic thicknesses of θ′ precipitates in Sn micro-alloyed Al–Cu. Acta Mater. 2012, 60, 633–644. [Google Scholar] [CrossRef]
- Bourgeois, L.; Nie, J.F.; Muddle, B.C. On the role of tin in promoting nucleation of the θ′ phase in Al-Cu-Sn. Mater. Sci. Forum 2002, 396–402, 789–794. [Google Scholar]
- Honma, T.; Saxey, D.W.; Ringer, S.P. Effect of trace addition of Sn in Al-Cu alloy. Mater. Sci. Forum 2006, 519–521, 203–208. [Google Scholar]
- Homma, T.; Moody, M.P.; Saxey, D.W.; Ringer, S.P. Effect of Sn addition in precipitation stage in Al-Cu alloys: A correlative transmission electron microscopy and atom probe tomography study Metall. Mater. Trans. A 2012, 43, 2192–2202. [Google Scholar] [CrossRef]
- Shu, J.; Chen, Z.G.; Zhang, J.S. Effects of microalloying with Sn on the precipitation process of Al-3.5Cu-0.4Mg (wt%) alloy. In Proceedings of the 13th International Conference on Aluminum Alloys, Pittsburgh, PA, USA, 3–7 June 2012; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Banerjee, S.; Robi, P.S.; Srinivasan, A. Calorimetric study of precipitation kinetics of Al-Cu-Mg and Al-Cu-Mg-0.06 wt.% Sn alloys. Met. Mater. Int. 2010, 16, 523–531. [Google Scholar] [CrossRef]
- Banerjee, S.; Robi, P.S.; Srinivasan, A.; Lakavath, P.K. Effect of trace additions of Sn on microstructure and mechanical properties of Al–Cu–Mg alloys. Mater. Des. 2010, 31, 4007–4015. [Google Scholar] [CrossRef]
- Banerjee, S.; Robi, P.S.; Srinivasan, A.; Kumar, L.P. High-temperature deformation behavior of Al–Cu–Mg alloys micro-alloyed with Sn. Mater. Sci. Eng. A 2010, 527, 2498–2503. [Google Scholar] [CrossRef]
- Poon, I.; Marceau, R.K.W.; Xia, J.; Liao, X.Z.; Ringer, S.P. Precipitation processes in Al-Cu-Mg-Sn and Al-Cu-Mg-Sn-Ag. Mater. Des. 2016, 96, 385–391. [Google Scholar] [CrossRef]
- Wolverton, C. Solute–vacancy binding in aluminum. Acta Mater. 2007, 55, 5867–5872. [Google Scholar] [CrossRef]
- Dai, S.; Bian, Z.; Wu, W.; Tao, J.; Cai, L.; Wang, M.; Xia, C.; Wang, H. The role of Sn element on the deformation mechanism and precipitation behavior of the Al–Cu–Mg alloy. Mater. Sci. Eng. A 2020, 792, 139838. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; John Wiley: New York, NY, USA, 1996; p. 78. [Google Scholar]
- Embury, J.; Lloyd, D.; Ramachandran, R. 22—Strengthening mechanisms in aluminum alloys. Treatise Mater. Sci. Technol. 1989, 31, 579–601. [Google Scholar]
- Polmear, I.J. Aluminium Alloys-A Century of Age hardening. Mater. Sci. Forum 2004, 28, 1–14. [Google Scholar]
- Hirth, J.P.; Lothe, J. Theory of Dislocations, 2nd ed.; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Foreman, A.J.E.; Makin, M.J. Dislocation movement through random arrays of obstacles. Philos. Mag. 1966, 14, 911–924. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Ashby, M.F. Work hardening of dispersion-hardened crystals. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1966, 14, 1157–1178. [Google Scholar] [CrossRef]
- Bacon, D.J.; Osetsky, Y.N.; Rodney, D. Dislocations in Solids; Elsevier: North-Holland, The Netherlands, 2009; Volume 15. [Google Scholar]
- Dérès, J.; Proville, L.; Marinica, M.C. Dislocation depinning fromnano-sized irradiation defects in a bcc iron model. Acta Mater. 2015, 99, 99–105. [Google Scholar] [CrossRef]
- Queyreau, S.; Monnet, G.; Devincre, B. Orowan strengtheningand forest hardening superposition examined by dislocation dynamics simulations. Acta Mater. 2010, 58, 5586–5595. [Google Scholar] [CrossRef]
- Nembach, E. Particle Strengthening of Metals and Alloys; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Szajewski, B.; Crone, J.C. Analytic model for the Orowan dislocation-precipitate bypass mechanism. Materialia 2020, 11, 100671. [Google Scholar] [CrossRef]

| Alloys: Nomenclature | Ag/Sn Ratio | Chemical Composition (wt.%) | ||||
|---|---|---|---|---|---|---|
| Cu | Mg | Ag | Sn | Al | ||
| Alloy-1 | 32 | 4 | 0.50 | 0.30 | 0.01 | Balance |
| Alloy-2 | 63 | 4 | 0.50 | 0.70 | 0.01 | Balance |
| Alloy-3 | 13 | 4 | 0.50 | 0.30 | 0.03 | Balance |
| Alloy-4 | 23 | 4 | 0.50 | 0.70 | 0.03 | Balance |
| Alloy | Peak Hardness HV | σUTS MPa. | Ref. |
|---|---|---|---|
| Pure Al | 25 | 90 | [8] |
| 7005 T-6 aluminum | 197 | 350 | [9] |
| 6061 T-6 aluminum | 95 | 310 | [10] |
| Al-4% Cu-0.5% Mg-0.7% Ag-0.03%Sn(Alloy-4) | 142 | 447 | Present study |
| Al-4% Cu-0.5% Mg-0.3% Ag-0.03%Sn(Alloy-3) | 138 | 434 | Present study |
| Al-4% Cu-0.5% Mg-0.7% Ag-0.01%Sn(Alloy-2) | 137 | 432 | Present study |
| Al-4% Cu-0.5% Mg-0.3% Ag-0.01%Sn(Alloy-1) | 136 | 429 | Present study |
| Alloy | Avg. Yield Stress σYS (MPa) | Avg. Tensile—Stress σUTS (MPa) | Avg. Fracture Stress σf (MPa) | Avg. Elongation (%) | Avg. Hardness (HV) | Avg. Grain Size (µm) | Avg. Precipitate Size (µm) | Avg. Peak Aging Time (h) |
|---|---|---|---|---|---|---|---|---|
| Alloy-1 | 355 ± 9 | 429 ± 11 | 400 ± 3 | 12 | 136 | 125 | 5.83 | 2 |
| Alloy-2 | 384 ± 7 | 432 ± 9 | 348 ± 8 | 8 | 137 | 121 | 5.17 | 2 |
| Alloy-3 | 386 ± 4 | 434 ± 7 | 421 ± 5 | 8 | 138 | 120 | 5.43 | 2 |
| Alloy-4 | 387 ± 6 | 446 ± 8 | 415 ± 7 | 9 | 142 | 106 | 4.04 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijaz, M.F.; Hashmi, F.H. Revisiting Alloy Design of Al-Base Alloys for Potential Orthotics and Prosthetics Applications. Crystals 2022, 12, 1699. https://doi.org/10.3390/cryst12121699
Ijaz MF, Hashmi FH. Revisiting Alloy Design of Al-Base Alloys for Potential Orthotics and Prosthetics Applications. Crystals. 2022; 12(12):1699. https://doi.org/10.3390/cryst12121699
Chicago/Turabian StyleIjaz, Muhammad Farzik, and Faraz Hussain Hashmi. 2022. "Revisiting Alloy Design of Al-Base Alloys for Potential Orthotics and Prosthetics Applications" Crystals 12, no. 12: 1699. https://doi.org/10.3390/cryst12121699
APA StyleIjaz, M. F., & Hashmi, F. H. (2022). Revisiting Alloy Design of Al-Base Alloys for Potential Orthotics and Prosthetics Applications. Crystals, 12(12), 1699. https://doi.org/10.3390/cryst12121699







