The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2
2.3. Characterization of TiO2 Analysis
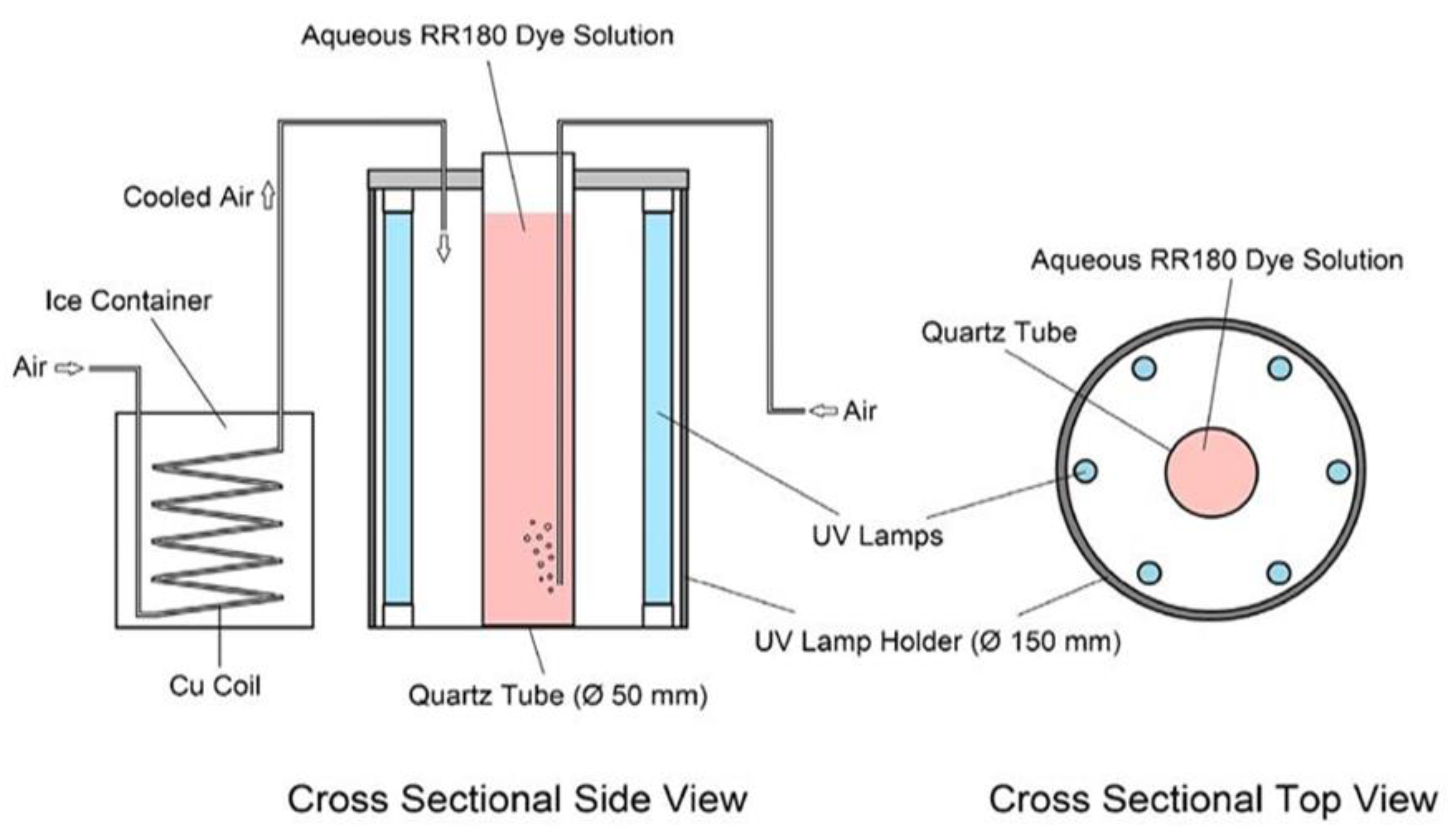
2.4. Photocatalytic Runs
3. Results and Discussion
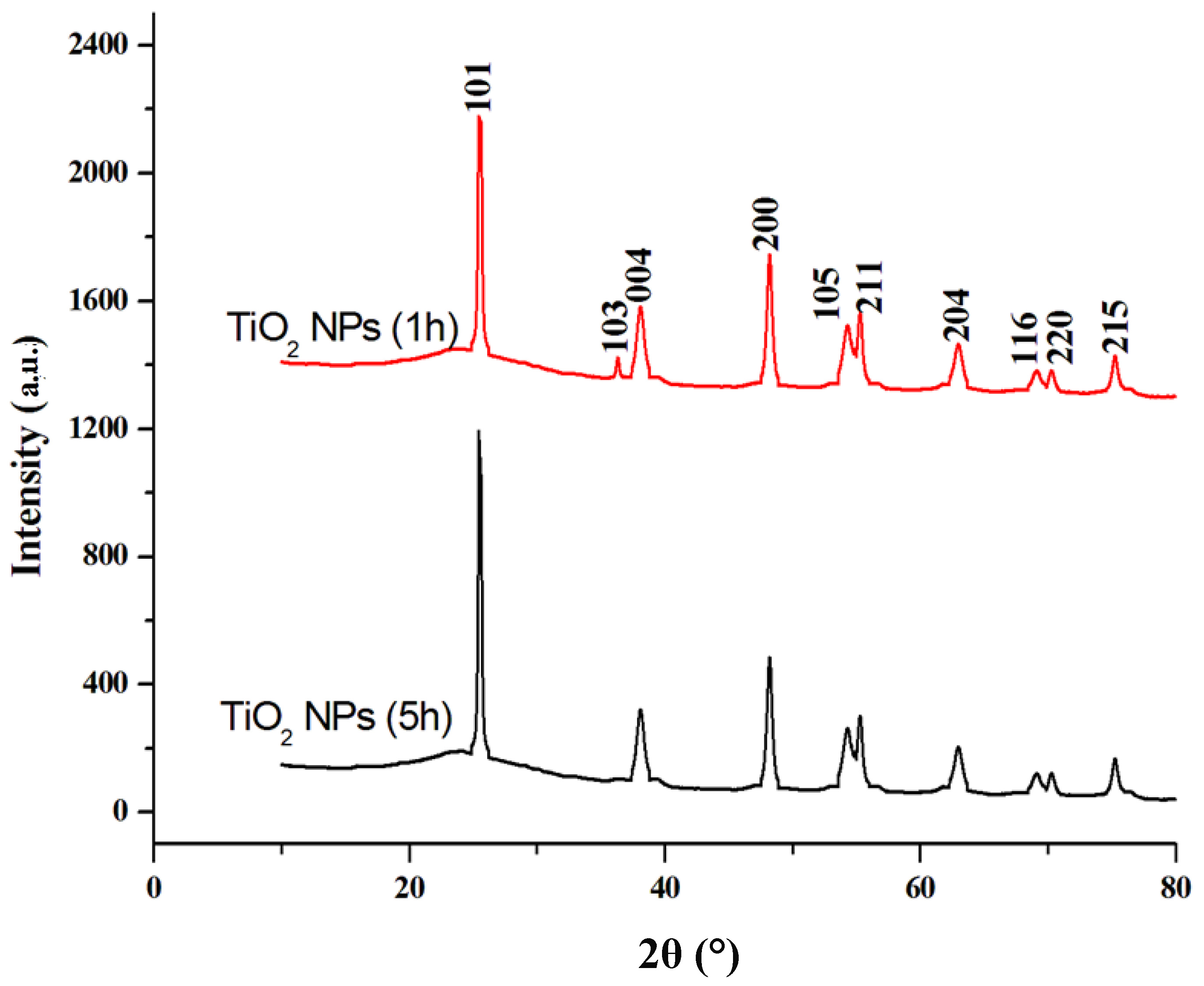
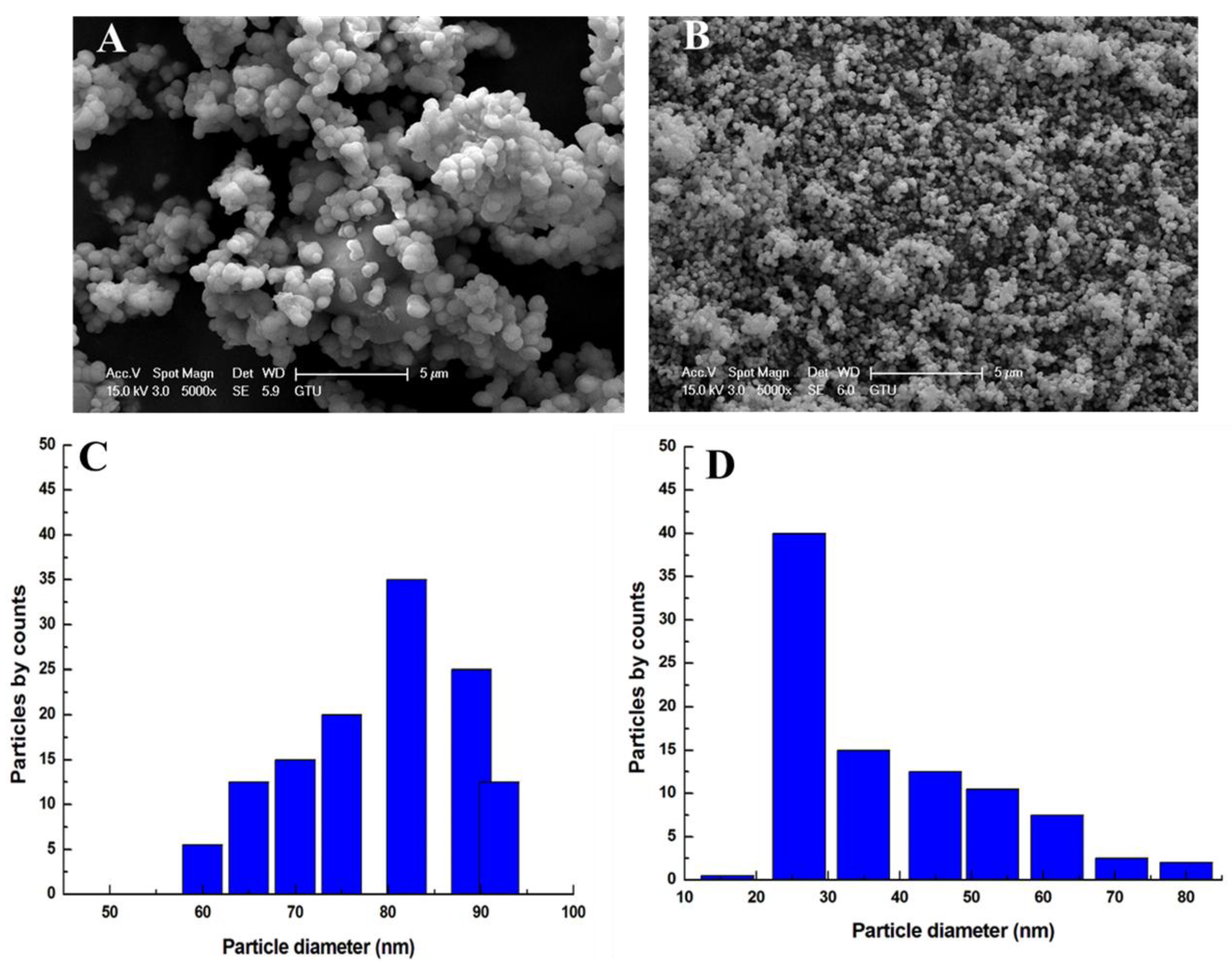
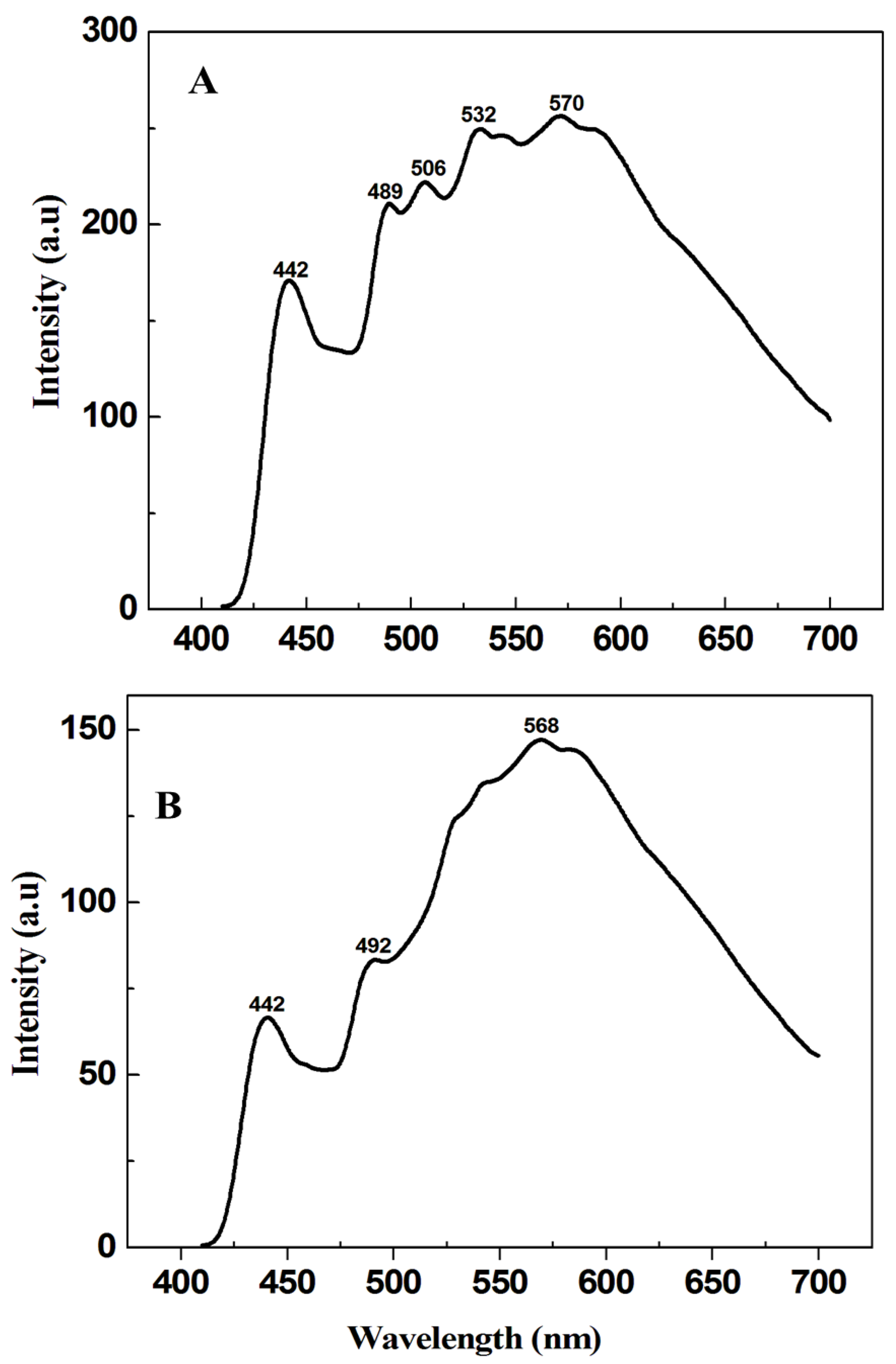
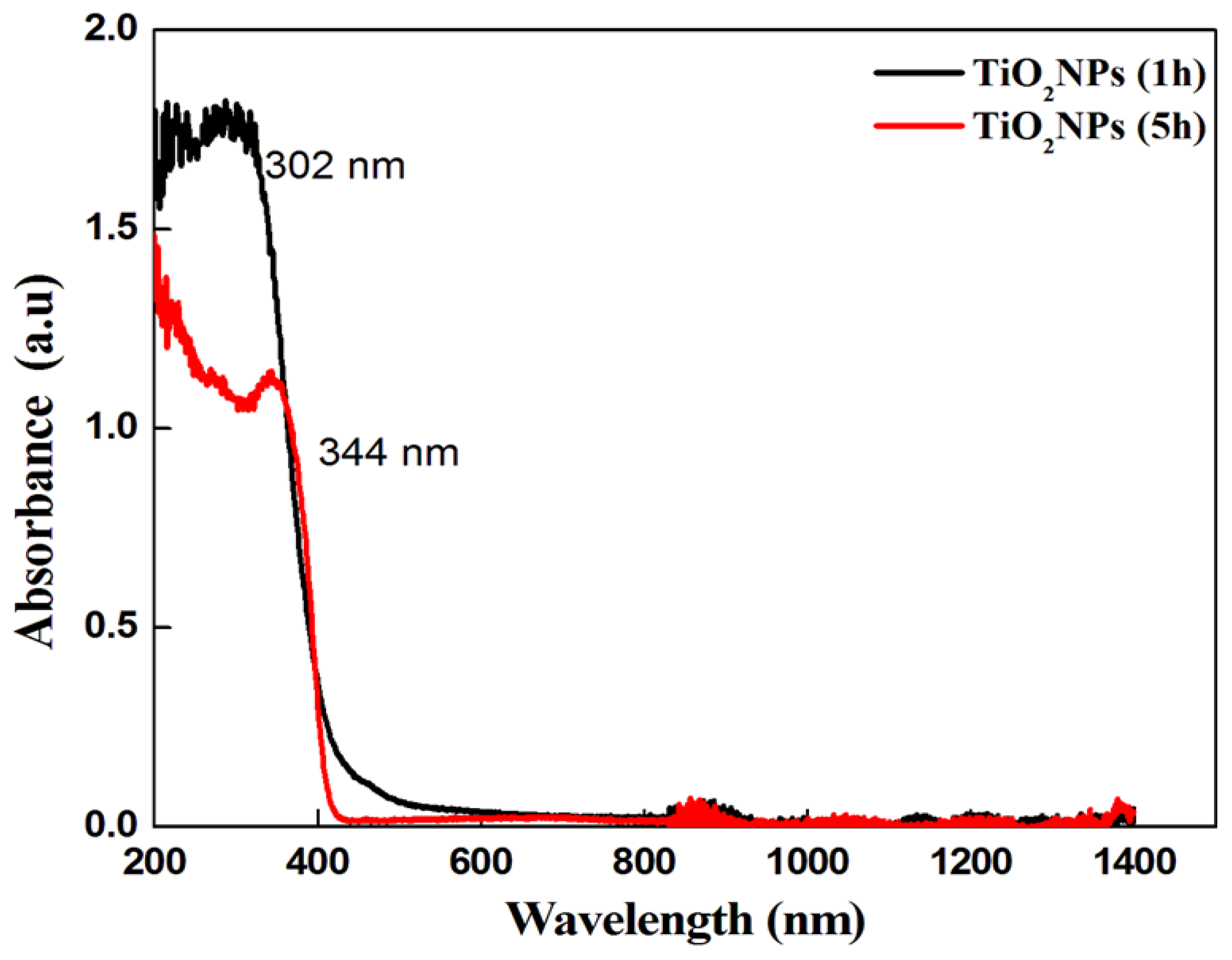
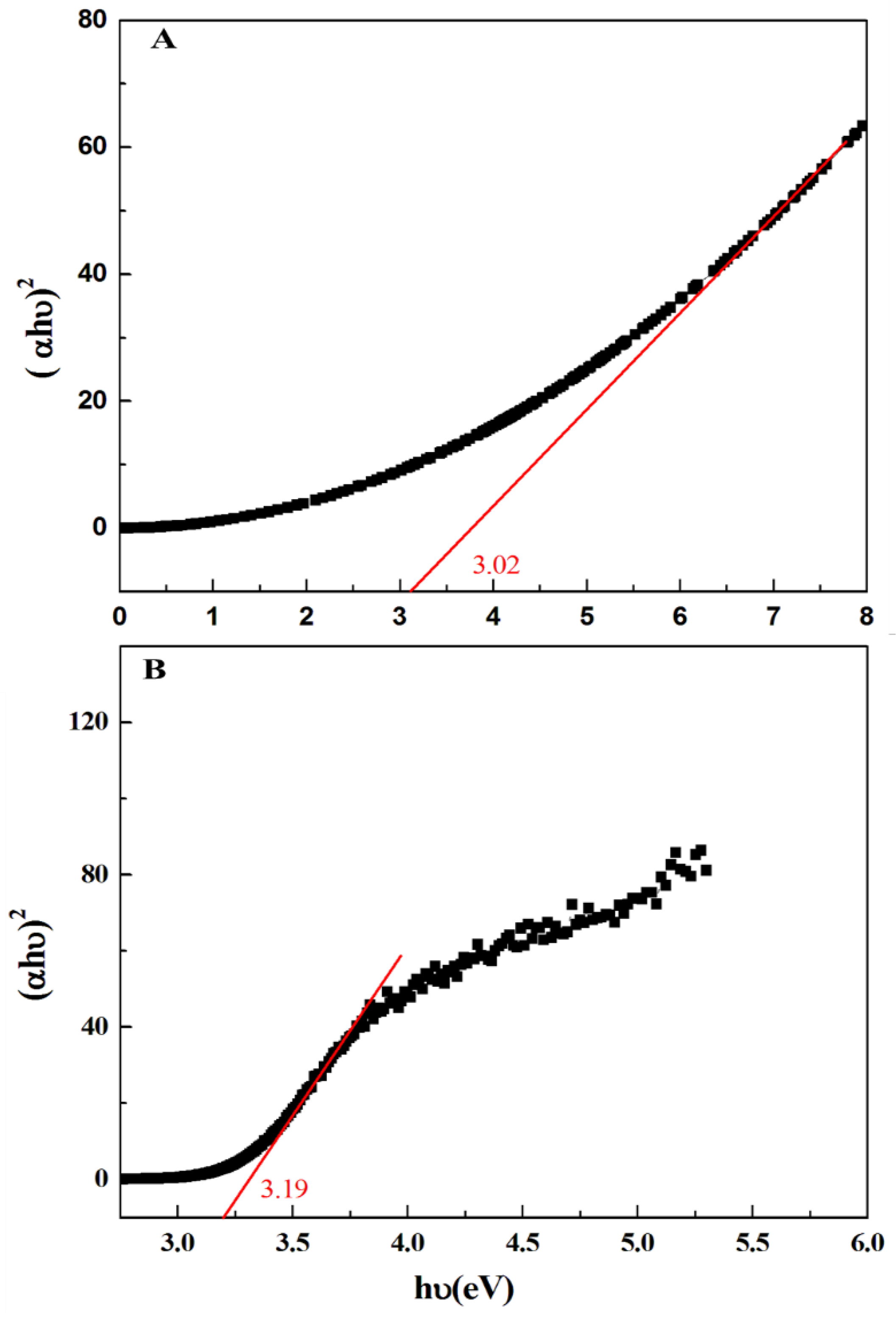
3.1. Characterization Results
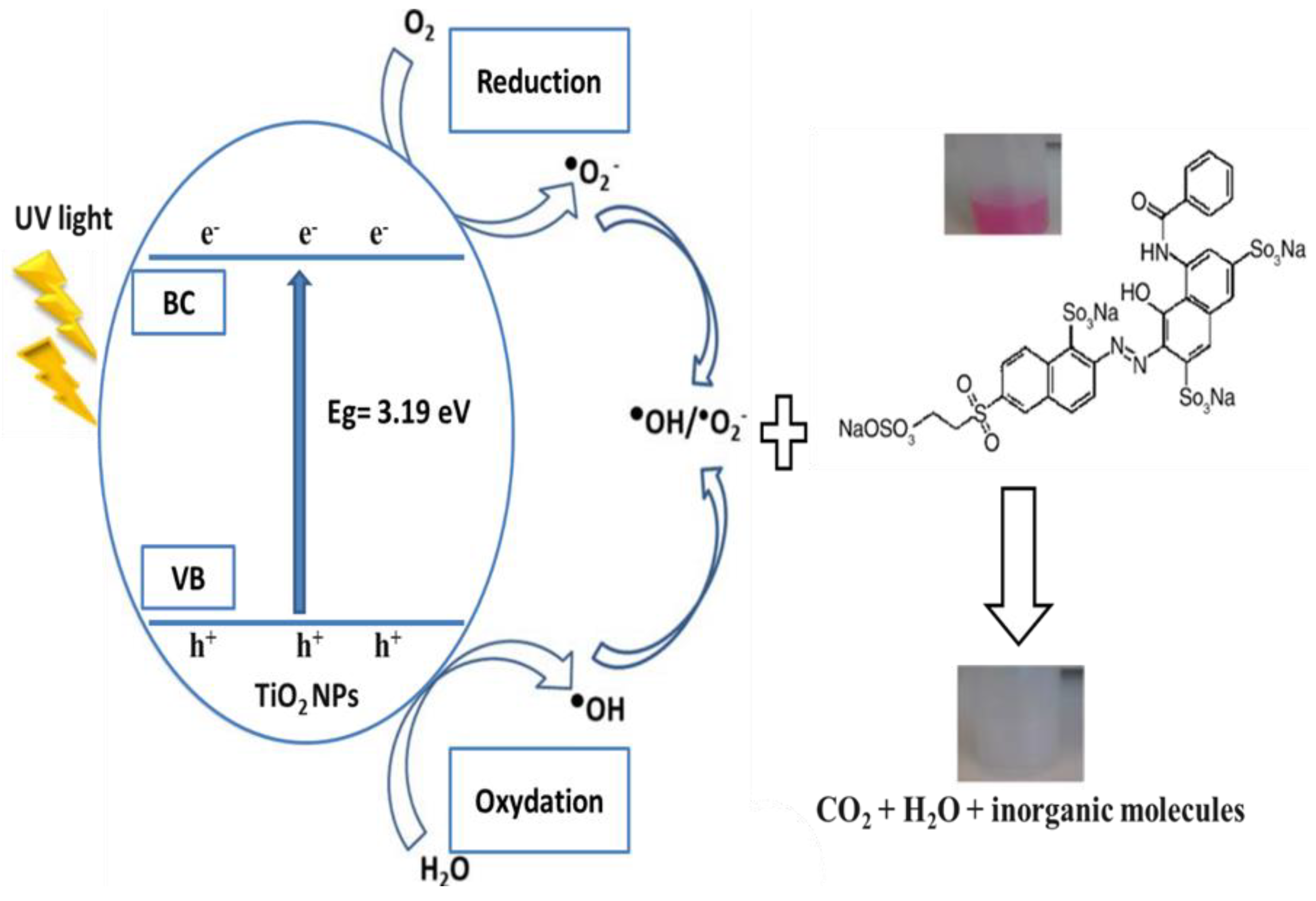
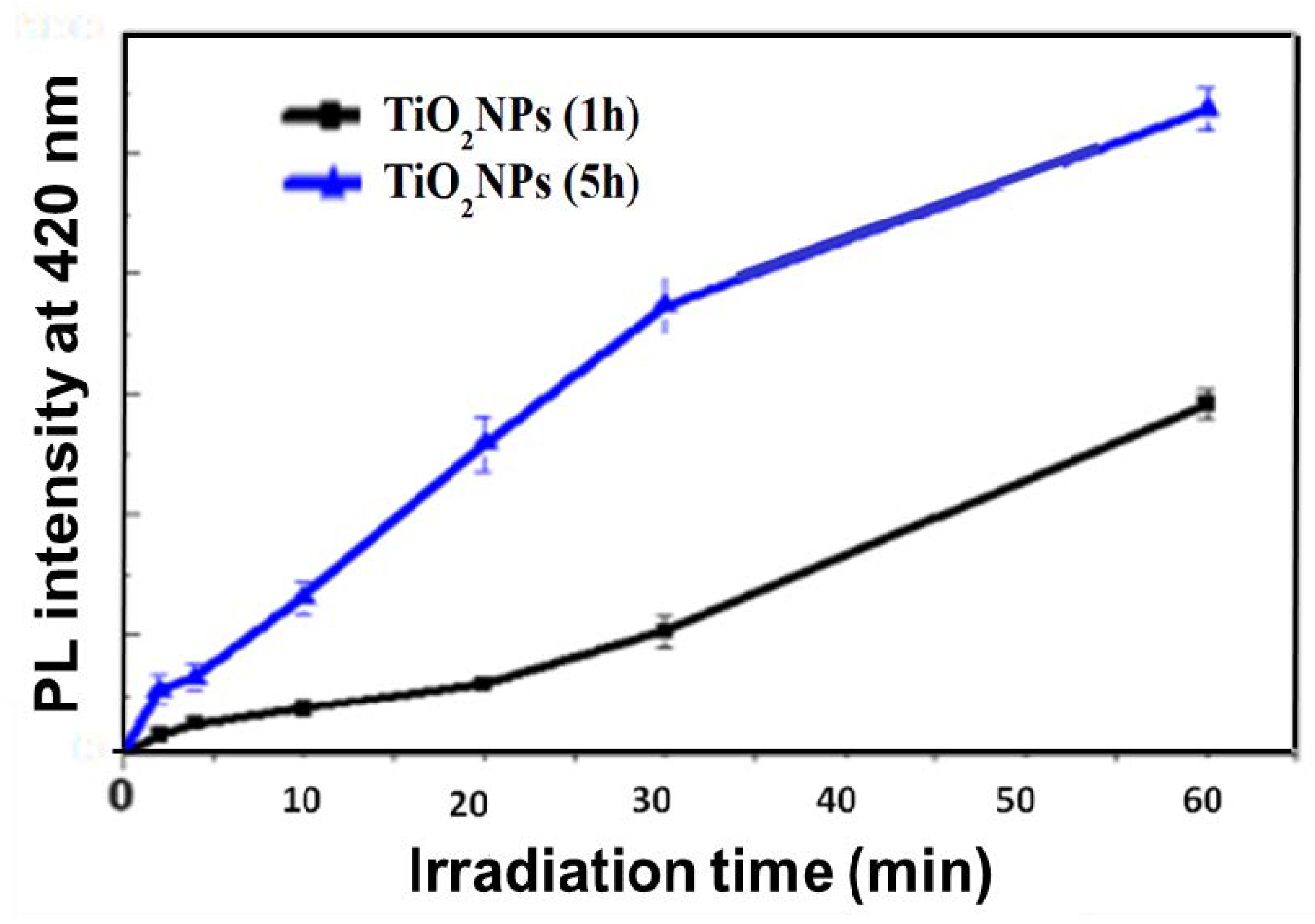
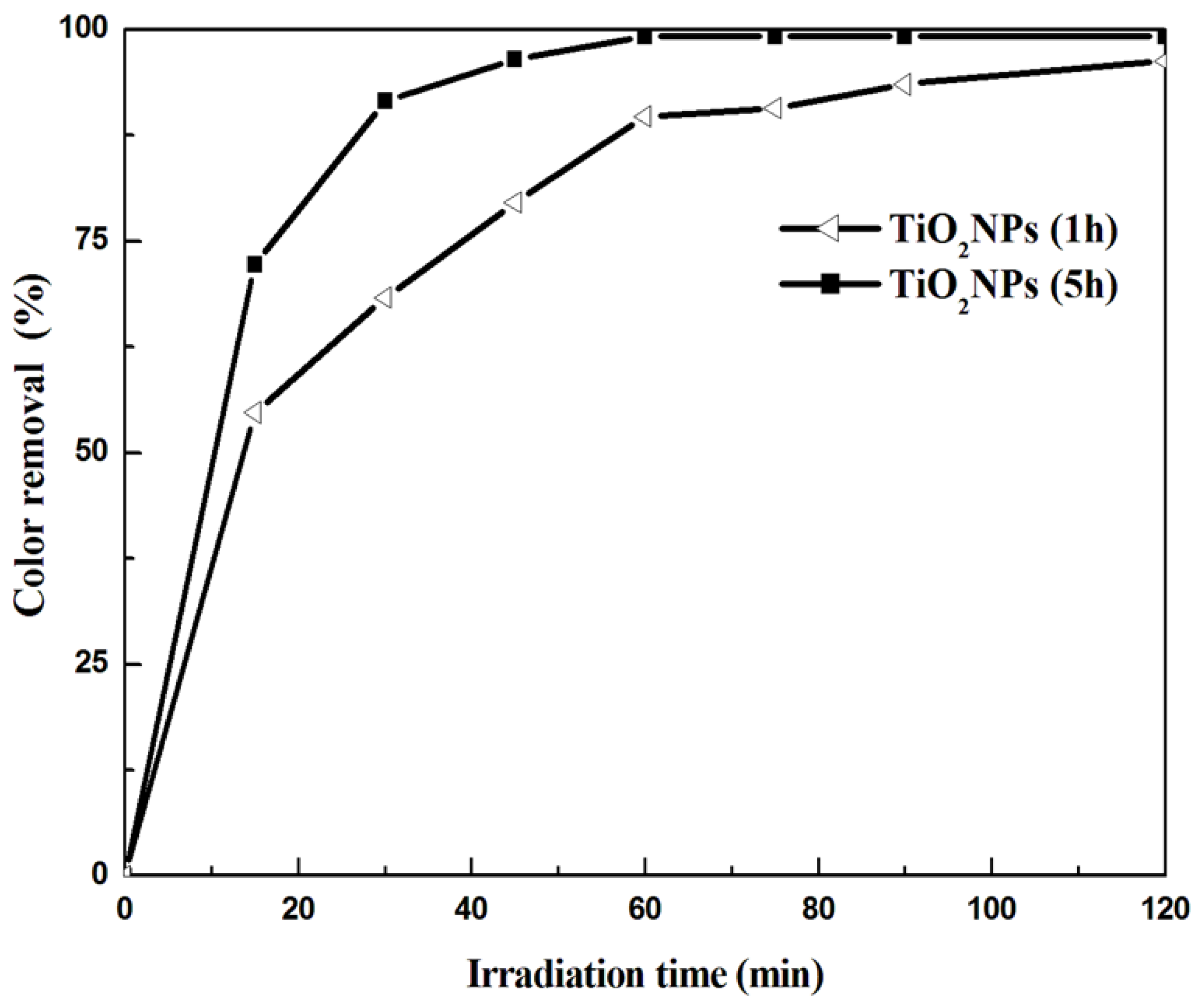
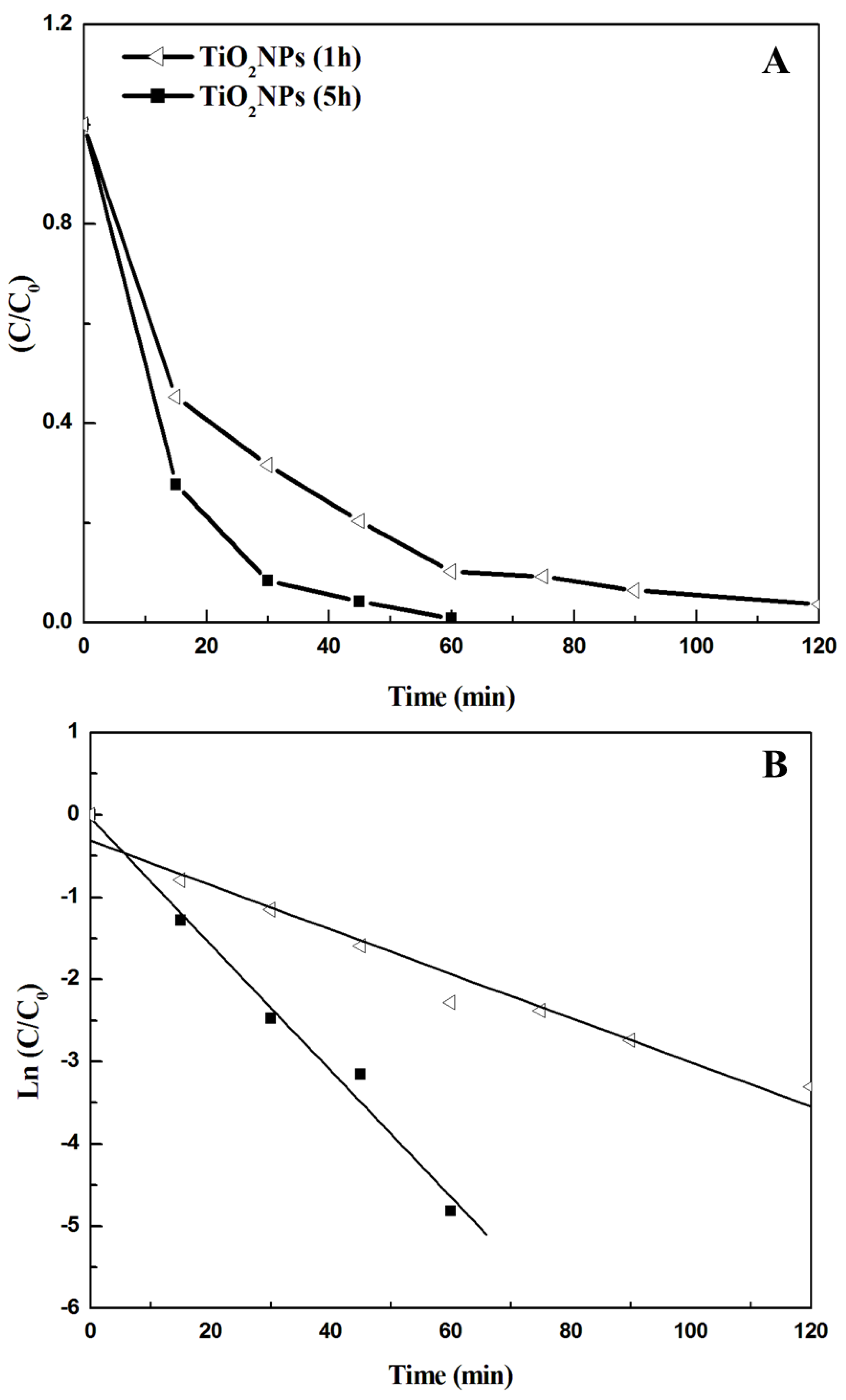
3.2. Photocatalytic Results
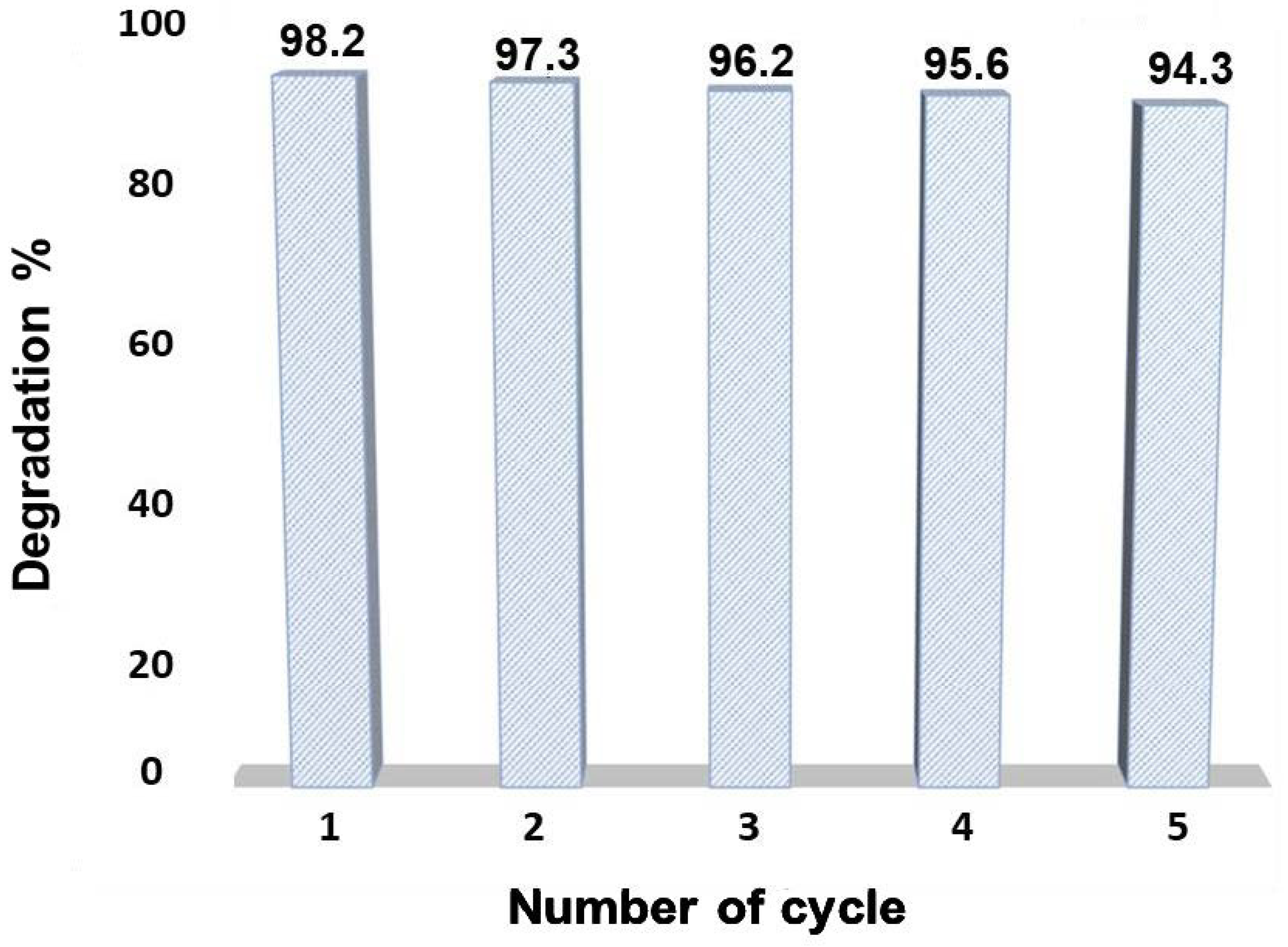
3.3. Reusability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes. Int. Biodeterior. Biodeg. 2015, 104, 21. [Google Scholar] [CrossRef]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Calenciuc, C.; Fdez-Sanromán, A.; Lama, G.; Annamalai, S.; Sanromán, A.; Pazos, M. Recent Developments in Advanced Oxidation Processes for Organics-Polluted Soil Reclamation. Catalysts 2022, 12, 64. [Google Scholar] [CrossRef]
- Kiendrebeogo, M.; Karimi Estahbanati, M.R.; Ouarda, Y.; Drogui, P.; Tyagi, R.D. Electrochemical degradation of nanoplastics in water: Analysis of the role of reactive oxygen species. Sci. Total Environ. 2022, 808, 151897. [Google Scholar] [CrossRef] [PubMed]
- Tsarev, S.; Collins, R.N.; Ilton, E.S.; Fahy, A.; Waite, T.D. The short-term reduction of uranium by nanoscale zero-valent iron (nZVI): Role of oxide shell, reduction mechanism and the formation of U(v)-carbonate phases. Environ. Sci. Nano 2017, 4, 1304. [Google Scholar] [CrossRef]
- Dey, A.S.; Bose, H.; Mohapatra, B.; Sar, P. Biodegradation of Unpretreated Low-Density Polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., Isolated from Waste Dumpsite and Drilling Fluid. Front. Microbiol. 2020, 11, 603210. [Google Scholar] [CrossRef] [PubMed]
- Okonji, S.O.; Yu, L.; Dominic, J.A.; Pernitsky, D.; Achari, G. Adsorption by Granular Activated Carbon and Nano Zerovalent Iron from Wastewater: A Study on Removal of Selenomethionine and Selenocysteine. Water 2021, 13, 23. [Google Scholar] [CrossRef]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton Process for Wastewater Treatment. In Advanced Oxidation Processes—Applications, Trends, and Prospects; IntechOpen: London, UK, 2020; Volume 90256, p. 182. [Google Scholar]
- Palanisamy, S.; Nachimuthu, P.; Awasthi, M.K.; Ravindran, B.; Chang, S.W.; Palanichamy, M.; Nguyen, D.D. Application of electrochemical treatment for the removal of triazine dye using aluminium electrodes. J. Water Supply Res. Technol. 2020, 69, 345–354. [Google Scholar] [CrossRef]
- Franca, R.; Oliveira, M.C.; Pinheiro, H.M.; Lourenço, N.D. Biodegradation products of a sulfonated azo dye in aerobic granular sludge sequencing batch reactors treating a simulated textile wastewater. ACS. Sustain. Chem. Eng. 2019, 7, 14697. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Wang, J.; Tang, A.; Yang, H. Coated clay presenting super high adsorption capacity for Congo Red. Chem. Eng. J. 2021, 406, 126784. [Google Scholar] [CrossRef]
- Li, H.; Meng, R.; Guo, Y.; Chen, B.; Jiao, Y.; Ye, C.; Long, Y.; Tadich, A.; Yang, Q.-H.; Jaroniec, M.; et al. Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al−S batteries. Nat. Commun. 2021, 12, 5714. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.; Awad, M.; Al-Farraj, A.S.; Al-Turki, A.M. Stability and Dynamic Aggregation of Bare and Stabilized Zero-Valent Iron Nanoparticles under Variable Solution Chemistry. Nanomaterials 2020, 10, 192. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.; Hou, Y.; Lv, H.; Li, J.; Cheng, T.; Yang, L.; Wu, H. Adsorption of low-concentration organic pollutants from typical coal-fired power plants by activated carbon injection. Process Saf. Environ. Prot. 2022, 159, 1174. [Google Scholar] [CrossRef]
- Nagaraj, G.; Dhayal Raj, A.; Irudayaraj, A.A.; Josephine, R. Tuning the Optical Band Gap of Pure TiO2 via Photon Induced Method. Optik 2018, 179, 889–894. [Google Scholar]
- Wang, S.; Ding, Z.; Chang, X.; Xu, J. Modified Nano-TiO2 Based Composites for Environmental Photocatalytic Applications. Catalysts 2020, 10, 759. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.-K.; Kim, S.-J. Effect of Particle Size and Phase Composition of titanium Dioxide Nanoparticles on the Photocatalytic Properties. J. Nanopart. Res. 2001, 3, 141. [Google Scholar] [CrossRef]
- Hao, W.C.; Zheng, S.K.; Wang, T.M. Comparison of the photocatalytic activity of TiO2 powder. J. Mater. Sci. Lett. 2002, 21, 1627. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size Effects in Gas-Phase Photo-oxidation of Trichloroethylene Using Nanometer-Sized TiO2 Catalysts. J. Catal. 2000, 196, 185. [Google Scholar] [CrossRef]
- Krýsa, J.; Keppert, M.; Štengl, V.; Šubrt, J. The effect of thermal treatment on the properties of TiO2 photocatalyst. Mater. Chem. Phys. 2004, 86, 333. [Google Scholar] [CrossRef]
- Ropers, M.-H.; Terrisse, H.; Mercier-Bonin, M.; Humbert, B. Titanium Dioxide as Food Additive. In Application of Titanium Dioxide; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Nabi, G.; Raza, W.; Tahir, M.B. Green Synthesis of TiO2 Nanoparticle Using Cinnamon Powder Extract and the Study of Optical Properties. J. Inorg. Org. Pol. Mat. 2020, 30, 1425. [Google Scholar] [CrossRef]
- Diriba, T.F.; Deresa, E.M. Botanical description, ethnomedicinal uses, phytochemistry, and pharmacological activities of genus Kniphofia and Aloe. Arab. J. Chem. 2022, 15, 9. [Google Scholar]
- Zhong, X.; Yang, B.; Zhang, X.; Jia, J.; Yi, G. Effect of calcining temperature and time on the characteristics of Sb-doped SnO2 nanoparticles synthesized by the sol–gel method. Particuology 2012, 10, 365. [Google Scholar] [CrossRef]
- Lai, C.; An, Z.; Yi, H.; Huo, X.; Qin, L.; Liu, X.; Li, B.; Zhang, M.; Liu, S.; Li, L.; et al. Enhanced visible-light-driven photocatalytic activity of bismuth oxide via the decoration of titanium carbide quantum dots. J. Coll. Inter. Sci. 2021, 600, 161. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lai, C.; Zhang, M.; Liu, S.; Yi, H.; Liu, X.; An, N.; Zhou, X.; Li, L.; Fu, Y.; et al. N, S-GQDs and Au nanoparticles co-modified ultrathin Bi2MoO6 nanosheet with enhanced charge transport dynamics for full-spectrum-light-driven molecular oxygen activation. Chem. Eng. J. 2021, 409, 128281. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Ashok, M.; Kashinath, L.; Sanjeeviraja, C.; Rajendran, A. Phytosynthesis and characterization of TiO2 nanoparticles using diospyros ebenum leaf extract and their antibacterial and photocatalytic degradation of crystal violet. Smart Sci. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Synthesis and Applications in Photocatalysis and Antimicrobial. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Alhalili, Z.; Romdhani, C.; Chemingui, H.; Smiri, M. Removal of dithioterethiol (DTT) from water by membranes of cellulose acetate (AC) and AC doped ZnO and TiO2 nanoparticles. J. Saudi Chem. Soc. 2021, 25, 8. [Google Scholar] [CrossRef]
- Alhalili, Z.; Romdhani, C.; Elarbaoui, S.; Smiri, M. Identification and removal of sulfhydryl groups from wastewaters. J. Saudi Chem. Soc. 2021, 25, 11. [Google Scholar] [CrossRef]
- Alhalili, Z.; Souli, H.; Smiri, M. Effect of LEO (Lycium Essential Oils) as green inhibitors of calcium carbonate scale on nanoparticles-doped ultrafiltration membrane (UFM) and water treatment. Arab. J. Sci. Eng. 2022, 47, 6233. [Google Scholar] [CrossRef]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Jain, A.; Vaya, D. Photocatalytic activity of TiO2 nanomaterial. J. Chil. Chem. Soc. 2017, 62, 3683–3690. [Google Scholar] [CrossRef]
- Aphairaj, D.; Wirunmongkol, T.; Pavasupree, S.; Limsuwan, P. Effect of calcination temperatures on structures of TiO2 powders prepared by hydrothermal method using thai leucoxene mineral. Energy Procedia 2011, 9, 539–544. [Google Scholar] [CrossRef]
- Indrayana, I.P.T.; Tjuana, L.A.; Tuny, M.T.; Kurnia. Nanostructure and optical propertiesof Fe3O4: Effect of calcination temperature and dwelling time. J. Phys. Conf. Ser. 2019, 1341, 082044. [Google Scholar] [CrossRef]
- Xing, S.; Song, S.; Xiang, J. Low temperature combustion synthesis and photoluminescence mechanism of ZnO/ZnAl2O4 composite phosphors. Opt. Int. J. Light Electron. Opt. 2020, 208, 164526. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 2007, 61, 79. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269. [Google Scholar] [CrossRef]
- Massima Mouele, E.S.; Dinu, M.; Cummings, F.; Fatoba, O.O.; Zar Myint, M.T.; Kyaw, H.H.; Parau, A.C.; Vladescu, A.; Francesconi, M.G.; Pescetelli, S.; et al. Effect of Calcination Time on the Physicochemical Properties and Photocatalytic Performance of Carbon and Nitrogen Co-Doped TiO2 Nanoparticles. Catalysts 2020, 10, 847. [Google Scholar] [CrossRef]
- Salah, N.H. Etude de la dégradation photocatalytique de polluants organiques en présence de dioxyde de titane, en suspension aqueuse et en lit fixe. Ph.D. Thesis, Université de Grenoble, Grenoble, France, Université Mentouri, Constantine, Algeria, 2012; 175p. [Google Scholar]
- Zhang, Y.; Miao, B.; Chen, Q.; Bai, Z.; Cao, Y.; Davaa, B. Synthesis, structure, and photocatalytic activity of TiO2-montmorillonite composites. Catalysts 2022, 12, 486. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, S.; Yu, X.; Gao, Y.; Wen, Z. In Situ Synthesis and Photocatalytic Properties of Titanium Dioxide Nanoparticles on Cotton Fabrics. Water Air Soil Pollut. 2019, 230, 169. [Google Scholar] [CrossRef]
- Wahi, R.K.; Yu, W.W.; Liu, Y.; Mejia, M.L.; Falkner, J.C.; Nolte, W.; Colvin, V.L. Photodegradation of Congo Red catalyzed by nanosized TiO2. J. Mol. Catal. A Chem. 2005, 242, 48. [Google Scholar] [CrossRef]
- Bilal, M.; Umar, M.; Bakhsh, E.M.; Ali, J.; Ahmad, R.; Akhtar, K.; Khan, S.B. Structural, optical and photocatalytic properties of silver-doped magnesia: Computational and experimental study. J. Mol. Liq. 2021, 339, 117176. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Heidalgo-lópz, M.C.; López-gonzález, R.; Frías-márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Jawed, A.; Verma, R.; Saxena, V.; Pandey, L.M. Photocatalytic metal nanoparticles: A green approach for degradation of dyes. In Photocatalytic Degradation of Dyes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 251–275. [Google Scholar]
- Lee, S.Y.; Kang, D.; Jeong, S.; Do, H.T.; Kim, J.H. Photocatalytic Degradation of Rhodamine B Dye by TiO2 and Gold Nanoparticles Supported on a Floating Porous Polydimethylsiloxane Sponge under Ultraviolet and Visible Light Irradiation. ACS Omega 2020, 5, 4233. [Google Scholar] [CrossRef]
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A. Applied sciences diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Kabachkov, E.N.; Balikhin, I.L.; Kurkin, E.N.; Troitskii, V.N.; Smirniotis, P.G. Correlation of surface area with photocatalytic activity of TiO2. J. Adv. Oxi. Tech. 2018, 21, 127. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of Particle Size on the Structure and Photocatalytic Performance by Alkali-Treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Venkatesh, K.S.; Krishnamoorthi, S.R.; Palani, N.S.; Thirumal, V.; Jose, S.P.; Wang, F.-M.; Ilangovan, R. Facile one step synthesis of novel TiO2 nanocoral by sol–gel method using Aloe vera plant extract. Indian J. Phys. 2015, 89, 445. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Kang, C.W.; Kolya, H. Green synthesis of Ag-Au bimetallic nanocomposites using waste tea leaves extract for degradation congo red and 4-Nitrophenol. Sustainability 2021, 13, 3318. [Google Scholar] [CrossRef]
- Geun, C.P.; Tae, Y.S.; Chae, H.P.; Jun, H.L.; Jinho, J. Effects of Calcination Temperature on Morphology, Microstructure, and Photocatalytic Performance of TiO2 Mesocrystals. Ind. Eng. Chem. Res. 2017, 56, 8235–8240. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhalili, Z.; Smiri, M. The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C. Crystals 2022, 12, 1629. https://doi.org/10.3390/cryst12111629
Alhalili Z, Smiri M. The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C. Crystals. 2022; 12(11):1629. https://doi.org/10.3390/cryst12111629
Chicago/Turabian StyleAlhalili, Zahrah, and Moez Smiri. 2022. "The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C" Crystals 12, no. 11: 1629. https://doi.org/10.3390/cryst12111629
APA StyleAlhalili, Z., & Smiri, M. (2022). The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C. Crystals, 12(11), 1629. https://doi.org/10.3390/cryst12111629






