Isostructural Crystals of Bis(Guanidinium) Trioxofluoro-Phosphate/Phosphite in the Ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1—Crystal Structures, Vibrational Spectra and Second Harmonic Generation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization
2.2. Structure Determination and Refinement
2.3. Vibrational Spectroscopy
2.4. Second Harmonic Generation
3. Results
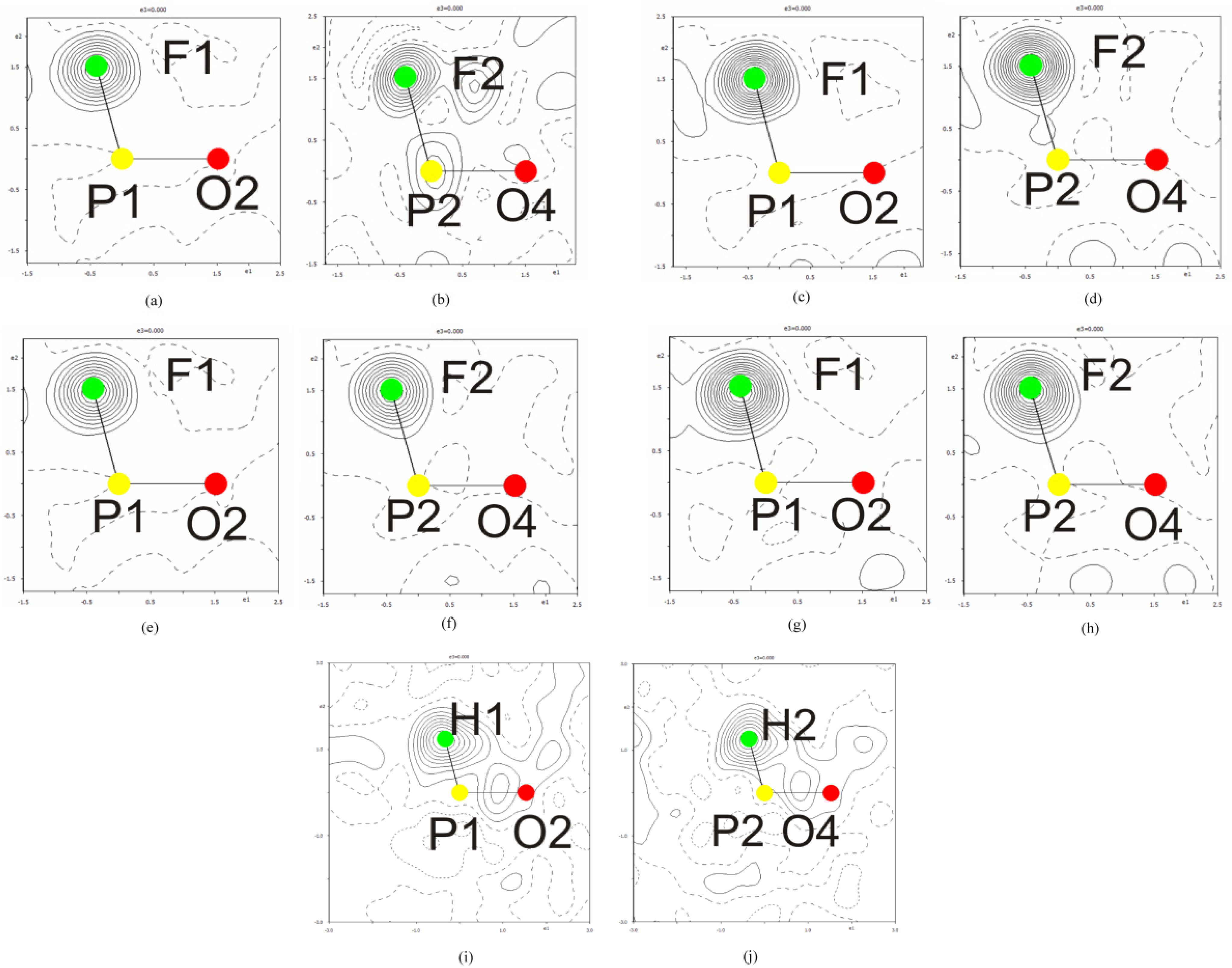
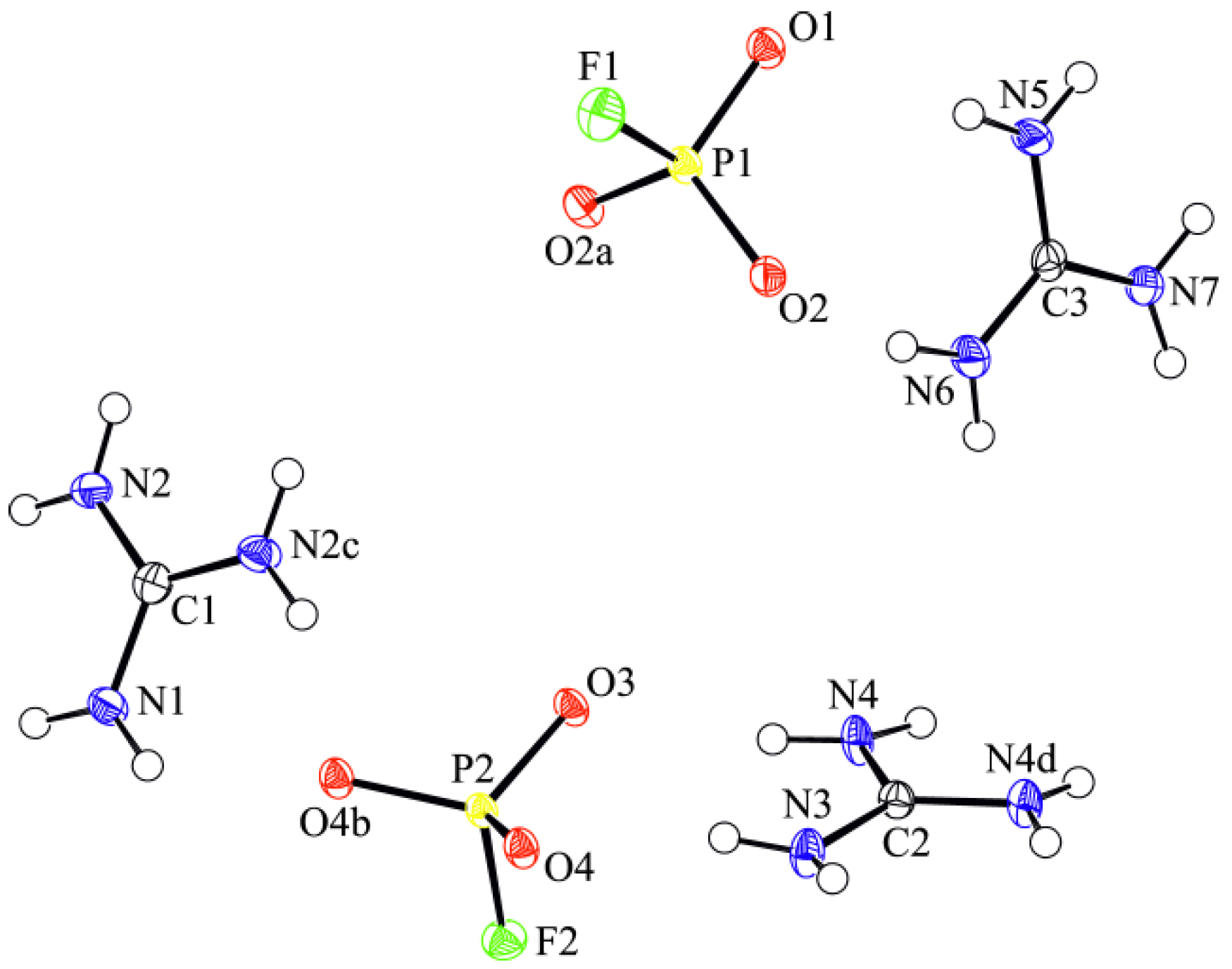
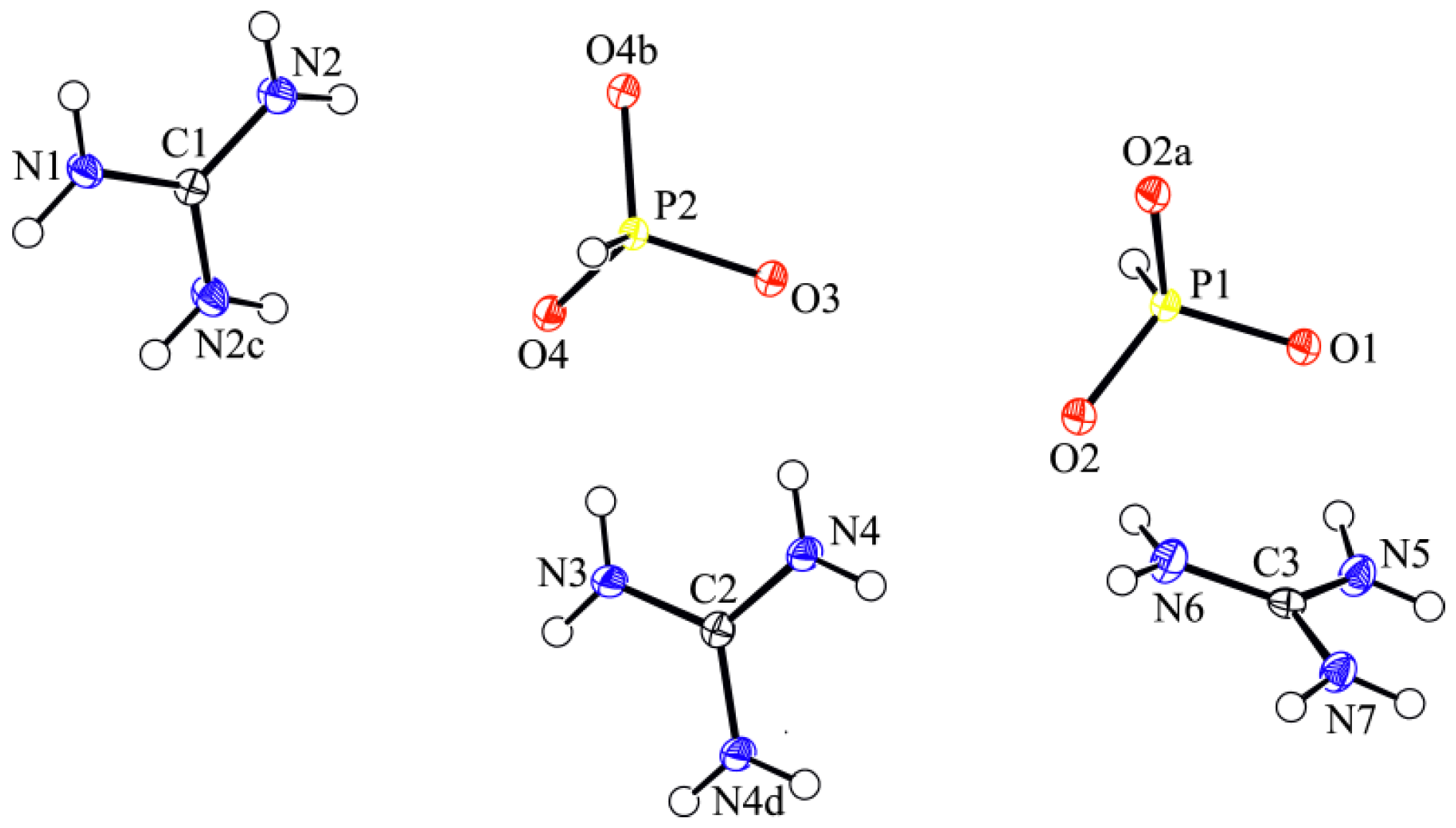
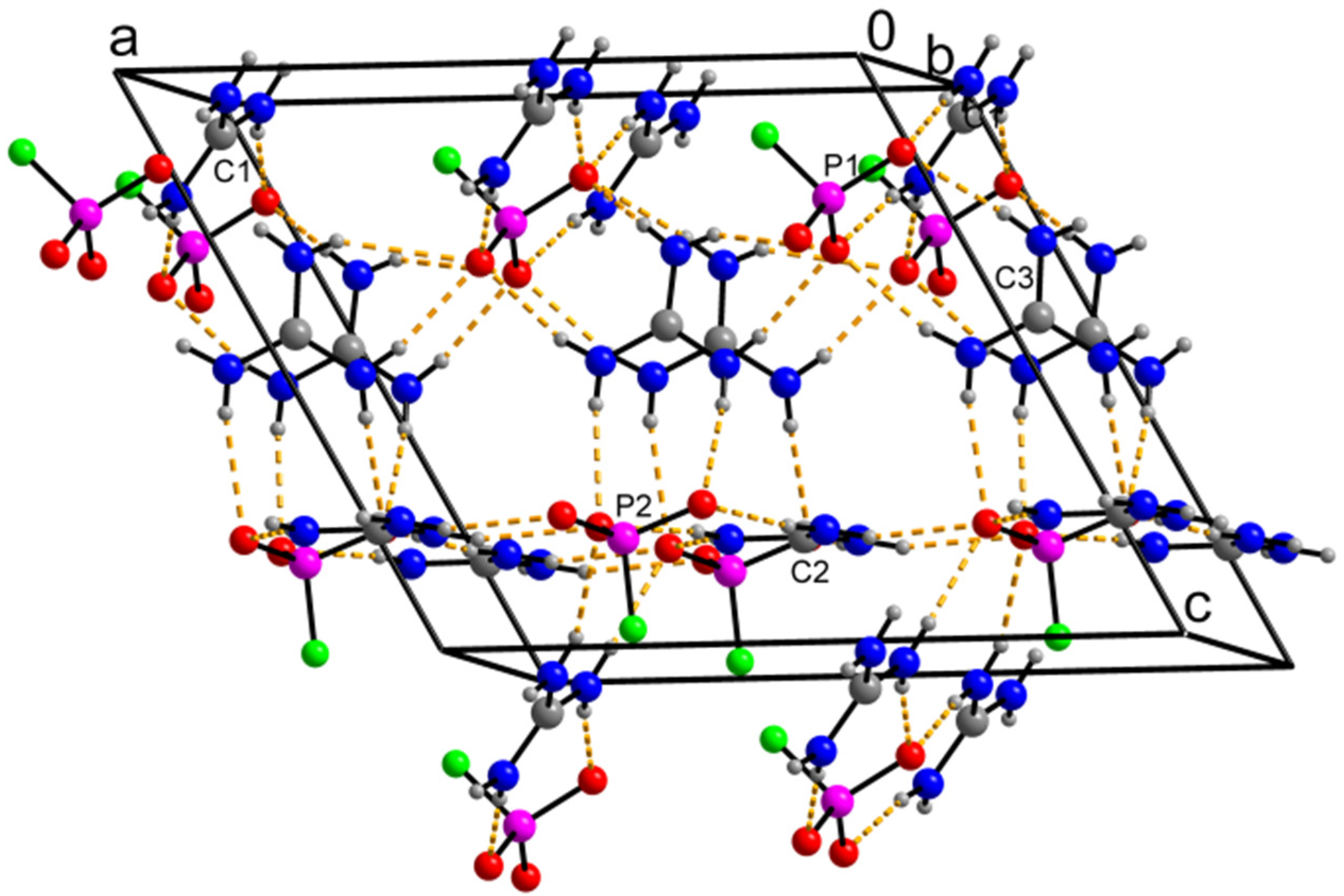
3.1. Crystal Structure
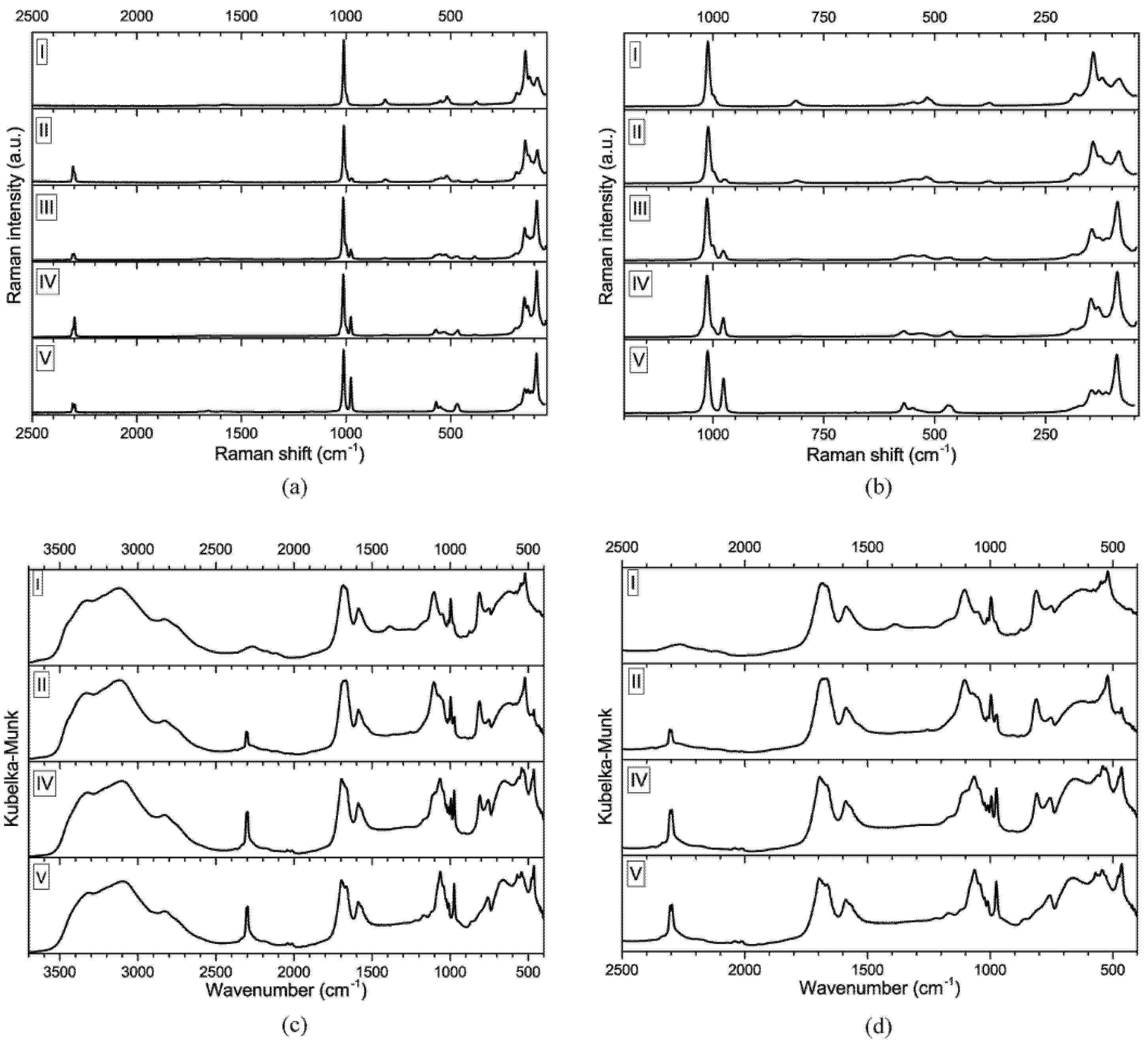
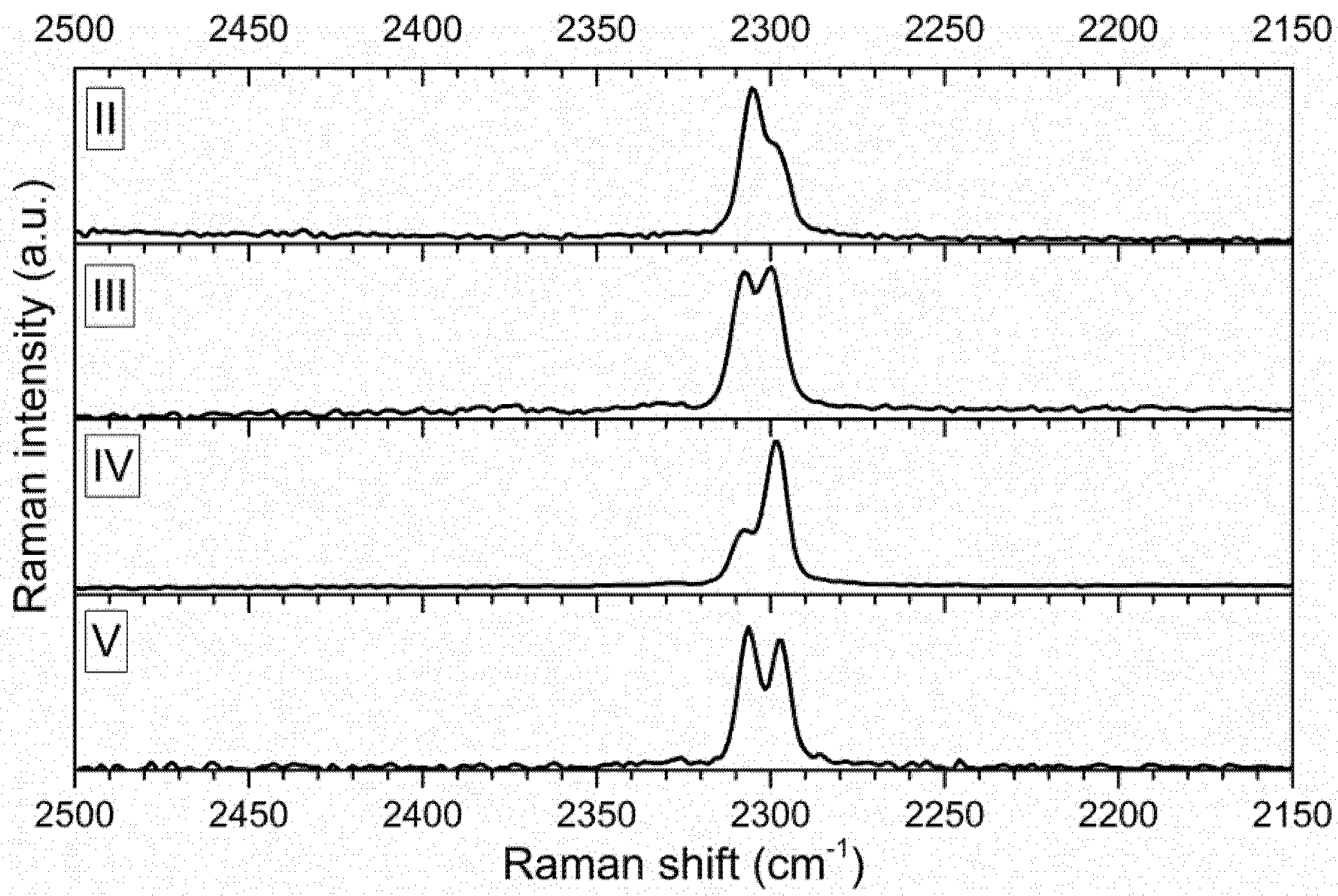
3.2. Vibrational Spectroscopy
3.3. Second Harmonic Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fábry, J.; Fridrichova, M.; Dušek, M.; Fejfarová, K.; Krupková, R. Mixed crystals of 2-carbamoylguanidinium with hydrogen fluorophosphonate and hydrogen phosphite in the ratios 1:0, 0.76 (2):0.24 (2) and 0.115 (7):0.885 (7). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2012, 68, o76–o83. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Databases, I.C.S. Version 2017-1. Fachinformationzentrum Karlsruhe, Germany, and the U.S. Department of Commerce on the behalf of the United States 2017. Available online: https://icsd.products.fiz-karlsruhe.de/ (accessed on 10 October 2022).
- Prescott, H.A.; Troyanov, S.I.; Kemnitz, E. The crystal structures of the potassium hydrogen monofluorophosphates, KHPO3F and K3[H(PO3F)2], and the α modification of RbHPO3F. Z. Krist.—Cryst. Mater. 2003, 218, 604–611. [Google Scholar] [CrossRef]
- Flack, H.D. Chiral and Achiral Crystal Structures. Helvet. Chim. Acta 2003, 86, 905–921. [Google Scholar] [CrossRef]
- Fridrichová, M.; Kroupa, J. Spontaneous noncollinear second harmonic generation in GUHP. J. Opt. 2011, 13, 035204. [Google Scholar] [CrossRef]
- Fridrichová, M.; Němec, I.; Císařová, I.; Němec, P. Guanylurea(1+) hydrogen phosphite: A novel promising phase-matchable material for second harmonic generation. CrystEngComm 2010, 12, 2054–2056. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, J.; Lu, J.; Pan, C.-Y.; Wu, L.-M. Monofluorophosphates: A New Source of Deep-Ultraviolet Nonlinear Optical Materials. Chem. Mater. 2018, 30, 7823–7830. [Google Scholar] [CrossRef]
- Němec, I.; Matulková, I.; Held, P.; Kroupa, J.; Němec, P.; Li, D.; Bohatý, L.; Becker, P. Crystal growth, crystal structure, vibrational spectroscopy, linear and nonlinear optical properties of guanidinium phosphates. Opt. Mater. 2017, 69, 420–431. [Google Scholar] [CrossRef]
- Němec, I.; Matulková, I.; Krumbe, W.; Andersen, L.; Císařová, I.; Kroupa, J.; Němec, P.; Bohatý, L.; Becker, P. Linear and nonlinear optical properties, pyroelectricity and vibrational spectroscopy of polar guanidinium hydrogen phosphite, GuH2PO3, and hydrogen selenite, GuHSeO3. Opt. Mater. 2021, 111, 110722. [Google Scholar] [CrossRef]
- Schülke, U.; Kayser, R. Herstellung von Fluorophosphaten, Difluorophosphaten, Fluorophsophonaten und Fluorophosphiten in fluoridhaltigen Harnstoffschmelzen. Z. Anorg. Allg. Chem. 1991, 600, 221–226. [Google Scholar] [CrossRef]
- Diffraction, R.O. CrysAlisPro. Single Crystal X-ray Diffraction Data Collection and Processing Software. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 10 October 2022).
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist.—Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Becker, P.J.; Coppens, P. Extinction within the limit of validity of the Darwin transfer equations. I. General formalism for primary and secondary extinction and their applications to spherical crystals. Acta Crystallogr. A 1974, 30, 129–147. [Google Scholar] [CrossRef]
- Müller, P.; Herbst-Irmer, R.; Spek, A.L.; Schneider, T.R.; Sawaya, R. Crystal Refinement. A Crystallographer’s Guide to SHELXL; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K. Diamond Version 4.6.8; Crystal Impact GbR: Bonn, Germany, 2022. [Google Scholar]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; The University of Western Australia: Crawley, WA, Australia, 2017; Available online: https://wiki.crystalexplorer.net (accessed on 10 October 2022).
- Kurtz, S.K.; Perry, T.T. A Powder Technique for Evaluation of Nonlinear Optical Materials. J. Appl. Phys. 1968, 39, 3798–3814. [Google Scholar] [CrossRef]
- Buijs, K.; Schutte, C.J.H. The infra-red spectra el the anhydrous nitrates of some alkali and alkaline earth metals. Spectrochim. Acta 1962, 18, 307–313. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond, Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK; New York, NY, USA, 2009. [Google Scholar]
- Etter, M.C.; MacDonald, J.C. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- Dunitz, J.D. Organic fluorine: Odd man out. ChemBioChem 2004, 5, 614–621. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Matulková, I.; Fábry, J.; Němec, I.; Císařová, I.; Vaněk, P. Migrating hydrogen in 2,4,6-triaminopyrimidinium(1+)x hydrogen trioxofluorophosphate(-)x monohydrate/2,4,6-triaminopyrimidinium(2+)1–x trioxofluorophosphate(2–)1–x monohydrate (0.0 < x < 0.73) with changing temperature. Acta Crystallogr. Sect. B Struct. Sci. 2017, 73, 1114–1124. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in moelcular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Rousseau, D.L.; Bauman, R.P.; Porto, S.P.S. Normal Mode Determination in Crystals. J. Raman Spectrosc. 1981, 10, 253–290. [Google Scholar] [CrossRef]
- Bühler, K.; Bues, W. Schwingungsspektren von Fluorophosphatschmelzen und -kristallen. Z. Anorg. Allg. Chem. 1961, 308, 62–71. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A: Theory and Applications in Inorganic Chemistry; John Wiley and Son, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Weil, M.; Baran, E.J.; Kremer, R.K.; Libowitzky, E. Synthesis, Crystal Structure, and Properties of Mn(PO3F)(H2O)2. Z. Anorg. Allg. Chem. 2015, 641, 184–191. [Google Scholar] [CrossRef]
- Weil, M.; Puchberger, M.; Baran, E.J. Preparation and Characterization of Dimercury(I) Monofluorophosphate(V), Hg2PO3F: Crystal Structure, Thermal Behavior, Vibrational Spectra, and Solid-State 31P and 19F NMR Spectra. Inorg. Chem. 2004, 43, 8330–8335. [Google Scholar] [CrossRef]
- Baran, J.; Czapla, Z.; Drozd, M.K.; Ilczyszyn, M.M.; Marchewka, M.; Ratajczak, H. Polarised FTIR and Raman spectra of betaine phosphite single crystal. I. Paraelectric phase. J. Mol. Struct. 1997, 403, 17–37. [Google Scholar] [CrossRef]
- Bickley, R.I.; Edwards, H.G.M.; Knowles, A.; Tait, J.K.F. Vibrational spectroscopic study of the hypophosphite and phosphite anions, H2PO2− and HPO32−, and of their deuteriated analogues in the solid state and in aqueous solution. Spectrochim. Acta Part A 1994, 50, 1277–1285. [Google Scholar] [CrossRef]
- Fridrichová, M.; Němec, I.; Matulková, I.; Gyepes, R.; Borodavka, F.; Kroupa, J.; Hlinka, J.; Gregora, I. Vibrational spectra of guanylurea(1+) hydrogen phosphite—Novel remarkable material for nonlinear optics. Vib. Spectrosc. 2012, 63, 485–491. [Google Scholar] [CrossRef]
- Matulková, I.; Cihelka, J.; Pojarová, M.; Fejfarová, K.; Dušek, M.; Císařová, I.; Vaněk, P.; Kroupa, J.; Němec, P.; Tesařová, N.; et al. Molecular Crystals of 2-amino-1,3,4-thiadiazole with Inorganic Oxyacids—Crystal Engineering, Phase Transformations and NLO Properties. CrystEngComm 2014, 16, 1763–1776. [Google Scholar] [CrossRef]
- Matulková, I.; Cihelka, J.; Pojarová, M.; Fejfarová, K.; Dušek, M.; Vaněk, P.; Kroupa, J.; Krupková, R.; Fábry, J.; Němec, I. A new series of 3,5-diamino-1,2,4-triazolium(1+) inorganic salts and their potential in crystal engineering of novel NLO materials. CrystEngComm 2012, 14, 4625–4636. [Google Scholar] [CrossRef]
- Matulková, I.; Němec, I.; Císařová, I.; Němec, P.; Mička, Z. Inorganic salts of biguanide—Searching for new materials for second harmonic generation. J. Mol. Struct. 2008, 886, 103–120. [Google Scholar] [CrossRef]
- Tsuboi, M. Vibrational Spectra of Phosphite and Hypophosphite Anions, and the Characteristic Frequencies of PO32− and PO2− Groups. J. Am. Chem. Soc. 1957, 79, 1351–1354. [Google Scholar] [CrossRef]
- Lautié, A.; Froment, F.; Novak, A. Relationship between NH Stretching Frequencies and N...O Distances of Crystals Containing NH...O Hydrogen-Bonds. Spectrosc. Lett. 1976, 9, 289–299. [Google Scholar] [CrossRef]
- Marchewka, M.K.; Drozd, M. Ethylenediammonium dication: H-bonded complexes with terephthalate, chloroacetate, phosphite, selenite and sulfamate anions. Detailed vibrational spectroscopic and theoretical studies of ethylenediammonium terephthalate. Spectrochim. Acta Part A 2012, 99, 223–233. [Google Scholar] [CrossRef]
- Arjunan, V.; Kalaivani, M.; Marchewka, M.K.; Mohan, S. Crystal structure, vibrational and DFT simulation studies of melaminium dihydrogen phosphite monohydrate. J. Mol. Struct. 2013, 1045, 160–170. [Google Scholar] [CrossRef]

| Compound | I | II | III |
|---|---|---|---|
| Chemical formula | FO3P·2(CH6N3) | (CH6N3)4(PO3F)0.744(PO3H)0.256 (PO3F)0.687(PO3H)0.313 | (CH6N3)4(PO3F)0.507(PO3H)0.493 (PO3F)0.494(PO3H)0.506 |
| Diffractometer | SUPERNOVA Dual | SUPERNOVA Dual | SUPERNOVA Dual |
| Detector | AtlasS2 (CCD) | AtlasS2 (CCD) | AtlasS2 (CCD) |
| Shape and size of the sample (mm) | prism 0.183 × 0.232 × 0.399 | prism 0.047 × 0.227 × 0.414 | prism 0.068 × 0.096 × 0.447 |
| colour | colourless | colourless | colourless |
| Mr | 218.1 | 426.02 | 418.29 |
| Crystal system, space group | Monoclinic, Cm | Monoclinic, Cm | Monoclinic, Cm |
| T (K) | 95 | 95 | 95 |
| a, b, c (Å) | 13.191 (3) | 13.1423 (4) | 13.1069 (7) |
| 7.2940 (6) | 7.2997 (1) | 7.3028 (3) | |
| 11.644 (3) | 11.6364 (10) | 11.6406 (16) | |
| β (°) | 119.80 (4) | 119.614 (6) | 119.560 (11) |
| V (Å3) | 972.2 (5) | 970.51(11) | 969.18 (18) |
| Z | 4 | 2 | 2 |
| X-ray density (g/cm3) | 1.4903 | 2.63 | 2.59 |
| F000 | 456 | 447 | 440 |
| μ (mm−1) | 2.681 | 2.627 | 2.587 |
| Radiation, λ(Å) | Cu Kα, 1.54184 | Cu Kα, 1.54184 | Cu Kα, 1.54184 |
| monochromator | mirror | mirror | mirror |
| θ – limit (°) | 4.38 – 75.08 | 4.37 – 75.02 | 4.37 – 74.82 |
| hmin, hmax | −16, 11 | −15, 16 | −16, 16 |
| kmin, kmax | −8, 8 | −9, 9 | −8, 8 |
| lmin, lmax | −11, 14 | −14, 14 | −14, 14 |
| Absorption correction | Analytical [12] | Analytical [12] | Analytical [12] |
| Tmin, Tmax | 0.559, 0.713 | 0.554, 0.908 | 0.536, 0.842 |
| No. of measured, independent and observed [I > 3σ(I)] | 3702, 1519, 1512 | 6952, 2050, 2047 | 7256, 2129, 2124 |
| Rint | 0.018 | 0.02 | 0.016 |
| (sin θ/λ)max (Å−1) | 0.627 | 0.627 | 0.626 |
| Refinement/ Weighting scheme | |F|2, w = 1/[σ2(I) + 0.0016(I)2] | |F|2, w = 1/[σ2(I) + 0.0004(I)2] | |F|2, w = 1/[σ2(I) + 0.0004(I)2] |
| R[F2 > 3σ(F2)] | 0.036 | 0.022 | 0.021 |
| wR(F2) | 0.102 | 0.06 | 0.056 |
| S | 2.17 | 2.16 | 2.07 |
| No. of reflections | 1520 | 2050 | 2129 |
| No. of parameters | 171 | 177 | 177 |
| Extinction correction [14] | 2000(200) | 730(100) | 660(90) |
| No. of constraints | 14 | 16 | 16 |
| No. of restraints | 12 | 16 | 16 |
| Δρmax, Δρmin (e Å−3) | 0.55, −0.25 | 0.16, −0.18 | 0.24, −0.27 |
| No. of the Friedel pairs | 460 | 968 | 1052 |
| Flack param. | 0.06 (3) | 0.003 (14) | −0.006 (12) |
| Compound | IV | V |
|---|---|---|
| Chemical formula | (CH6N3)4(PO3F)0.271(PO3H)0.729 (PO3F)0.265(PO3H)0.735 | HO3P·2(CH6N3) |
| Diffractometer | SUPERNOVA Dual | SUPERNOVA Dual |
| Detector | AtlasS2 (CCD) | AtlasS2 (CCD) |
| Shape and size of the sample (mm) | prism 0.183 × 0.232 × 0.399 | prism 0.047 × 0.227 × 0.414 |
| colour | colourless | colourless |
| Mr | 409.92 | 200.1 |
| Crystal system, space group | Monoclinic, Cm | Monoclinic, Cm |
| T (K) | 95 | 95 |
| a, b, c (Å) | 13.0598 (4) | 13.0231 (4) |
| 7.3078 (2) | 7.3185 (3) | |
| 11.6352 (9) | 11.6405 (4) | |
| β (°) | 119.509 (6) | 119.484 (3) |
| V (Å3) | 966.39(10) | 965.77 (7) |
| Z | 2 | 4 |
| X-ray density (g/cm3) | 1.4087 | 1.3765 |
| F000 | 433 | 424 |
| μ (mm−1) | 2.546 | 2.493 |
| Radiation, λ(Å) | Cu Kα, 1.54184 | Cu Kα, 1.54184 |
| monochromator | mirror | mirror |
| θ – limit (°) | 4.37 - 74.69 | 4.37 - 75.02 |
| hmin, hmax | −15, 16 | −15, 16 |
| kmin, kmax | −8, 8 | −9, 9 |
| lmin, lmax | −14, 13 | −14, 14 |
| Absorption correction | Analytical [12] | Multi-scan [12] |
| Tmin, Tmax | 0.586, 0.850 | 0.672, 0.779 |
| No. of measured, independent and observed [I > 3σ(I)] | 4134, 1766, 1760 | 6465, 2066, 2047 |
| Rint | 0.013 | 0.018 |
| (sin θ/λ)max (Å−1) | 0.626 | 0.624 |
| Refinement/ Weighting scheme | |F|2, w = 1/[σ2(I) + 0.0004(I)2] | |F|2, w = 1/[σ2(I) + 0.0004(I)2] |
| R[F2 > 3σ(F2)] | 0.022 | 0.016 |
| wR(F2) | 0.069 | 0.044 |
| S | 2.35 | 1.45 |
| No. of reflections | 1766 | 2066 |
| No. of parameters | 177 | 163 |
| Extinction correction [14] | 1200(120) | 1970(80) |
| No. of constraints | 16 | 16 |
| No. of restraints | 16 | 12 |
| Δρmax, Δρmin (e Å−3) | 0.22, −0.28 | 0.16, −0.10 |
| No. of the Friedel pairs | 694 | 1005 |
| Flack param. | −0.023 (19) | 0.016 (11) |
| Compound | H-Bond | D-H (Å) | H-A (Å) | D···A (Å) | D-H···A (°) | Compound | H-Bond | D-H (Å) | H-A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | N1-H1n1···O2 ii | 0.92(3) | 1.97(3) | 2.874(3) | 167(3) | I | N5-H1n5···O2 iv | 0.82(3) | 2.39(4) | 3.118(5) | 148(4) |
| II | 0.874(16) | 1.998(16) | 2.8677(12) | 173.0(17) | II | 0.860(19) | 2.33(2) | 3.096(2) | 149(2) | ||
| III | 0.879(15) | 1.993(15) | 2.8656(11) | 171.8(14) | III | 0.85(2) | 2.14(2) | 2.980(2) | 170(2) | ||
| IV | 0.90(2) | 1.97(2) | 2.8610(17) | 174(3) | IV | 0.86(2) | 2.30(3) | 3.066(3) | 148(3) | ||
| V | 0.889(13) | 1.973(13) | 2.8564(10) | 171.8(12) | V | 0.826(16) | 2.31(2) | 3.0541(16) | 149.7(16) | ||
| I | N2-H1n2···O1 iii | 0.86(3) | 2.06(3) | 2.911(3) | 172(5) | I | N5-H2n5···O1 | 0.85(5) | 2.17(5) | 2.988(5) | 162(3) |
| II | 0.847(16) | 2.070(16) | 2.9106(12) | 171(3) | II | 0.85(3) | 2.14(3) | 2.988(2) | 171(2) | ||
| III | 0.840(15) | 2.072(16) | 2.9079(12) | 173(2) | III | 0.85(2) | 2.14(2) | 2.980(2) | 170(2) | ||
| IV | 0.867(19) | 2.05(2) | 2.9049(17) | 171(3) | IV | 0.87(3) | 2.13(3) | 2.980(3) | 167(3) | ||
| V | 0.849(13) | 2.066(14) | 2.9051(11) | 170(2) | V | 0.87(2) | 2.12(2) | 2.9733(18) | 169.4(16) | ||
| I | N2-H2n2···O4 x | 0.85(3) | 2.22(3) | 2.976(4) | 148(3) | I | N6-H1n6···O2 | 0.85(5) | 2.10(5) | 2.940(5) | 171(3) |
| II | 0.853(14) | 2.214(16) | 2.9676(15) | 147.1(18) | II | 0.85(3) | 2.10(3) | 2.939(2) | 169.0(16) | ||
| III | 0.847(13) | 2.221(15) | 2.9686(16) | 147.1(17) | III | 0.82(2) | 2.13(2) | 2.946(2) | 170.2(14) | ||
| IV | 0.862(16) | 2.21(2) | 2.967(2) | 147(2) | IV | 0.85(3) | 2.10(3) | 2.945(3) | 169.5(19) | ||
| V | 0.838(11) | 2.213(13) | 2.9650(12) | 149.4(15) | V | 0.85(2) | 2.11(2) | 2.9475(18) | 171.0(12) | ||
| I | N3-H1n3···O4 i | 0.85(3) | 2.08(3) | 2.915(4) | 169(3) | I | N6-H2n6···O4 iv | 0.84(3) | 2.18(3) | 2.972(4) | 158(4) |
| II | 0.852(19) | 2.06(2) | 2.9106(17) | 173.5(16) | II | 0.854(15) | 2.123(17) | 2.9698(17) | 171(2) | ||
| III | 0.843(17) | 2.067(18) | 2.9040(15) | 171.6(19) | III | 0.863(14) | 2.121(16) | 2.9668(18) | 166(2) | ||
| IV | 0.85(2) | 2.06(2) | 2.901(2) | 170(2) | IV | 0.861(17) | 2.123(19) | 2.965(2) | 166 (3) | ||
| V | 0.848(15) | 2.055(16) | 2.8953(13) | 170.6(17) | V | 0.828(13) | 2.154(15) | 2.9650(13) | 166.3(18) | ||
| I | N4-H1n4···O3 | 0.88(4) | 2.07(4) | 2.943(4) | 171(4) | I | N7-H1n7···O3 v | 0.86(3) | 2.17(3) | 2.987(4) | 161(3) |
| II | 0.86(2) | 2.08(2) | 2.9343(16) | 171.9(17) | II | 0.861(16) | 2.129(18) | 2.9791(17) | 169.1(18) | ||
| III | 0.852(18) | 2.086(19) | 2.9300(15) | 170.7(18) | III | 0.865(15) | 2.128(17) | 2.9752(17) | 166.1(17) | ||
| IV | 0.85(2) | 2.09(2) | 2.931(2) | 170(3) | IV | 0.859(19) | 2.13(2) | 2.970(2) | 167(2) | ||
| V | 0.841(15) | 2.089(16) | 2.9268(14) | 174.3(15) | V | 0.837(13) | 2.144(14) | 2.9671(13) | 167.6(14) | ||
| I | N4-H2n4···O4 iv | 0.90(4) | 2.03(4) | 2.909(5) | 166(3) | I | N7-H2n7···O2 iv | 0.85(3) | 1.97(3) | 2.810(4) | 167(4) |
| II | 0.87(2) | 2.05(2) | 2.897(2) | 167.5(19) | II | 0.855(15) | 1.990(14) | 2.8225(15) | 164(2) | ||
| III | 0.87(2) | 2.03(2) | 2.8914(17) | 168.1(17) | III | 0.852(15) | 2.005(13) | 2.8277(15) | 162(2) | ||
| IV | 0.88(3) | 2.01(3) | 2.884(3) | 171(2) | IV | 0.856(17) | 1.982(16) | 2.826(2) | 169(3) | ||
| V | 0.846(19) | 2.035(18) | 2.8761(15) | 172.8(15) | V | 0.848(13) | 2.013(12) | 2.8372(11) | 163.8(18) |
| Compound | H-interaction | D-H (Å) | H-A (Å) | D···A (Å) | D-H···A (°) |
|---|---|---|---|---|---|
| I | N5-H1n5···F1 xi | 0.83(4) | 2.86(3) | 3.421(4) | 126(3) |
| II | N5-H1n5···F1 xi | 0.860(19) | 2.824(18) | 3.419(2) | 127.8(18) |
| II | N5-H1n5···H1 xi | 0.860(19) | 2.82(13) | 3.45(16) | 131(5) |
| III | N5-H1n5···F1 v | 0.824(18) | 2.838(18) | 3.425(3) | 129.9(18) |
| III | N5-H1n5···H1 v | 0.824(18) | 2.87(6) | 3.50(7) | 134(3) |
| IV | N5-H1n5···F1 v | 0.86(2) | 2.81(2) | 3.407(7) | 128(2) |
| IV | N5-H1n5···H1 v | 0.86(2) | 2.86(6) | 3.50(7) | 132(3) |
| V | N5-H1n5···H1 v | 0.826(16) | 2.883(19) | 3.524(16) | 136.0(15) |
| I | N2-H1n2···F2 viii | 0.80 (3) | 3.05(4) | 3.293 (4) | 101(3) |
| II | N2-H1n2···F2 viii | 0.847(16) | 3.05(2) | 3.289 (2) | 98.7(15) |
| II | N2-H1n2···H2 viii | 0.847(16) | 3.14(14) | 3.34 (12) | 96.3(19) |
| III | N2-H1n2···F2 x | 0.840 (15) | 3.035(18) | 3.425(3) | 100.1(13) |
| III | N2-H1n2···H2 x | 0.840 (15) | 3.08(8) | 3.50(7) | 98.0(14) |
| IV | N2-H1n2···F2 x | 0.867(19) | 3.04(3) | 3.407(7) | 99.6(18) |
| IV | N2-H1n2···H2 x | 0.867(19) | 2.98(6) | 3.50(7) | 98.7(18) |
| V | N2-H1n2···H2 x | 0.849(13) | 3.09(2) | 3.524(16) | 97.4(11) |
| I | N2-H2n2···F2 viii | 0.83 (3) | 2.93(4) | 3.293(4) | 109(3) |
| II | N2-H2n2···F2 viii | 0.853 (14) | 2.97(2) | 3.289(2) | 104.4(15) |
| II | N2-H2n2···H2 viii | 0.853 (14) | 2.97(11) | 3.34(12) | 109(2) |
| III | N2-H2n2···F2 x | 0.847 (13) | 2.95(2) | 3.288(3) | 105.9(15) |
| III | N2-H2n2···H2 x | 0.847 (13) | 2.92(6) | 3.31(6) | 110.0(17) |
| IV | N2-H2n2···F2 x | 0.826(16) | 2.99(3) | 3.294(6) | 103.3(19) |
| IV | N2-H2n2···H2 x | 0.826(16) | 2.88(5) | 3.23(5) | 106(2) |
| V | N2-H2n2···H2 x | 0.838 (11) | 2.92(2) | 3.305(14) | 110.3(13) |
| gu2PO3F | gu2(PO3F)x(HPO3)y | gu2HPO3 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound (II) | Compound (III) | Compound (IV) | |||||||||||||||||
| Compound (I) | x = 0.716 y = 0.284 | x = 0.501 y = 0.499 | x = 0.268 y = 0.732 | Compound (V) | |||||||||||||||
| FTIR | Raman | FTIR | Raman | Raman | FTIR | Raman | FTIR | Raman | |||||||||||
| Assignment | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | cm−1 | Int. * | Assignment |
| v N-H(…O) | 3325 | 71 | 3330 | 91 | 3330 | 73 | 3310 | 70 | v N-H(…O) | ||||||||||
| 3120 | 84 | 3115 | 99 | 3110 | 86 | 3100 | 83 | ||||||||||||
| 2830 | 50 | 2830 | 72 | 2835 | 48 | 2835 | 48 | ||||||||||||
| ? | 2377 | 10 | ? | ||||||||||||||||
| 2307 | 62 | 2305 | 33 | 2307 | 9 | 2305 | 50 | 2308 | 10 | 2305 | 52 | 2306 | 14 | v PH | |||||
| 2299 | 62 | 2298 | 17 | 2300 | 9 | 2297 | 51 | 2298 | 29 | 2296 | 54 | 2297 | 13 | ||||||
| ? | 2266 | 21 | |||||||||||||||||
| 2182 | sh | ||||||||||||||||||
| 2120 | sh | ||||||||||||||||||
| 2040 | 8 | 2040 | 12 | ? | |||||||||||||||
| 2015 | 8 | 2015 | 12 | ||||||||||||||||
| δ NH2, v CN | 1683 | 87 | 1682 | 5 | 1694 | 67 | 1697 | 6 | 1697 | 84 | 1697 | 2 | δ NH2, v CN | ||||||
| 1669 | sh | 1660 | sh | 1667 | 99 | 1677 | 5 | 1665 | 3 | 1658 | 27 | 1659 | 6 | 1663 | 77 | 1660 | 3 | ||
| 1588 | 62 | 1586 | 6 | 1588 | 80 | 1592 | 6 | 1591 | 2 | 1589 | 61 | 1587 | sh | 1588 | 59 | 1595 | 2 | ||
| 1568 | sh | 1567 | 4 | 1564 | 5 | 1571 | sh | ||||||||||||
| ? | 1389 | 43 | |||||||||||||||||
| 1381 | 35 | ||||||||||||||||||
| 1256 | 62 | ||||||||||||||||||
| 1221 | 12 | ? | |||||||||||||||||
| ρ NH2 | 1166 | sh | 1160 | 2 | 1170 | 44 | 1165 | 2 | ρ NH2 | ||||||||||
| vas PO3, ρ NH2 | 1104 | 80 | 1104 | 98 | 1110 | sh | vas PO3, ρ NH2 | ||||||||||||
| ρ NH2, δ PO3 | 1053 | sh | 1059 | 5 | 1065 | 88 | 1064 | 93 | 1059 | 3 | δ PO3, ρ NH2 | ||||||||
| 1046 | sh | ||||||||||||||||||
| 1022 | 72 | 1022 | 59 | 1022 | 62 | δ PH, ρ NH2 | |||||||||||||
| vs CN3 | 1012 | 49 | 1013 | 100 | 1010 | 74 | 1011 | 134 | 1013 | 100 | 1010 | 56 | 1013 | 95 | 1010 | 57 | 1012 | 100 | vs CN3 |
| vs PO3 | 997 | 72 | 1000 | sh | 997 | 89 | 1000 | 25 | 995 | 66 | 1001 | sh | |||||||
| 979 | sh | 975 | 5 | 973 | 75 | 973 | 13 | 977 | 16 | 974 | 75 | 977 | 30 | 975 | 80 | 976 | 56 | vs PO3 | |
| ? | 916 | 7 | |||||||||||||||||
| 876 | 37 | 859 | sh | ? | |||||||||||||||
| v PF | 811 | 79 | 813 | 13 | 811 | 86 | 811 | 11 | 816 | 3 | 810 | 67 | 811 | 3 | |||||
| δ NCN | 750 | 62 | 752 | 73 | 756 | 64 | 757 | 64 | δ NCN | ||||||||||
| γ N-H(…O), τ NH2 | 675 | 6 | 678 | 2 | 661 | 85 | 660 | 84 | 678 | 3 | γ N-H(…O), τ NH2 | ||||||||
| 649 | 86 | ||||||||||||||||||
| 625 | 80 | 621 | 85 | ||||||||||||||||
| δ PO3 | 580 | 80 | 576 | 8 | 581 | 85 | 572 | 3 | 564 | sh | 560 | 90 | 570 | 11 | 570 | 90 | 569 | 17 | δs PO3 |
| 556 | 2 | 552 | 9 | 560 | 88 | ||||||||||||||
| δ NCN, τ NH2 | 549 | 89 | 547 | 11 | 546 | 5 | 541 | 100 | 544 | 93 | 550 | 9 | δ NCN, τ NH2 | ||||||
| 525 | 9 | 529 | 98 | 534 | 7 | 532 | sh | ||||||||||||
| δ FPO3, δ NCN, τ NH2 | 521 | 100 | 517 | 18 | 521 | 100 | 519 | 19 | |||||||||||
| 481 | 77 | 473 | 6 | 478 | 87 | δ PO3 | |||||||||||||
| 464 | 80 | 463 | 8 | 466 | 6 | 464 | 98 | 465 | 10 | 463 | 100 | 469 | 14 | ||||||
| ρ PO3 | 377 | 10 | 377 | 9 | 385 | 6 | 387 | 3 | |||||||||||
| External modes | 183 | 23 | 185 | 27 | 189 | 10 | 189 | 13 | 188 | sh | External modes | ||||||||
| 143 | 84 | 143 | 100 | 146 | 51 | 147 | 59 | 146 | 38 | ||||||||||
| 123 | 47 | 130 | sh | 130 | 38 | 131 | 47 | 130 | 37 | ||||||||||
| 112 | 36 | 115 | 34 | ||||||||||||||||
| 84 | 45 | 84 | 78 | 88 | 95 | 88 | 100 | 90 | 94 | ||||||||||
| Compound | Stoicheiometric Coefficients x/y | Area of the Band at 2300 cm−1 | Area of the Band at 2306 cm−1 | Ratio of the Band Areas |
|---|---|---|---|---|
| II | 0.716/0.284 | 941 | 1679 | 0.56 |
| III | 0.501/0.499 | 1046 | 763 | 1.37 |
| IV | 0.268/0.732 | 3195 | 1137 | 2.81 |
| V | 0/1 | 763 | 680 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulková, I.; Fábry, J.; Eigner, V.; Dušek, M.; Kroupa, J.; Němec, I. Isostructural Crystals of Bis(Guanidinium) Trioxofluoro-Phosphate/Phosphite in the Ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1—Crystal Structures, Vibrational Spectra and Second Harmonic Generation. Crystals 2022, 12, 1694. https://doi.org/10.3390/cryst12121694
Matulková I, Fábry J, Eigner V, Dušek M, Kroupa J, Němec I. Isostructural Crystals of Bis(Guanidinium) Trioxofluoro-Phosphate/Phosphite in the Ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1—Crystal Structures, Vibrational Spectra and Second Harmonic Generation. Crystals. 2022; 12(12):1694. https://doi.org/10.3390/cryst12121694
Chicago/Turabian StyleMatulková, Irena, Jan Fábry, Václav Eigner, Michal Dušek, Jan Kroupa, and Ivan Němec. 2022. "Isostructural Crystals of Bis(Guanidinium) Trioxofluoro-Phosphate/Phosphite in the Ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1—Crystal Structures, Vibrational Spectra and Second Harmonic Generation" Crystals 12, no. 12: 1694. https://doi.org/10.3390/cryst12121694
APA StyleMatulková, I., Fábry, J., Eigner, V., Dušek, M., Kroupa, J., & Němec, I. (2022). Isostructural Crystals of Bis(Guanidinium) Trioxofluoro-Phosphate/Phosphite in the Ratio 1/0, 0.716/0.284, 0.501/0.499, 0.268/0.732, 0/1—Crystal Structures, Vibrational Spectra and Second Harmonic Generation. Crystals, 12(12), 1694. https://doi.org/10.3390/cryst12121694







