Biogenic Synthesis of Zero Valent Fe/Magnetite Fe3O4 Nanoparticles Using Caralluma acutangula and Application for Methylene Blue Dye Degradation under UV Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
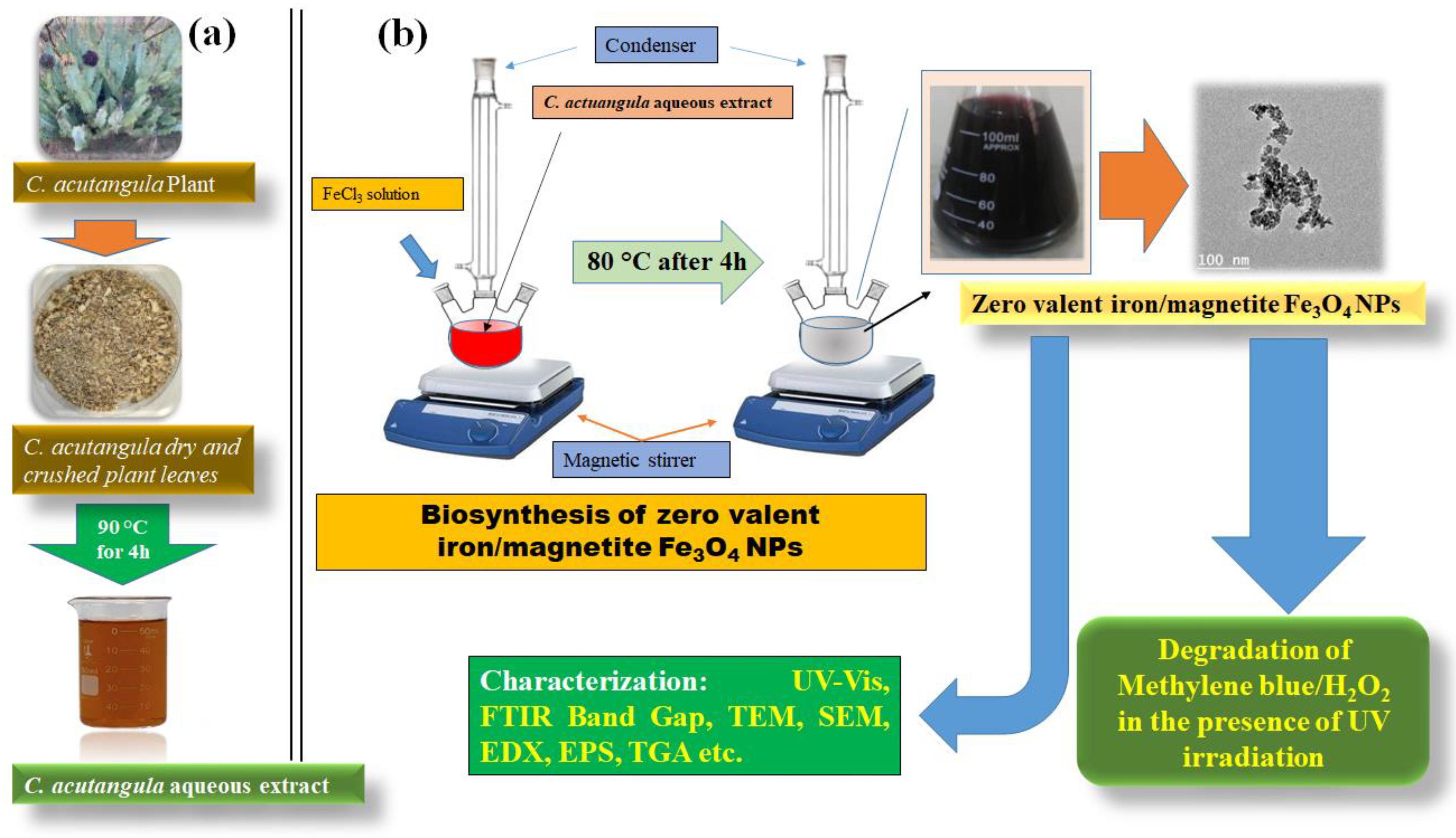
2.2. Preparation of Fe0/Fe3O4 NPs
2.3. Catalytic Degradation of MB Dye
2.4. Characterization of Fe0/Fe3O4 NPs
3. Results and Discussions
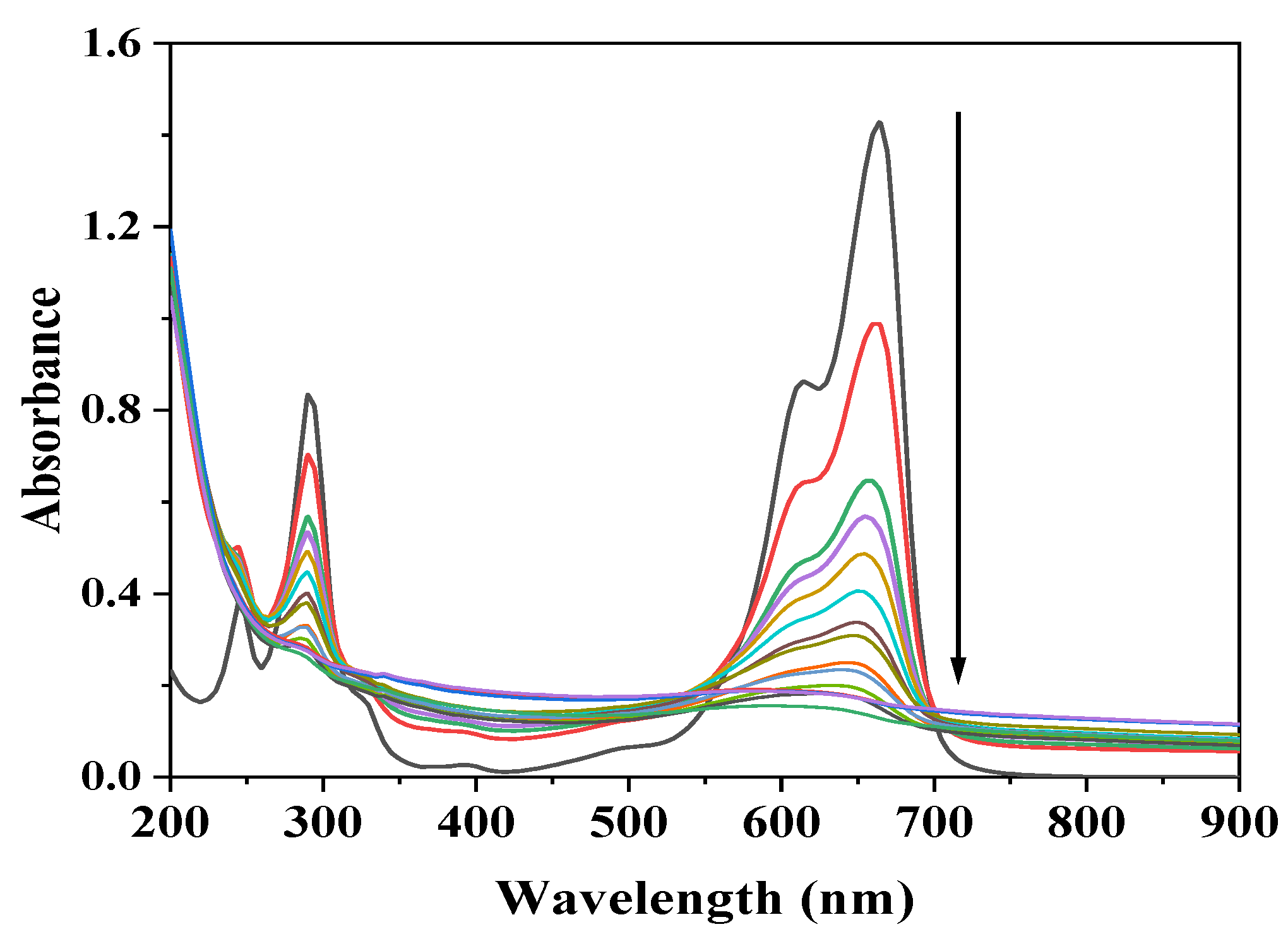
3.1. UV-VIS and Band Gap Analysis
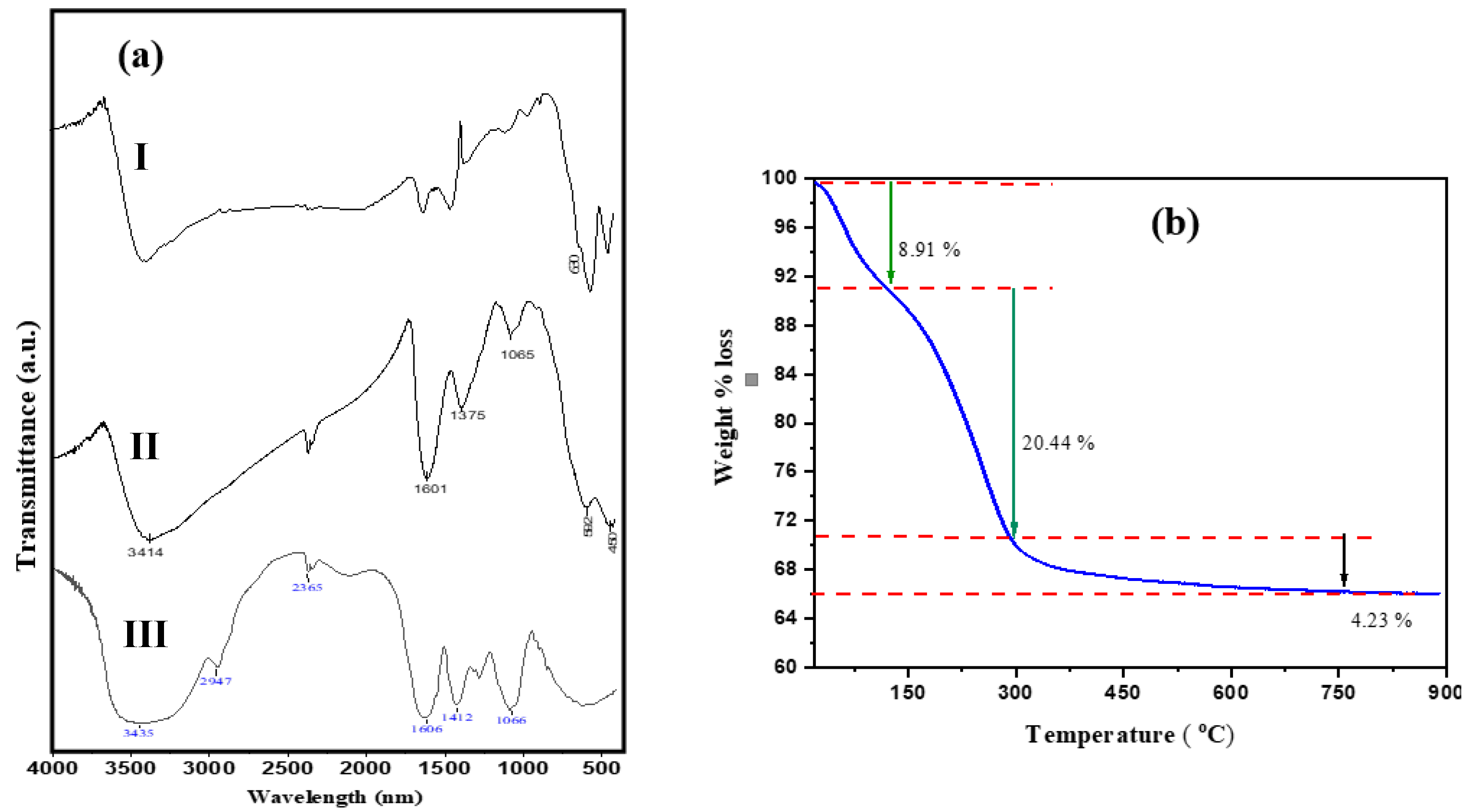
3.2. FTIR and TGA Analysis
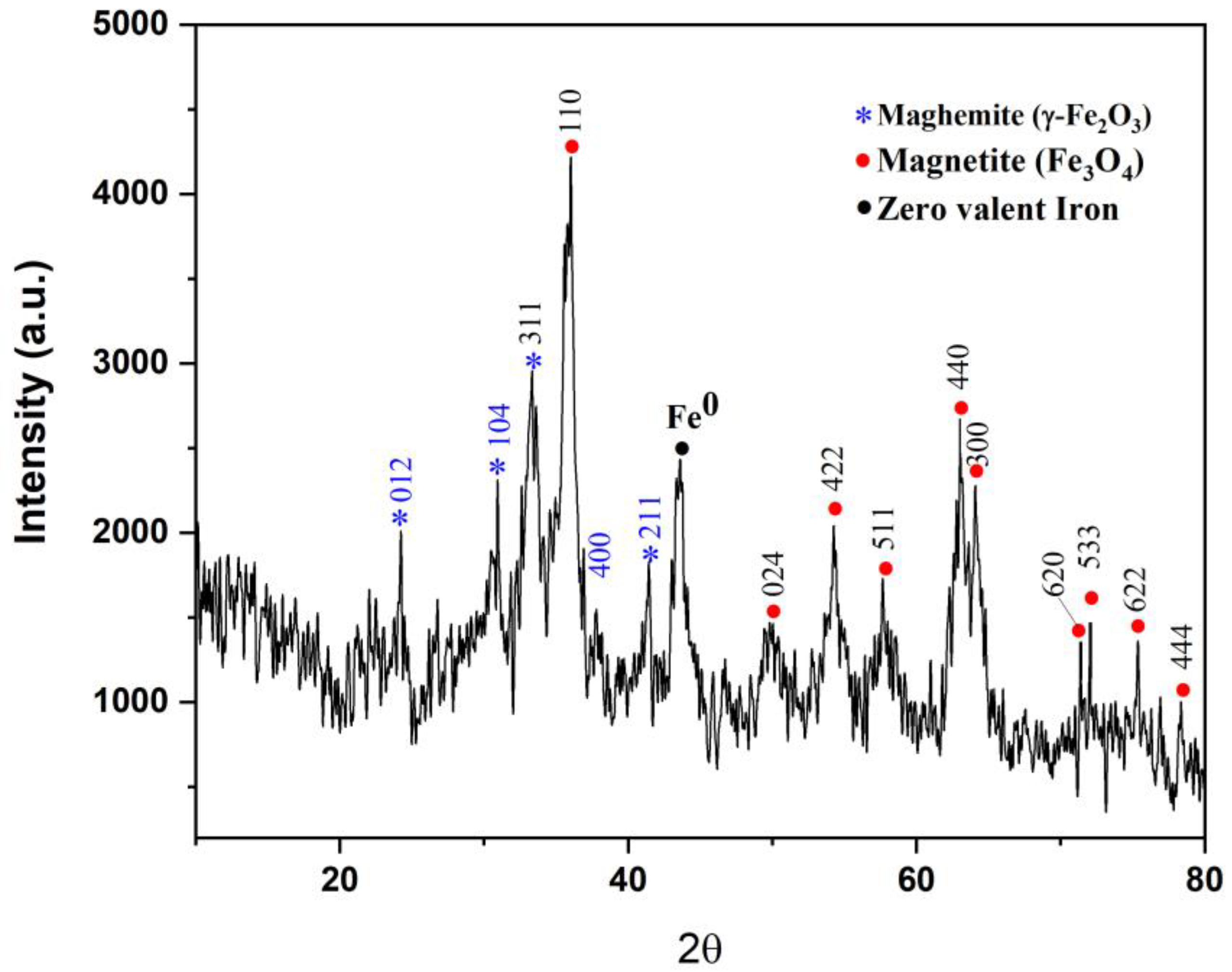
3.3. XRD Analysis
3.4. TEM and Particles Size Distribution
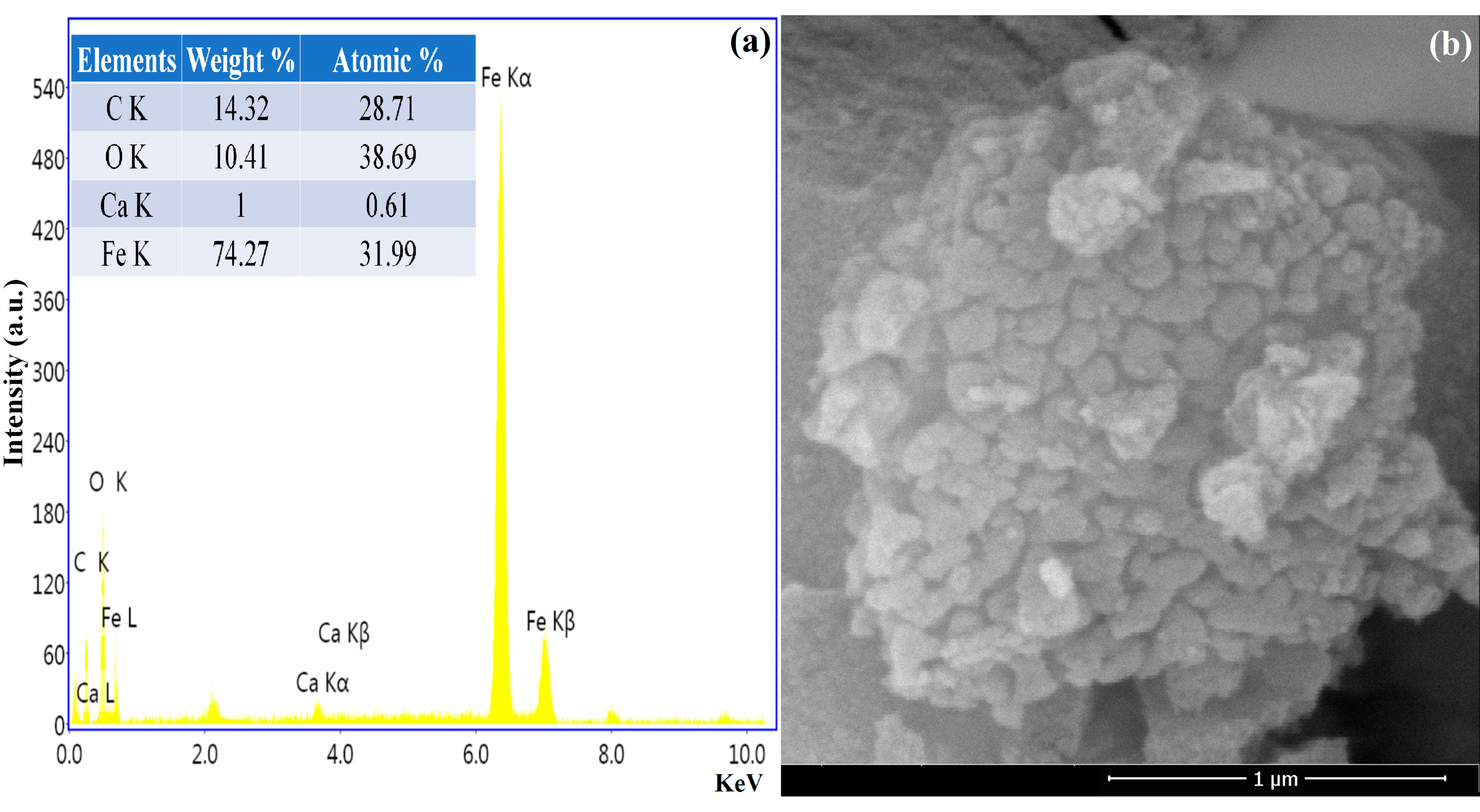
3.5. SEM and EDX Analysis
3.6. XPS Analysis
3.7. Surface Area Measurement of Fe0/Fe3O4 NPs
3.8. Catalytic Activity of Fe0/Fe3O4 NPs
3.8.1. Photolysis of Methylene Blue (MB)
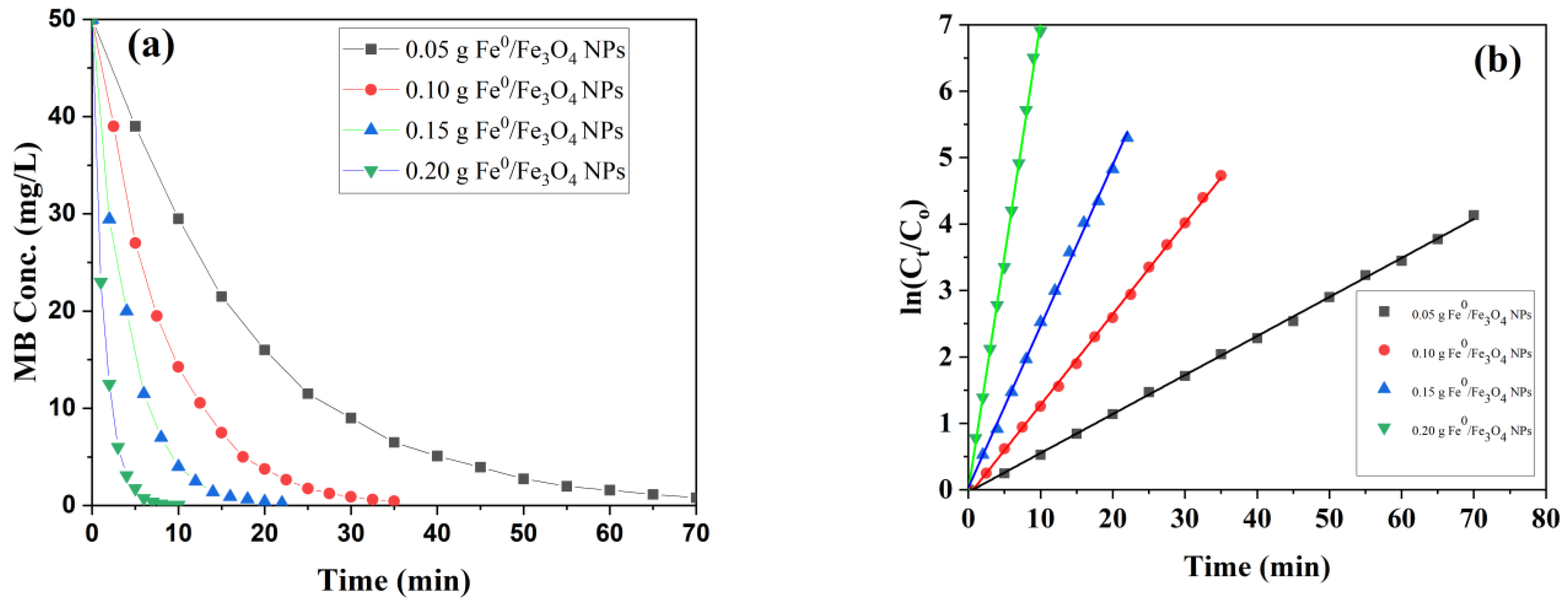
3.8.2. Effect of Fe0/Fe3O4 NPs Catalyst on the Photocatalytic Degradation of MB
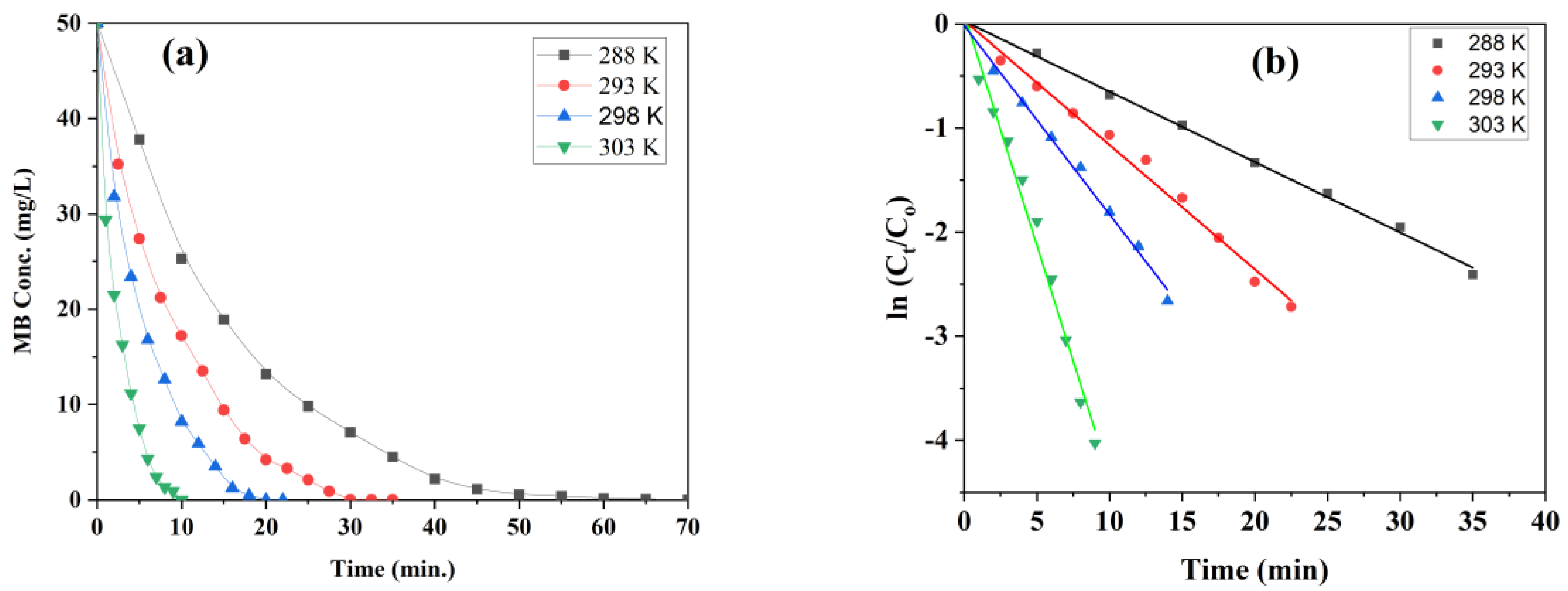
3.8.3. Thermodynamic Study of MB Degradation with Fe0/Fe3O4 NPs
3.8.4. Thermodynamic Parameters Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdeen, M.; Sabry, S.; Ghozlan, H.; El-Gendy, A.A.; Carpenter, E.E. Microbial-Physical Synthesis of Fe and Fe3O4 Magnetic Nanoparticles Using Aspergillus niger YESM1 and Supercritical Condition of Ethanol. J. Nanomater. 2016, 2016, 9174891. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Arya, A.; Mishra, V.; Chundawat, S.T. Green synthesis of silver nanoparticles from green algae (Botryococcus braunii) and its catalytic behavior for the synthesis of benzimidazoles. Chem. Data Collect. 2019, 20, 100190. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Iqbal, M.; Aslam, S. Applications of Plant Flavonoids in the Green Synthesis of Colloidal Silver Nanoparticles and Impacts on Human Health, Iranian Journal of Science and Technology. Trans. A Sci. 2019, 43, 1381–1392. [Google Scholar]
- Saleh, T.A.; Fadillah, G.; Saputra, O.A. Nanoparticles as components of electrochemical sensing platforms for the detection of petroleum pollutants: A review. TrAC Trends Anal. Chem. 2019, 118, 194–206. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the aquatic environment: Usage, properties, transformation and toxicity—A review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Campos, E.A.; Pinto, D.V.B.S.; Oliveira, J.I.S.d.; Mattos, E.d.C.; Dutra, R.d.C.L. Synthesis, characterization and applications of iron oxide nanoparticles-a short review. J. Aerosp. Technol. Manag. 2015, 7, 267–276. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Abhilash, K.; Revati, B.D. Pandey, Microbial synthesis of iron-based nanomaterials—A review. Bull. Mater. Sci. 2011, 34, 191–198. [Google Scholar] [CrossRef]
- Bhatia, S. Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural Polymer Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2016; pp. 33–93. [Google Scholar]
- Ealia, S.A.M.; Saravanakumar, M. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 032019. [Google Scholar]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Kharanaghi, E.A.; Arough, J.M.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mei, W.; Wu, Q. Applications of metal nanoparticles in medicine/metal nanoparticles as anticancer agents. In Metal Nanoparticles: Synthesis and Applications in Pharmaceutical Sciences; Wiley: Hoboken, NJ, USA, 2018; pp. 169–190. [Google Scholar]
- Borah, D.; Das, N.; Das, N.; Bhattacharjee, A.; Sarmah, P.; Ghosh, K.; Chandel, M.; Rout, J.; Pandey, P.; Ghosh, N.N. Alga-mediated facile green synthesis of silver nanoparticles: Photophysical, catalytic and antibacterial activity. Appl. Organomet. Chem. 2020, 34, e5597. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic nanoparticles: Synthesis, characterisation and applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef]
- Sharma, P.; Kumari, S.; Ghosh, D.; Yadav, V.; Vij, A.; Rawat, P.; Kumar, S.; Sinha, C.; Saini, S.; Sharma, V. Capping agent-induced variation of physicochemical and biological properties of α-Fe2O3 nanoparticles. Mater. Chem. Phys. 2021, 258, 123899. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron oxide nanozyme: A multifunctional enzyme mimetics for biomedical application. Nanozymology 2020, 105–140. [Google Scholar]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Li, X.-Q.; Zhang, W.-X. Iron nanoparticles: The core−shell structure and unique properties for Ni (II) sequestration. Langmuir 2006, 22, 4638–4642. [Google Scholar] [CrossRef] [PubMed]
- Radini, I.A.; Hasan, N.; Malik, M.A.; Khan, Z. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J. Photochem. Photobiol. B 2018, 183, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, A.; Malik, M.A.; Khan, Z.; Al-Thabaiti, S.A.; Hasan, N. Biofabrication of Fe nanoparticles in aqueous extract of Hibiscus sabdariffa with enhanced photocatalytic activities. RSC Adv. 2017, 7, 25149–25159. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yan, X.; Fan, K. Advances in chiral nanozymes: A review. Microchim. Acta 2019, 186, 782. [Google Scholar] [CrossRef]
- Jubran, A.S.; Al-Zamely, O.M.; Al-Ammar, M.H. A study of iron oxide nanoparticles synthesis by using bacteria. Int. J. Pharm. Qual. Assur. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Liu, R. Superparamagnetic α-Fe2O3/Fe3O4 Heterogeneous Nanoparticles with Enhanced Biocompatibility. Nanomaterials 2021, 11, 834. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yakkate, S.; Balaji, T.; Prathima, B.; Jyothi, N.V. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater. Lett. 2013, 100, 241–244. [Google Scholar] [CrossRef]
- Silva, C.; Marchetti, S.; Farro, A., Jr.; Silva, T.; Assaf, J.; Rangel, M. Effect of gadolinium on the catalytic properties of iron oxides for WGSR. Catal. Today 2013, 213, 127–134. [Google Scholar] [CrossRef][Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Adelere, I.A.; Lateef, A. A novel approach to the green synthesis of metallic nanoparticles: The use of agro-wastes, enzymes, and pigments. Nanotechnol. Rev. 2016, 5, 567–587. [Google Scholar] [CrossRef]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Latif, M.; Abbas, S.; Kormin, F.; Mustafa, M. Green synthesis of plant-mediated metal nanoparticles: The role of polyphenols. Asian J. Pharm. Clin. Res. 2019, 12, 75–84. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Chaudhary, S.A.; Waṭaniyah, M.; Miyah, M.W.A.Z. Flora of the Kingdom of Saudi Arabia, Illustrated; Ministry of Agriculture & Water National Agriculture and Water Research Center: Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Al-Fifi, Z.; Masrahi, Y.; Aly, M.; Al-Turki, T. In vitro Anticancer Activity of Caralluma acutangula (Decne.) N.E.Br. Extract. Int. J. Pharm. Sci. Rev. Res. 2016, 38, 59–63. [Google Scholar]
- Alamier, W.M.; Hasan, N.; Ali, S.K.; Oteef, M.D.Y. Biosynthesis of Ag Nanoparticles Using Caralluma acutangula Extract and Its Catalytic Functionality towards Degradation of Hazardous Dye Pollutants. Crystals 2022, 12, 1069. [Google Scholar] [CrossRef]
- Albaghdadi, W.A.; Abdulridha, W.A.; Latif, D. Synthesis and Some Physical Properties of Magnetite (Fe3O4) NPS. J. Multidiscip. Eng. Sci. Stud. 2016, 2, 341–345. [Google Scholar]
- Korpany, K.; Majewski, D.; Chiu, C.; Cross, S.; Blum, A. Iron Oxide Surface Chemistry: The Effect of Chemical Structure on Binding in Benzoic Acid and Catechol Derivatives. Langmuir 2017, 33, 3000–3013. [Google Scholar] [CrossRef]
- Gusrizal, G.; Santosa, S.; Kunarti, E.; Rusdiarso, B. Synthesis of Silver Nanoparticles by Reduction of Silver Ion with m-Hydroxybenzoic Acid. Asian J. Chem. 2017, 29, 1417–1422. [Google Scholar] [CrossRef]
- Fadliah, D.S.; Wahyuni, E.; Santosa, S. Synthesis of Silver Nanoparticles Using o-Hydroxybenzoic, p-Hydroxybenzoic, and o,p-Dihydroxybenzoic Acids as Reducing Agents. Mater. Sci. Forum 2017, 901, 26–31. [Google Scholar]
- Sharma, G.; Jeevanandam, P. Synthesis of self-assembled prismatic iron oxide nanoparticles by a novel thermal decomposition route. RSC Adv. 2012, 3, 189–200. [Google Scholar] [CrossRef]
- Aksimentyeva, O.; Savchyn, V.; Dyakonov, V.; Piechota, S.; Horbenko, Y.; Demchenko, P.; Popov, A.; Szymczak, H. Modification of Polymer-Magnetic Nanoparticles by Luminescent and Conducting Substances. Mol. Cryst. Liq. Cryst. 2014, 590, 35–42. [Google Scholar] [CrossRef]
- Jozwiak, W.; Kaczmarek, E.; Maniecki, T.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A-Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Cai, N.; Larese-Casanova, P. Facile Synthesis and Reuse of Magnetic Black Carbon Magnetite (BC-Mag) for Fast Carbamazepine Removal from Water. Nanomaterials 2020, 10, 213. [Google Scholar] [CrossRef]
- Dalal, U.; Reddy, S.N. A novel nano zero-valent iron biomaterial for chromium (Cr(6+) to Cr(3+)) reduction. Environ. Sci. Pollut. Res. Int. 2019, 26, 10631–10640. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Ali, O.A.A.; Saleh, D.I.; Abu-Saied, M.A.; Ahmed, M.K.; Abdel-Fattah, E.; Mansour, S.F. Degradation of methylene blue using co-dopants Mg and Se in an hydroxyapatite composite. Luminescence 2022, 37, 399–407. [Google Scholar] [CrossRef]
- Emara, M.M.; Tourky, A.S.; El-Moselhy, M.M. Structural modification of mordenite zeolite with Fe for the photo-degradation of EDTA. J. Hazard. Mater. 2009, 166, 514–522. [Google Scholar] [CrossRef]
- Elsayed, M.S.; Ahmed, I.A.; Bader, D.M.D.; Hassan, A.F. Green Synthesis of Nano Zinc Oxide/Nanohydroxyapatite Composites Using Date Palm Pits Extract and Eggshells: Adsorption and Photocatalytic Degradation of Methylene Blue. Nanomaterials 2021, 12, 49. [Google Scholar] [CrossRef]
- El-Moselhy, M.M. Methylene blue dye degradation using C-100 polymeric material modified with ZnO nanoparticles. Desalination Water Treat. 2016, 57, 25800–25811. [Google Scholar] [CrossRef]
- El-Moselhy, M.M. Photo-degradation of acid red 44 using Al and Fe modified silicates. J. Hazard. Mater. 2009, 169, 498–508. [Google Scholar] [CrossRef]
- Liu, C.; Yang, B.; Chen, J.; Jia, F.; Song, S. Synergetic degradation of Methylene Blue through photocatalysis and Fenton reaction on two-dimensional molybdenite-Fe. J. Environ. Sci. 2022, 111, 11–23. [Google Scholar] [CrossRef]
- Perumal, V.; Inmozhi, C.; Uthrakumar, R.; Robert, R.; Chandrasekar, M.; Mohamed, S.B.; Honey, S.; Raja, A.; Al-Mekhlafi, F.A.; Kaviyarasu, K. Enhancing the photocatalytic performance of surface—Treated SnO2 hierarchical nanorods against methylene blue dye under solar irradiation and biological degradation. Environ. Res. 2022, 209, 112821. [Google Scholar] [CrossRef]



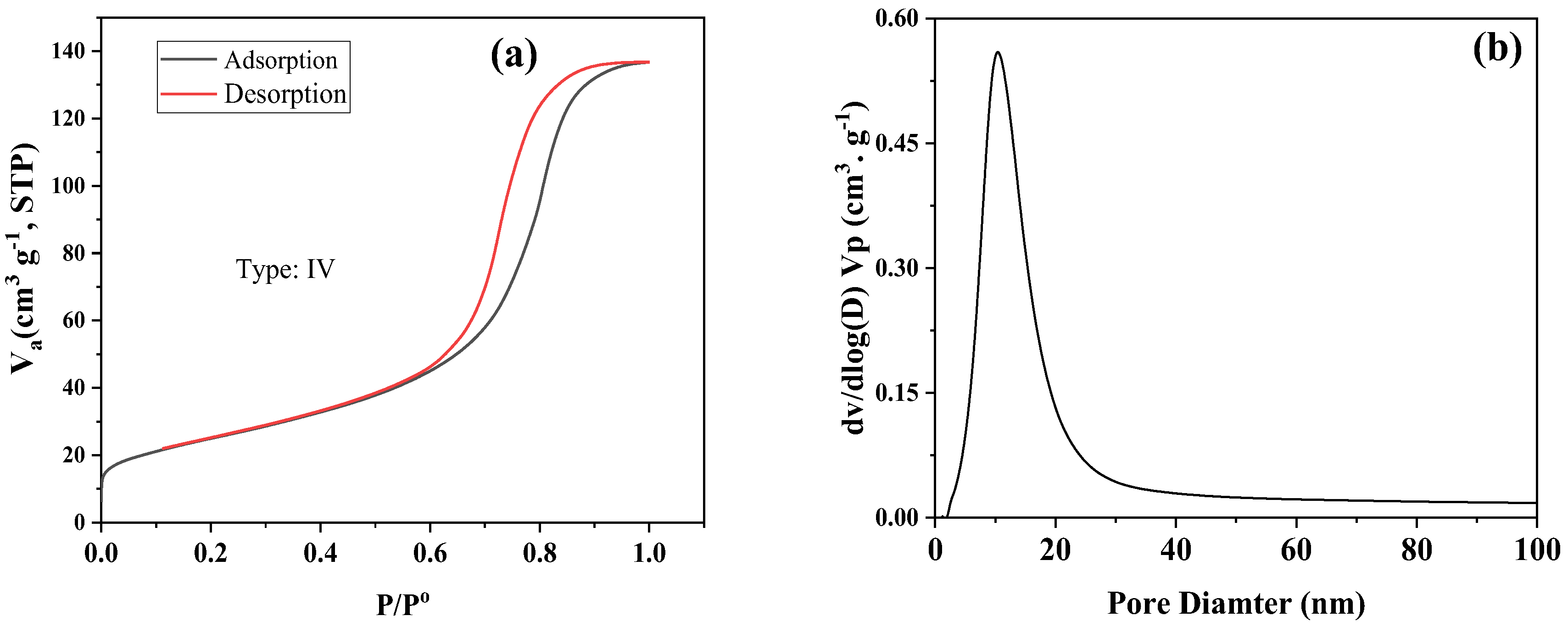


| Sample | SBET (m2. G−1) | Vp (cm3. G−1) | Pore Size (nm) |
|---|---|---|---|
| Fe3O4 | 89.1 | 0.2264 | 10.75 |
| H2O2 (mmol/L) | Kapp | R2 | Fe Oxide NPs (g/L) | Kapp | R2 |
|---|---|---|---|---|---|
| 0.005 | 0.03361 | 0.996 | 0.05 | 0.058 | 0.999 |
| 0.010 | 0.04514 | 0.998 | 0.10 | 0.137 | 0.999 |
| 0.015 | 0.06690 | 0.998 | 0.15 | 0.243 | 0.998 |
| 0.020 | 0.13343 | 0.999 | 0.20 | 0.704 | 0.998 |
| T(K) | T−1( 10−3) | Kapp | Ln(K) | Ea. | ΔH | ΔS | ΔG |
|---|---|---|---|---|---|---|---|
| (min−1) | (KJ. mol−1) | (KJ. mol−1) | (J. K−1. mol−1) | (KJ. K−1.mol−1) | |||
| 288 | 3.47 | 0.069 | −2.67 | 87.78 | 308.4 | −323 | 92.9864 |
| 293 | 3.41 | 0.120 | −2.12 | 93.9656 | |||
| 298 | 3.36 | 0.200 | −1.61 | 95.8964 | |||
| 303 | 3.30 | 0.440 | −0.82 | 97.5054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamier, W.M.; El-Moselhy, M.M.; Bakry, A.M.; Hasan, N.; Alamri, A.A. Biogenic Synthesis of Zero Valent Fe/Magnetite Fe3O4 Nanoparticles Using Caralluma acutangula and Application for Methylene Blue Dye Degradation under UV Light Irradiation. Crystals 2022, 12, 1510. https://doi.org/10.3390/cryst12111510
Alamier WM, El-Moselhy MM, Bakry AM, Hasan N, Alamri AA. Biogenic Synthesis of Zero Valent Fe/Magnetite Fe3O4 Nanoparticles Using Caralluma acutangula and Application for Methylene Blue Dye Degradation under UV Light Irradiation. Crystals. 2022; 12(11):1510. https://doi.org/10.3390/cryst12111510
Chicago/Turabian StyleAlamier, Waleed M., Medhat Mohamed El-Moselhy, Ayyob M. Bakry, Nazim Hasan, and Abdullah Ali Alamri. 2022. "Biogenic Synthesis of Zero Valent Fe/Magnetite Fe3O4 Nanoparticles Using Caralluma acutangula and Application for Methylene Blue Dye Degradation under UV Light Irradiation" Crystals 12, no. 11: 1510. https://doi.org/10.3390/cryst12111510
APA StyleAlamier, W. M., El-Moselhy, M. M., Bakry, A. M., Hasan, N., & Alamri, A. A. (2022). Biogenic Synthesis of Zero Valent Fe/Magnetite Fe3O4 Nanoparticles Using Caralluma acutangula and Application for Methylene Blue Dye Degradation under UV Light Irradiation. Crystals, 12(11), 1510. https://doi.org/10.3390/cryst12111510







