Abstract
Four new rare-earth metal–organic frameworks containing thieno[3,2b]thiophene-2,5-dicarboxylate (ttdc2−) with general formulae [M2(DMF)4(ttdc)3] (M3+ = Y3+ for 1, La3+ for 2, Tb3+ for 3) and [M2(H2O)2(ttdc)3] (M3+ = Lu3+ for 4) were synthesized. Their crystal structures were determined by performing a single-crystal X-ray diffraction analysis. Coordination polymers 1–3 are based on the binuclear metal-carboxylate building units with the formulae {M2(DMF)4(OOCR)6}. The six-connected blocks in 1–3 form a three-dimensional network with the primitive cubic (pcu) topology. Coordination framework 4 is based on chains comprised by stretched pseudo-binuclear metal-carboxylate building units. The chains are interconnected in four directions with ttdc2− linkers forming the 3D framework. The luminescent properties were studied for the synthesized frameworks in the solid state. All the coordination frameworks show a broad blue emission band (λex = 380 nm) typical for intra-ligand electron transitions. The sensing properties of 3 dispersions in solutions were investigated in detail and the luminescent response (quenching) was discovered in the presence of cinnamaldehyde and quinoline in diluted solutions at concentrations of as low as 4 × 10−1 vol.% and 4 × 10−2 vol.% (~3 × 10−3 M), respectively.
1. Introduction
Metal–organic frameworks (MOFs) represent a prominent class of coordination compounds. The vast variety of available organic linkers and metal nodes creates opportunity to design materials with highly tunable characteristics such as porosity [1,2,3], chemical affinity [4,5], catalytic activity [6,7,8,9], and optical properties [10,11,12] etc.
Lanthanide(III) MOFs attract special interest due to the high versatility of their coordination modes and presence of the partially occupied f-sublevel in metal centers which is responsible for magnetism [13,14,15,16], luminescence [17,18,19,20,21,22,23,24,25] and other properties. However, since f-f electron transitions are forbidden by the Laporte selection rule, lanthanide cations demonstrate low molar absorption coefficients and low quantum yields. The incorporation of a ligand with an extended π-system to effectively absorb light energy and to transfer it to the Ln3+ cation is a promising approach to overcome the Laporte prohibition. This photosensitization phenomenon is also known as the «antenna effect» [25,26].
Thieno[3,2b]thiophene-2,5-dicarboxylic acid (trans-H2ttdc, Scheme 1) is based on two conjugated thiophene heterocycles, containing the 10 e− aromatic π-system. It is a linear, structurally robust molecule with two carboxylate functional groups. Even though its structure closely resembles naphthalene-2,6-dicarboxylic acid—one of the extensively used linkers in MOF chemistry, sulfur heteroatoms predetermine a number of unique features. The polarizable sulfur atoms were shown to enhance host–guest interactions and thus increase the gas adsorption capacity and selectivity of the corresponding MOFs [27,28,29,30]. Additionally, the electron-rich aromatic thiophene heterocycle is an effective photon trap [31,32,33] and may serve as a proper photosensitizer for luminescence centers provided that HOMO/LUMO energy levels are appropriately arranged. Although the sulfur atom in five-membered aromatic rings might be considered as a potential ligand, S coordination is not usually observed in cases of the presence of stronger donor atoms in the heterocycle [34,35] or strong electron acceptor substituents, such as carboxylic groups [36,37,38]. Therefore, combining lanthanide cations with thiophene-containing ligands is a potential approach for obtaining materials with high adsorption uptakes, effective luminescence and, as a consequence, prospective sensing applications. Of all the range of organic compounds, acetone, amides and several strong-donor or strong-acceptor aromatic compounds [18,25,26,39,40,41,42,43,44,45,46] are most often described in the literature.
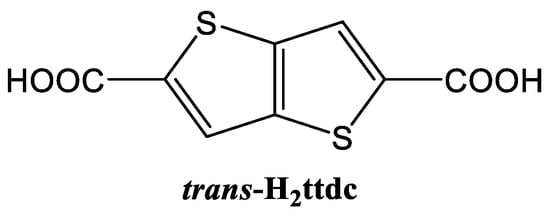
Scheme 1.
Structural formula of thieno[3,2b]thiophene-2,5-dicarboxylic acid (trans-H2ttdc).
The present work reports the synthesis and characterization of four new rare-earth-based MOFs containing thieno[3,2b]thiophene-2,5-dicarboxylate with the formulae [M2(DMF)4(ttdc)3]·xDMF, where M3+ = Y3+ (1), La3+ (2), Tb3+ (3), and [Lu2(H2O)2(ttdc)3]·4DMF (4). A luminescence study revealed a guest-dependent quenching which allows for the semi-quantitative determination of quinoline or cinnamaldehyde at low concentrations in solutions.
2. Materials and Methods
2.1. Materials
Thieno[3,2b]thiophene-2,5-dicarboxylic acid (H2ttdc, >97.0%) was synthesized according to the previously published procedure [37]. Y(NO3)3∙6H2O (99.9% REO) and La(NO3)3∙6H2O (99.9% REO) were received from Strem Chemicals. Lu(NO3)3∙7H2O (reagent grade) was received from Reakhim. Tb(NO3)3∙5H2O (reagent grade) and N,N-dimethylformamide (DMF, reagent grade) were supplied by Vekton. Trans-Cinnamaldehyde (99%) was supplied by Sigma Aldrich. Quinoline (>97.0%) was received from TCI. All the reagents were used as received without further purification.
2.2. Instruments
Infrared (IR) spectra were obtained in the 4000−400 cm−1 range using a Bruker Scimitar FTS 2000 spectrometer in KBr pellets. Elemental CHNS analyses were carried out using a VarioMICROcube device. Powder X-ray diffraction (PXRD) data were acquired with a Shimadzu XRD-7000 diffractometer (Cu-Kα radiation, λ = 1.54178 Å) at room temperature. Thermogravimetric analyses (TGA) were performed on a Netzsch TG 209 F1 Iris instrument at a 10 K∙min−1 heating rate under an inert atmosphere. Photoluminescence excitation and emission spectra were recorded with a spectrofluorometer Horiba Jobin Yvon Fluorolog 3 equipped with 450W power ozone-free Xe-lamp, cooled R928/1860 PFR technologies photon detector with a PC177CE-010 refrigerated chamber and double grating monochromators. Standard correction curves were used for the spectra correction for source intensity and detector response. Diffraction data for single crystals of 1–4 were collected with an automated Agilent Xcalibur diffractometer equipped with AtlasS2 area detector and graphite monochromator (λ(MoKα) = 0.71073 Å). The CrysAlisPro program package [47] was used for the integration, absorption correction and determination of unit cell parameters. Dual space algorithm (SHELXT [48]) was used for structure solution and the full-matrix least squares technique (SHELXL [49]) was used for structure refinement. Anisotropic approximation was applied for all atoms, except hydrogens. Positions of hydrogen atoms of organic ligands were calculated geometrically and refined in the riding model. Details for single crystal structure determination experiments and structure refinements are summarized in Appendix A, Table A1. CCDC 2191621–2191624 entries contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/ (accessed at 27 September 2022).
2.3. Synthetic Methods
Synthesis of [Y2(DMF)4(ttdc)3]·5DMF (1) First, 23.0 mg (0.06 mmol) of Y(NO3)3∙6H2O and 20.6 mg (0.09 mmol) of H2ttdc were mixed and dissolved in 2.0 mL of DMF in a 5 mL screw-capped glass vial using an ultrasonic bath. The obtained solution was thermostated at 80 °C for 48 h. After cooling in air to room temperature, the resultant colorless crystals were filtered, washed twice with DMF to remove unreacted metal salt and H2ttdc, and dried in air. Yield: 23.3 mg (54%). IR spectrum (KBr, cm−1) main bands: 3084 (w., νCsp2–H); 2928 (m., νCsp3–H); 1660 (s., νCODMF); 1485 (s., νCOOas); 1387 (s., νCOOs). Elemental CHNS analysis data, calculated for [Y2(DMF)4(ttdc)3]·3.5DMF (%): C, 39.5; H, 4.6; N, 7.4; S, 13.6. Found (%): C, 39.8; H, 4.3; N, 7.7; S, 14.2. PXRD of the filtered sample: Figure S1.
Synthesis of [La2(DMF)4(ttdc)3]·3.7DMF (2) First, 26.0 mg (0.06 mmol) of La(NO3)3∙6H2O and 20.6 mg (0.09 mmol) of H2ttdc were mixed and dissolved in 2.0 mL of DMF in a 5 mL screw-capped glass vial via ultrasonic bath assistance. The obtained solution was thermostated at 80 °C for 48 h. After cooling in air to room temperature, the resultant colorless crystals were filtered, washed twice with DMF to remove unreacted metal salt and H2ttdc, and dried in air. Yield: 26.4 mg (57%). IR spectrum (KBr, cm−1) main bands: 3090 and 3082 (w., νCsp2–H); 2926 (m., νCsp3–H); 1653 (m., νCODMF); 1485 (s., νCOOas); 1387 (s., νCOOs). Elemental CHNS analysis data, calculated for [La2(DMF)4(ttdc)3]·4DMF (%): C, 37.4; H, 4.1; N, 7.3; S, 12.5. Found (%): C, 37.8; H, 4.3; N, 7.1; S, 13.0. PXRD of the filtered sample: Figure S2.
Synthesis of [Tb2(DMF)4(ttdc)3]·5.3DMF (3) First, 26.1 mg (0.06 mmol) of Tb(NO3)3∙5H2O and 20.6 mg (0.09 mmol) of H2ttdc were mixed and dissolved in 2.0 mL of DMF in a 5 mL screw-capped glass vial with ultrasonic bath assistance. The obtained solution was thermostated at 100 °C for 48 h. After cooling in air to room temperature, the resultant colorless crystals were filtered off, washed twice with DMF to remove unreacted metal salt and H2ttdc, and dried in air. Yield: 22.3 mg (47%). IR spectrum (KBr, cm−1) main bands: 3078 and 3090 (w., νCsp2–H); 2920 (m., νCsp3–H); 1659 (s., νCODMF); 1485 (s., νCOOas); 1387 (s., νCOOs). Elemental CHNS analysis data, calculated for [Tb2(DMF)4(ttdc)3]·3DMF (%): C, 35.8; H, 3.7; N, 6.5; S, 12.8. Found (%): C, 35.9; H, 3.7; N, 6.8; S, 12.9. PXRD of the filtered sample: Figure S3.
Synthesis of [Lu2(H2O)2(ttdc)3]·4DMF (4) A total of 29.2 mg (0.06 mmol) of Lu(NO3)3∙7H2O and 20.6 mg (0.09 mmol) of H2ttdc were mixed and dissolved in 2.0 mL of DMF in a 5 mL screw-capped glass vial with ultrasonic bath assistance. The obtained solution was thermostated at 80 °C for 48 h. After cooling in air to room temperature, the resultant colorless crystals were filtered, washed twice with DMF to remove unreacted metal salt and H2ttdc, and dried in air. Yield: 25.6 mg (63%). IR spectrum (KBr, cm−1) main bands: 3427 (s., br., νO–H); 3099 and 3082 (w., νCsp2–H); 2928 (m., νCsp3–H); 1655 (s., νCODMF); 1484 (s., νCOOas); 1417 (s., νCOOs). Elemental CHNS analysis data, calculated for [Lu2(H2O)2(ttdc)3]·3.5DMF (%): C, 31.4; H, 2.6; N, 3.7; S, 14.6. Found (%): C, 31.6; H, 2.6; N, 4.1; S, 15.5. PXRD of the filtered sample: Figure S4.
Synthesis of [Tb2(DMF)4(ttdc)3]·2.5DMF·1.3cinnamal (3⊃cinnamal). A sample of [Tb2(DMF)4(ttdc)3]·3DMF was placed in a glass vial and soaked in cinnamaldehyde for 48 h at room temperature. The obtained yellow paste was filtered, washed with a minimal amount of DMF to remove cinnamaldehyde from the surface of the crystals and dried in air. IR spectrum (KBr, cm−1) main bands: 3086 (w., νCsp2–H); 2932 (m., νCsp3–H); 2810 (w., νC(O)-H); 1672 (s., νCOcinnamal); 1647 (s., νCODMF); 1485 (s., νCOOas); 1391 (s., νCOOs). Elemental CHNS analysis data, calculated for [Tb2(DMF)4(ttdc)3]·2.5DMF·1.3cinnamal (%): C, 40.3; H, 3.8; N, 5.5; S, 11.7. Found (%): C, 40.1; H, 4.0; N, 5.2; S, 11.7.
Synthesis of [Tb2(DMF)4(ttdc)3]·DMF·2.5quinoline (3⊃quinoline). A sample of [Tb2(DMF)4(ttdc)3]·3DMF was placed in a glass vial and soaked in quinoline for 48 h at room temperature. The obtained brown paste was filtered, washed with minimal amount of DMF to remove quinoline from the surface of the crystals and dried in air dried in air. IR spectrum (KBr, cm−1) main bands: 3090 (w., νCsp2–H); 2928 (m., νCsp3–H); 1618 (s., νCODMF); 1489 (s., νCOOas); 1389 (s., νCOOs). Elemental CHNS analysis data, calculated for [Tb2(DMF)4(ttdc)3]·DMF·2.5quinoline (%): C, 43.8; H, 3.5; N, 6.2; S, 11.4. Found (%): C, 43.5; H, 3.7; N, 5.9; S, 11.4.
Preparation of suspensions for luminescence measurements. Powder sample of 3 suspended in DMF was prepared according to the scaled synthetic method described above. A total of 130.5 mg (0.30 mmol) of Tb(NO3)3∙5H2O and 103.0 mg (0.45 mmol) of H2ttdc were mixed and dissolved in 10.0 mL of DMF in a 20 mL screw-capped glass vial with ultrasonic bath assistance. Then, the solution was thermostated at 100 °C for 48 h at continuous intensive stirring. After cooling in air to room temperature, the resultant thin powder dispersion was decanted, washed with 10 mL of DMF and decanted again. The washing procedure was repeated 5 times to remove unreacted Tb(III) salt and H2ttdc. A 1.0 mL of suspension of 3, prepared as described above, was sampled in a volumetric flask with 1.0 mL of the corresponding analyte solutions and diluted with DMF to a 25.0 mL total volume. No sedimentation of 3 to the bottom of the flask occurred for at least 24 h. The resultant diluted suspensions were transferred into cuvettes and analyzed with luminescent spectroscopy.
3. Results and Discussion
3.1. Synthesis and Crystal Structure Description
Compounds 1–4 were synthesized in similar solvothermal conditions, using N,N-dimethylformamide (DMF) as a solvent. Compound [Y2(DMF)4(ttdc)3]·5DMF (1) crystallizes in the P–1 space group and is similar to the previously reported MOF [Eu2(DMF)4(ttdc)3]·4DMF [44]. Unlike that Eu3+-based compound, frameworks containing Y3+, La3+ and Lu3+ appeared to form poor quality single crystals under similar synthetic conditions. Therefore, optimization experiments were carried out and the optimal temperature was found to be 80 °C, as opposed to the 100 °C for [Eu2(DMF)4(ttdc)3]·4DMF. An asymmetric unit of structure 1 is shown in Figure S5. Y(III) adopts a capped square-antiprismatic environment, which consists of two oxygen atoms of DMF, four oxygen atoms of chelate carboxylate groups and three oxygen atoms of bridging carboxylate groups. The selected coordination bond lengths are listed in Table 1. Two symmetry-equivalent Y(III) ions form binuclear metal-carboxylate blocks with the formula {Y2(DMF)4(RCOO-κ2)2(μ-RCOO-κ1,κ1)2(μ-RCOO-κ1,κ2)2} (Figure 1a). Such blocks represent six connected nodes which are interconnected by thieno[3,2b]thiophene-2,5-dicarboxylate bridges and form a three-dimensional coordination lattice bearing a pcu topology (Figure S6). The polymeric framework in 1 contains channels ca. 3 Å × 6 Å in size (Figure 2a) with a 38% total solvent accessible volume, as calculated with the PLATON [50] program. Only two DMF molecules per formula unit in 1 were located directly. The residual electron density in the voids was analyzed using the SQUEEZE [51] procedure in PLATON and the obtained electron count (121 e− in 404 Å3 void volume per formula unit) was assigned to three additional DMF molecules per f.u., resulting in [Y2(DMF)4(ttdc)3]⋅5DMF as the final composition of the crystal.

Table 1.
Selected bond lengths in the structures 1–3.
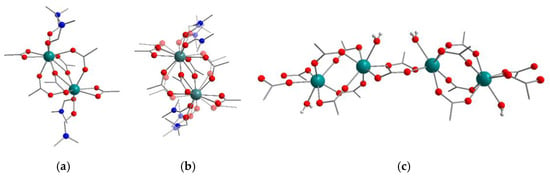
Figure 1.
Binuclear carboxylate secondary building units in 1 (a), 2 (b) and one-dimensional lutetium(III)-carboxylate chains in 4 (c). Metal atoms are turquoise, N atoms are blue, O atoms are red. H atoms (grey) are shown only in H2O ligands.
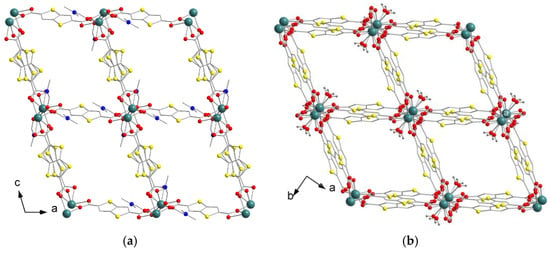
Figure 2.
Projections of three-dimensional coordination frameworks in 1 along b axis (a) and in 4 along c axis (b). Ln atoms are turquoise, N atoms are blue, O atoms are red, S atoms are yellow. H atoms (grey) are shown only in water molecules.
The asymmetric unit of compound [La2(DMF)4(ttdc)3]·3.7DMF (2) is shown in Figure S7. 2 has a similar form to 1 binuclear {La2(DMF)4(RCOO-κ2)2(μ-RCOO-κ1,κ1)2(μ-RCOO-κ1,κ2)2} building units and possesses the same pcu topology. However, coordinated DMF molecules, κ2- and κ1,κ1-carboxylic groups appear to be disordered between two positions (Figure 1b), which is apparently related to a larger ionic radius of La3+ (103 pm) compared to that of Y3+ (90 pm). Moreover, the metal node deformation in 2 results in the pronounced bending of the (κ1,κ1;κ1,κ1)-ttdc linker and a subsequent decrease in the total solvent accessible volume in 2 to 29%. Similar features were previously observed by our group in a cerium(III) thieno[3,2b]thiophene-2,5-dicarboxylate [52]. No guest molecules were located directly in the voids of 2. The residual electron density in these voids was analyzed using PLATON/SQUEEZE and the obtained electron count (148 e− in 453 Å3 void volume per formula unit) was assigned as 3.7 DMF molecules per f.u., leading to [La2(DMF)4(ttdc)3]⋅3.7DMF as the final composition of the crystal.
The compound [Tb2(DMF)4(ttdc)3]·5.3DMF (3) is isostructural to the yttrium-based 1, that can apparently be explained by the similarity of Tb3+ and Y3+ in the ionic radii (92 pm and 90 pm, respectively). Therefore, the lanthanide contraction phenomenon appears to be a significant factor manipulating the local structural features of the metal cation in otherwise chemically and topologically similar coordination frameworks [M2(DMF)4(ttdc)3] (M = Y3+, La3+, Tb3+). Bond lengths in the metal coordination environment for 1–3 are summarized in Table 1 and fit the typical values for Ln(III)-carboxylate complexes containing similar binuclear building blocks [53,54,55,56]. Similarly to 1, only two DMF molecules were located directly in the voids of 3. The residual electron density in these voids was analyzed by PLATON/SQUEEZE and the obtained electron count (131 e− in 422 Å3 void volume per formula unit) was assigned to 5.3 DMF molecules per f.u., giving [Tb2(DMF)4(ttdc)3]⋅5.3DMF as the final composition of the crystal.
Compound [Lu2(H2O)2(ttdc)3]·3.64DMF (4) crystallizes in the C2/c space group. The asymmetric unit of 4 is shown in Figure S8. The coordination environment of Lu(III) consists of six oxygen atoms of six carboxylate groups and one oxygen atom of a water molecule. The Lu3+ features the smallest ionic radius (86 pm) among the heavy rare-earth elements, which results in relatively short Lu–O bond lengths (2.211(3)–2.291(3) Å for Lu–O(COO), 2.349(3) Å for Lu–O(H2O)). Nevertheless, such metal–oxygen distances are typical for lutetium(III)-carboxylate MOFs or complexes containing Lu atom with a similar coordination environment [57,58,59]. The apparent coordination number (CN) of Lu3+ is reduced to 7, in comparison with CN = 9 for the other metal cations in 1–3, which again manifests the lanthanide contraction effect. Two symmetry-equivalent Lu(III) cations are linked by four (κ1,κ1)-carboxylate bridges to form a pseudo-binuclear metal-carboxylate block with the shortest Lu…Lu distance of 3.99 Å. Due to an insufficient space around the small Lu3+ cation, its coordination environment includes a H2O molecule in contrast to bulkier DMF ligands in 1–3. Neighboring pseudo-binuclear blocks are interlinked by two additional (κ1,κ1)-carboxylate groups creating an infinite chain (Figure 1c) with a 5.39 Å Lu…Lu distance between those blocks. The chains are interconnected in four directions by ttdc2− linkers forming 3D framework containing one-dimensional channels ca. 5 Å × 5 Å (Figure 2b) in size and a 46% calculated total solvent-accessible volume. Only two DMF molecules per formula unit were located directly, apparently due to the presence of hydrogen bonding between the coordinated water and DMF guests with 1.83 Å H(H2O)…O(DMF) contacts (see Figure S8). A non-ordered electron density present in the residual voids (320 e− in 1212 Å3 void volume per unit cell or 80 e− in 303 Å3 void volume per formula unit, as calculated by SQUEEZE) was attributed to two additional DMF molecules per f.u. resulting in [Lu2(H2O)2(ttdc)3]·4DMF as a general crystal composition for 4.
3.2. Thermal Properties and IR Spectra
The chemical identity and composition of compounds 1–4 were confirmed by performing elemental CHN analyses and IR spectroscopy. All the IR (Figure 3a) spectra contain typical bands corresponding to C(sp2)–H vibrations of an aromatic ligand core, C(sp3)–H vibrations of DMF molecule methyl groups, C=O vibrations in the DMF molecule, and antisymmetric and symmetric COO vibrations. The major difference between the spectra is a presence of strong O–H vibrations (broad band at ~3450 cm−1) in Lu-based compound 4, which corresponds to the coordinated water molecules, perfectly matching the crystal structure data.
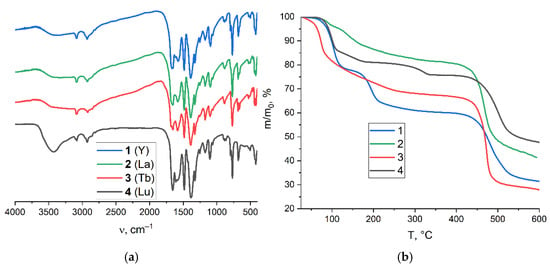
Figure 3.
IR spectra for compounds 1–4 (a). TG plots for compounds 1–4 (b). According to thermogravimetric analyses (TGA, Figure 3b), all the compounds slowly lose their guest and coordinated solvent molecules at heating temperatures of up to 340 °C. The stepwise curve for 1 shows the loss of 3.5 guest DMF molecules at ca. 100 °C (22% weight loss; calculated for 3.5DMF–18%) and then four coordinated DMF molecules at ca. 190 °C (18% weight loss; calculated for 4DMF–21%). The total weight losses for 2 and 3 at 400 °C correspond to four and seven DMF molecules, respectively. The stepwise TGA curve for 4 demonstrates the 20% weight loss in the 100–140 °C interval, corresponding to ~3.6 guest DMF molecules (calculated for 3.6 guest DMF–20%) followed by the removal of two coordinated H2O molecules at ca. 310 °C (4% weight loss; calculated for 2H2O–3%). Therefore, the thermogravimetric analysis agrees well with the elemental analysis data, except for in the case of 2, where some level of underestimation of the guest content based on TGA data might be attributed to the weathering of two DMF guest molecules during sample handling. An irreversible framework decomposition in 1–4 starts at ca. 440 °C. Such thermal stabilities of the coordination frameworks are comparable to those for other reported Ln(III)-based MOFs based on ttdc2− ligand and its derivatives [39,41,44,52].
3.3. Luminescence Spectrocopy
Solid-state luminescence measurements were performed for the synthesized compounds. All four coordination frameworks show a broad blue emission band (λex = 380 nm) typical for intra-ligand π-π* electron transitions. The corresponding emission spectra are presented in Figure 4. The spectral maxima for 1 (Y) and 3 (Tb) appear in a close range of λmax = 457–460 nm. The maximum spectra for 2 (La) were slightly red-shifted (λmax = 472 nm) in comparison to 1 and 3; this is apparently caused by the presence of three structurally different ttdc2− ligands in 1–3 and a subsequent variation in their vibrational modes which affects intra-ligand luminescence. As previously reported, [Eu2(DMF)4(ttdc)3]·4DMF [44] demonstrates the characteristic red emission, indicating an efficient energy transfer from the ttdc2− ligand to the Eu3+ center. On the contrary, no apparent energy transfer occurs from ttdc2− to Tb3+ likely because the LUMO π* orbitals of the ligand lie between the LUMO levels of green-emitting Tb3+ and red-emitting Eu3+.
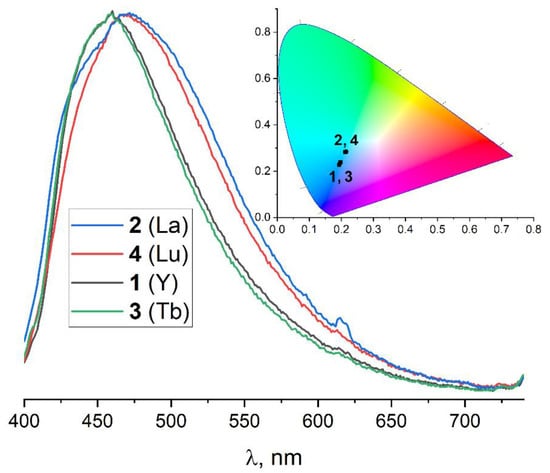
Figure 4.
The normalized emission spectra and CIE 1931 chromaticity diagram for 1–4 at λex = 380 nm.
Since the luminescence properties of the title MOFs are generally comparable, only Tb(III)-based compound 3 was chosen for further guest-dependent luminescence experiments. Several typical donors (o-, m- and p-cresoles) or typical acceptors (cinnamaldehyde, quinoline and pyridine), representing important industrial analytes, were selected. At first, the substrates were added to the suspension of 3 in DMF (see the Experimental section for details) at up to 4 vol.%. Compared to the crystalline sample, the emission spectrum of suspension 3 (Figure S9; λex = 320 and 360 nm) is more complex and contains additional bands in the UV range (λem = 360, 380, 410 nm). Such a difference may be explained again by the different vibrational modes of structurally independent ttdc2− ligands, as the lower excitation wavelengths (λex = 320 or 360 nm vs. λex = 380 nm) make possible the emergence of the more energy-demanding transitions in the emission spectra. More interestingly, the addition of cresoles and pyridine did not change luminescence properties of 3 in the visible range while an addition of cinnamaldehyde prevents luminescence completely. An addition of quinoline also quenches the luminescence but only at λex = 320 nm.
So long as both cinnamal and quinoline feature strong absorption in the near-UV region of their own, additional experiments are to be carried out to prove the luminescent detection of those substrates with a porous MOF instead of just competitive absorption. First, an incorporation of the guests into 3 was confirmed as the solid adducts 3⊃cinnamal and 3⊃quinoline were obtained. Elemental analysis data unambiguously show a partial substitution of DMF by cinnamal or quinoline resulting in the chemically reasonable formula [Tb2(DMF)4(ttdc)3]·2.5DMF·1.3cinnamal (3⊃cinnamal) or [Tb2(DMF)4(ttdc)3]·DMF·2.5quinoline (3⊃quinoline). IR spectra are also consistent with the presence of cinnamal and quinoline in the pores of 3. Absorbtion bands in the 3⊃cinnamal spectrum (Figure S10) at 2810 cm−1 and 1672 cm−1 match the oscillations of the aldehyde group conjugated with C=C double bond as in cinnamaldehyde. The 3⊃quinoline spectrum (Figure S10) shows a characteristic band at 1200 cm−1 indicating a presence of a moiety containing a pyridine core, i.e., quinoline. Next, DMF solutions of cinnamal and quinoline with lower concentrations were prepared to elucidate the minimal concentration at which the quenching response occurs. The excitation spectra for λem = 440 and 470 nm were recorded and shown in Figure S11 and Figure S12. For the spectra of pure suspension of 3 in DMF, a broad multimodal excitation peak appears in the range ca. 270–420 nm. Upon addition of cinnamal or quinoline, the lower-than-320 nm components of the spectra completely vanished, which might be attributed to the competitive absorption of an excess of cinnamal and quinoline, dissolved in solution. Such a decrease in the higher-energy excitation of 3 in quinoline solutions corresponds well with the UV/vis absorption spectrum of quinoline in the DMF solution represented in Figure S13. Moreover, a similar UV/vis spectrum for cinnamal shows very weak absorption in the λ > 330 nm region, so the quenching of 3 in cinnamal solutions at λex = 360 nm (Figure 5) indicates intensive energy transfer between the host and the adsorbed guest. At the same time, the excitation spectra at higher wavelengths increase substantially, most notably in the case of quinoline. All these data point to an efficient interplay between the luminescent MOF host and the guest substrate molecules through some short-range intermolecular interactions, additionally confirming the adsorption detection mechanism.
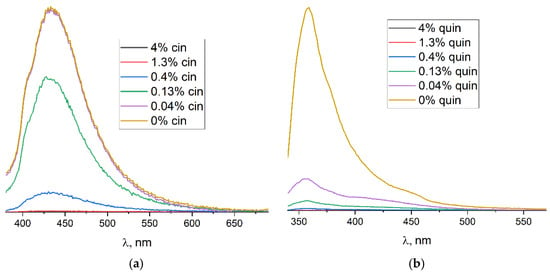
Figure 5.
Emission spectra for DMF suspension of 3 with cinnamaldehyde at λex = 360 nm (a) and with quinoline at λex = 320 nm (b) at different concentrations.
The emission spectra of the DMF suspension of 3 in the presence of the analytes are shown on Figure 5. As it can be clearly seen, the substantial decrease in luminescence intensity was already observed for 4 × 10−1% v/v of cinnamal (λex = 360 nm). A comparable level of cinnamal detection was reported previously for other Tb-based MOF [Tb2(phen)2(NO3)2(chdc)2]·2DMF (phen = 1,10-phenantroline, chdc2− = thans-1,4cyclohexanedicarboxylate) [60]. Remarkably, the reliable detection of quinoline through luminescence quenching is secured at as low as 4 × 10−2 % v/v (ca. 3 × 10−3 M) concentrations (λex = 320 nm). Interestingly, completely different luminescence behaviors were obtained upon sample excitation by λex = 360 nm (Figure S14) as the intensity of the emission spectra increased by up to three times upon an addition of the quinoline to the DMF suspension of 3. Such complex luminescence behavior is rather unusual and requires more experimental/theoretical efforts for its accurate explanation. Nevertheless, on the basis of the experimental data reported here, it is clear that compound 3 has a promising application potential in the detection or semi-quantitative determination of quinoline, cinnamal or, possibly, other important chemicals.
4. Conclusions
In summary, four new rare-earth metal–organic frameworks containing thieno[3,2b]thiophene-2,5-dicarboxylate (ttdc2−) were synthesized and characterized. Their crystal structures were established by performing an X-ray diffraction analysis of single crystals. A lanthanide contraction phenomenon was found to be a considerable factor driving the structural features of the frameworks along the rare earth metals row. All the compounds possess three-dimensional porous coordination networks with specific solvent-accessible volumes ranging from 30% to 46%. Luminescent properties measurements revealed the intra-ligand blue emission for all the synthesized frameworks. The luminescence properties of the terbium-based framework [Tb2(DMF)4(ttdc)3] in the presence of different organic guest molecules were investigated. The obtained experimental data show that a reliable detection of quinoline and cinnamal with luminescence quenching is possible at milimolar concentrations, suggesting promising sensing applications for the studied MOFs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12101374/s1. Figure S1: Experimental PXRD pattern for 1 in comparison to the theoretical pattern; Figure S2: Experimental PXRD pattern for 2 in comparison to the theoretical pattern; Figure S3: Experimental PXRD pattern for 3 in comparison to the theoretical pattern; Figure S4: Experimental PXRD pattern for 4 in comparison to the theoretical pattern; Figure S5: The asymmetric unit of Y-containing structure 1; Figure S6: Topological representation of coordination framework in 1; Figure S7: The asymmetric unit of La-containing structure 2; Figure S8: The asymmetric unit of Lu-containing structure 4; Figure S9: Emission spectra for 3 suspension with selected substrates at λex = 360 nm (left) and 320 nm (right); Figure S10: IR spectra for 3 soaked in quinoline and cinnamal; Figure S11: Excitation spectra for 3 suspensions in cinnamal solutions at λem = 440 nm (a) and λem = 470 nm (b); Figure S12: Excitation spectra for 3 suspensions in quinoline solutions at λem = 440 nm (a) and λem = 470 nm (b); Figure S13: UV/vis absorption spectra for DMF solutions of cinnamal (3·10−5 M) and quinoline (6·10−5 M); Figure S14: Emission spectra for 3 suspensions in quinoline solutions at λex = 360 nm; Figure S15: Solid-state luminescence excitation spectrum of 3 at λem = 450 nm.
Author Contributions
Y.A.Y.—writing (original draft preparation), synthesis, characterization, graphing. P.A.D.—writing (original draft preparation), graphing, single-crystal XRD. A.A.R.—solid-state luminescence measurements. V.P.F.—writing (manuscript review and editing), resources. D.N.D.—writing (manuscript review and editing), conceptualization, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by Russian Science Foundation, project № 18-13-00203, https://rscf.ru/project/18-13-00203/ (accessed at 27 September 2022). The analytical services were supported by the Ministry of Science and Higher Education of the Russian Federation, projects № 121031700321-3 and 121031700313-8.
Informed Consent Statement
Not applicable.
Data Availability Statement
Samples of compounds 1–4 are available from authors.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. The Crystallographic Data for 1–4

Table A1.
Single crystal X-ray diffraction analysis and structure refinement details.
Table A1.
Single crystal X-ray diffraction analysis and structure refinement details.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Chemical formula | C51H69N9O21S6Y2 | C47.1H59.9La2N7.7O19.7S6 | C51.9H71.1N9.3O21.3S6Tb2 | C36H38Lu2N4O18S6 |
| Mr, g/mol | 1514.33 | 1519.30 | 1676.28 | 1357.00 |
| Crystal system | Triclinic | Triclinic | Triclinic | Monoclinic |
| Space group | P¯1 | P¯1 | P¯1 | C2/c |
| Temperature, K | 150 | 150 | 150 | 170 |
| a, Å | 11.9443(6) | 10.8057(4) | 11.9884(4) | 20.8816(5) |
| b, Å | 12.1749(7) | 12.0790(4) | 12.1592(5) | 12.7885(3) |
| c, Å | 12.9163(7) | 13.1351(4) | 13.0067(3) | 18.4579(6) |
| α, ° | 101.820(4) | 105.625(3) | 101.739(3) | 90 |
| b, ° | 101.592(4) | 98.423(3) | 101.689(3) | 108.470(3) |
| γ, ° | 108.524(5) | 100.939(3) | 108.713(3) | 90 |
| V, Å3 | 1670.07(17) | 1585.27(10) | 1683.24(10) | 4675.2(2) |
| Z | 1 | 1 | 1 | 4 |
| F(000) | 780 | 764 | 844 | 2656 |
| D(calc.), g·cm−3 | 1.506 | 1.591 | 1.654 | 1.928 |
| μ, mm−1 | 1.99 | 1.60 | 2.35 | 4.54 |
| Crystal size, mm | 0.37 × 0.35 × 0.12 | 0.22 × 0.11 × 0.07 | 0.42 × 0.23 × 0.12 | 0.43 × 0.37 × 0.20 |
| θ range for data collection, ° | 2.1 ≤ θ ≤ 25.4 | 2.0 ≤ θ ≤ 25.4 | 2.1 ≤ θ ≤ 25.4 | 2.1 ≤ θ ≤ 25.4 |
| No. of reflections: measured/independent/observed [I > 2σ(I)] | 10061/ 6002/ 5090 | 11546/ 5795/ 4987 | 18265/ 5984/ 5470 | 8366/ 4277/ 3785 |
| Rint | 0.0497 | 0.0213 | 0.0355 | 0.0250 |
| Index ranges | –11 ≤ h ≤ 14 –14 ≤ k ≤ 14 –15 ≤ l ≤ 12 | –13 ≤ h ≤ 10 –14 ≤ k ≤ 14 –13 ≤ l ≤ 15 | –14 ≤ h ≤ 14 –14 ≤ k ≤ 14 –15 ≤ l ≤ 15 | –16 ≤ h ≤ 25 –15 ≤ k ≤ 13 –22 ≤ l ≤ 14 |
| Final R indices [I > 2σ(I)] | R1 = 0.0660 wR2 = 0.1743 | R1 = 0.0316 wR2 = 0.0746 | R1 = 0.0460 wR2 = 0.1227 | R1 = 0.0259 wR2 = 0.0652 |
| Final R indices (all data) | R1 = 0.0769 wR2 = 0.1821 | R1 = 0.0406 wR2 = 0.0784 | R1 = 0.0506 wR2 = 0.1252 | R1 = 0.0304 wR2 = 0.0667 |
| Goodness-of-fit on F2 | 1.054 | 1.043 | 1.033 | 1.060 |
| Largest diff. peak, hole, e/Å3 | 1.86/–1.04 | 0.59/–1.32 | 2.77/–1.29 | 1.60/–1.00 |
References
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Wei, R.; Gaggioli, C.A.; Li, G.; Islamoglu, T.; Zhang, Z.; Yu, P.; Farha, O.K.; Cramer, C.J.; Gagliardi, L.; Yang, D.; et al. Tuning the Properties of Zr6O8 Nodes in the Metal Organic Framework UiO-66 by Selection of Node-Bound Ligands and Linkers. Chem. Mater. 2019, 31, 1655–1663. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Bai, N.; Zhang, X.; Han, X.; Da Silva, I.; Morris, C.G.; Xu, S.; Wilary, D.M.; Sun, Y.; et al. Refinement of pore size at sub-angstrom precision in robust metal–organic frameworks for separation of xylenes. Nat. Commun. 2020, 11, 4280. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro- and mesoporous metal-organic coordination polymers for the separation of hydrocarbons. Russ. Chem. Rev. 2022, 91, RCR5026. [Google Scholar] [CrossRef]
- Agafonov, M.A.; Alexandrov, E.V.; Artyukhova, N.A.; Bekmukhamedov, G.E.; Blatov, V.A.; Butova, V.V.; Gayfulin, Y.M.; Garibyan, A.A.; Gafurov, Z.N.; Gorbunova, Y.G.; et al. Metal-organic frameworks in Russia: From the synthesis and structure to functional properties and materials. J. Struct. Chem. 2022, 63, 671–843. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically porous metal–organic frameworks: Synthetic strategies and applications. Natl. Sci. Rev. 2020, 7, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Bryliakov, K.P. Asymmetric catalysis using metal-organic frameworks. Coord. Chem. Rev. 2021, 437, 213845. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Lv, H.; Qin, Q.-P.; Fan, L.; Zhang, X. Chemorobust 4p–5p {InPb}-organic framework for efficiently catalyzing cycloaddition of CO2 with epoxides and deacetalization-Knoevenagel condensation. Mater. Today Chem. 2022, 24, 100984. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Zhang, X. Bifunctional {Pb10K2}—Organic Framework for High Catalytic Activity in Cycloaddition of CO2 with Epoxides and Knoevenagel Condensation. ACS Catal. 2022, 12, 10373–10383. [Google Scholar] [CrossRef]
- Yin, W.; Tao, C.; Wang, F.; Huang, J.; Qu, T.; Wang, J. Tuning optical properties of MOF-based thin films by changing the ligands of MOFs. Sci. China Mater. 2018, 61, 391–400. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Gontcharenko, V.E.; Lunev, A.M.; Sidoruk, A.V.; Arkhipov, I.A.; Taydakov, I.V.; Belousov, Y.A. New Carboxylate Anionic Sm-MOF: Synthesis, Structure and Effect of the Isomorphic Substitution of Sm3+ with Gd3+ and Tb3+ Ions on the Luminescent Properties. Inorganics 2022, 10, 104. [Google Scholar] [CrossRef]
- Demakov, P.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Zinc(II) metal-organic frameworks with 1,4-diazabicyclo [2.2.2]octane N,N′-dioxide: Control of the parameters of the cationic porous framework and optical properties. Russ. Chem. Bull. 2022, 71, 83–90. [Google Scholar] [CrossRef]
- Parker, D.; Suturina, E.A.; Kuprov, I.; Chilton, N.F. How the Ligand Field in Lanthanide Coordination Complexes Determines Magnetic Susceptibility Anisotropy, Paramagnetic NMR Shift, and Relaxation Behavior. Acc. Chem. Res. 2020, 53, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Saraci, F.; Quezada-Novoa, V.; Rafael Donnarumma, P.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef]
- Zairov, R.; Pizzanelli, S.; Dovzhenko, A.P.; Nizameev, I.; Orekhov, A.; Arkharova, N.; Podyachev, S.N.; Sudakova, S.; Mustafina, A.R.; Calucci, L. Paramagnetic Relaxation Enhancement in Hydrophilic Colloids Based on Gd(III) Complexes with Tetrathia-and Calix[4]arenes. J. Phys. Chem. C 2020, 124, 4320–4329. [Google Scholar] [CrossRef]
- Kumar, M.; Qiu, C.-Q.; Zareba, J.K.; Frontera, A.; Kaur Jassal, A.; Chandra Sahoo, S.; Liu, S.-J.; Nawaz Sheikh, H. Magnetic, luminescence, topological and theoretical studies of structurally diverse supramolecular lanthanide coordination polymers with flexible glutaric acid as a linker. New J. Chem. 2019, 43, 14546–14564. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Nonat, A.M.; Charbonnière, L. Upconversion of light with molecular and supramolecular lanthanide complexes. Coord. Chem. Rev. 2020, 409, 213192. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Eliseeva, S.V.; Gładysiak, A.; Petoud, S.; Stylianou, K.C. Design of lanthanide-based metal–organic frameworks with enhanced near-infrared emission. J. Mater. Chem. A 2020, 8, 10188–10192. [Google Scholar] [CrossRef]
- Cui, R.; Sun, W.; Liu, M.; Shi, J.; Liu, Z. Near-Infrared Emissive Lanthanide Metal–Organic Frameworks for Targeted Biological Imaging and pH-Controlled Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 59164–59173. [Google Scholar] [CrossRef]
- Kim, J.H.; Lepnev, L.S.; Utochnikova, V.V. Dual vis-NIR emissive bimetallic naphthoates of Eu-Yb-Gd: A new approach toward Yb luminescence intensity increase through Eu → Yb energy transfer. Phys. Chem. Chem. Phys. 2021, 23, 7213–7219. [Google Scholar] [CrossRef] [PubMed]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and mixed metal complexes of Eu3+, Gd3+, and Tb3+ with a diketone, bearing pyrazole moiety and CHF2-group: Structure, color tuning, and kinetics of energy transfer between lanthanide ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal—Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Bolotov, V.A.; Kovalenko, K.A.; Samsonenko, D.G.; Han, X.; Zhang, X.; Smith, G.L.; McCormick, L.J.; Teat, S.J.; Yang, S.; Lennox, M.J.; et al. Enhancement of CO2 Uptake and Selectivity in a Metal—Organic Framework by the Incorporation of Thiophene Functionality. Inorg. Chem. 2018, 57, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, Y.; Liu, J.; Gu, C.; Wu, D. High CO2 adsorption capacities in UiO type MOFs comprising heterocyclic ligand. Microporous Mesoporous Mater. 2018, 256, 25–31. [Google Scholar] [CrossRef]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A Selenophene-Incorporated Metal—Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kusaka, S.; Hijikata, Y.; Hosono, N.; Kitagawa, S. Development of a Porous Coordination Polymer with a High Gas Capacity Using a Thiophene-Based Bent Tetracarboxylate Ligand. ACS Appl. Mater. Interfaces 2017, 9, 33455–33460. [Google Scholar] [CrossRef]
- Xie, F.-Y.; Yang, Q.; Wang, J.-S.; Yu, H.-Y.; Li, Y.; Ruan, W.-J. Benzotrithiophene-based MOFs: Interchromophoric interactions affected Ln(III) crystallization selectivity and optoelectronic properties. Dalton Trans. 2021, 50, 17228–17234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lu, H.; Wang, Y.; Bao, H.; Li, Z.-J.; Xiao, G.-P.; Lin, J.; Qian, Y.; Wang, J.-Q. Tuning of the Network Dimensionality and Photoluminescent Properties in Homo- and Heteroleptic Lanthanide Coordination Polymers. Inorg. Chem. 2021, 60, 1359–1366. [Google Scholar] [CrossRef]
- Kumar, M.; Li, L.-Q.; Zaręba, J.K.; Tashi, L.; Chandra Sahoo, S.; Nyk, M.; Liu, S.-J.; Nawaz Sheikh, H. Lanthanide Contraction in Action: Structural Variations in 13 Lanthanide(III) Thiophene-2,5-dicarboxylate Coordination Polymers (Ln = La–Lu, Except Pm and Tm) Featuring Magnetocaloric Effect, Slow Magnetic Relaxation, and Luminescence-Lifetime-based Thermometry. Cryst. Growth Des. 2020, 20, 6430–6452. [Google Scholar] [CrossRef]
- Gul, Z.; Ud Din, N.; Khan, E.; Ullah, M.; Nawaz Tahir, M. Synthesis, molecular structure, anti-microbial, anti-oxidant and enzyme inhibition activities of 2-amino-6-methylbenzothiazole and its Cu(II) and Ag(I) complexes. J. Mol. Struct. 2020, 1199, 126956. [Google Scholar] [CrossRef]
- Rogovoy, M.I.; Tomilenko, A.V.; Samsonenko, D.G.; Nedolya, N.A.; Rakhmanova, M.I.; Artem’ev, A.V. New silver(I) thiazole-based coordination polymers: Structural and photophysical investigation. Mendeleev Commun. 2020, 30, 728–730. [Google Scholar] [CrossRef]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. 3D Metal—Organic Frameworks Based on Co(II) and Bithiophendicarboxylate: Synthesis, Crystal Structures, Gas Adsorption, and Magnetic Properties. Molecules 2021, 26, 1269. [Google Scholar] [CrossRef]
- Samsonova, A.M.; Bolotov, V.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Network Coordination Polymers Based on Thieno [3,2-b]Thiophene-2,5-Dicarboxylic Acid. J. Struct. Chem. 2019, 60, 1468–1473. [Google Scholar] [CrossRef]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Samsonov, V.A.; Ryadun, A.A.; Dybtsev, D.N.; Fedin, V.P. Structure and luminescent properties of coordination polymers containing lead(II) and thiophene ligands. J. Struct. Chem. 2020, 61, 1800–1809. [Google Scholar] [CrossRef]
- Wang, S.; Cao, T.; Yan, H.; Li, Y.; Lu, J.; Ma, R.; Li, D.; Dou, J.; Bai, J. Functionalization of Microporous Lanthanide-Based Metal–Organic Frameworks by Dicarboxylate Ligands with Methyl-Substituted Thieno [2,3-b]thiophene Groups: Sensing Activities and Magnetic Properties. Inorg. Chem. 2016, 55, 5139–5151. [Google Scholar] [CrossRef]
- Einkauf, J.D.; Ortega, R.E.; Mathivathanan, L.; De Lill, D.T. Nitroaromatic sensing with a new lanthanide coordination polymer [Er2(C10H4O4S2)3(H2O)6]n assembled by 2,2′-bithiophene-5,5′-dicarboxylate. New J. Chem. 2017, 41, 10929–10934. [Google Scholar] [CrossRef]
- Dou, A.-N.; Yang, L.-B.; Fang, X.-D.; Yin, Q.; Li, M.-D.; Li, J.; Wang, M.-Y.; Zhu, A.-X.; Xu, Q.-Q. Two luminescent lanthanide–organic frameworks containing bithiophene groups for the selective detection of nitrobenzene and Fe3+. CrystEngComm 2018, 20, 3609–3619. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, K.; Wang, X.; Xin, X.; Fan, W.; Dai, F.; Han, Y.; Sun, D. Solvent-induced terbium metal–organic frameworks for highly selective detection of manganese(II) ions. Dalton Trans. 2019, 48, 2569–2573. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Zhu, M.; Kosinova, M.; Fedin, V.P.; Gao, E. Three coordination polymers with regulated coordination interactions as fluorescent sensors for monitoring purine metabolite uric acid. Dalton Trans. 2020, 49, 4343–4351. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Structure and Luminescent Properties of Europium(III) Coordination Polymers with Thiophene Ligands. J. Struct. Chem. 2020, 61, 1965–1974. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, J.-Y.; Guo, R.; An, J.-D.; Huo, J.-Z.; Liu, Y.-Y.; Shi, W.; Ding, B. Solvothermal Preparation of a Lanthanide Metal-Organic Framework for Highly Sensitive Discrimination of Nitrofurantoin and l-Tyrosine. Molecules 2021, 26, 3673. [Google Scholar] [CrossRef]
- Wang, J.; Yu, M.; Chen, L.; Li, Z.; Li, S.; Jiang, F.; Hong, M. Construction of a Stable Lanthanide Metal-Organic Framework as a Luminescent Probe for Rapid Naked-Eye Recognition of Fe3+ and Acetone. Molecules 2021, 26, 1695. [Google Scholar] [CrossRef]
- CrysAlisPro, 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Yudina, Y.A.; Samsonova, A.M.; Bolotov, V.A.; Demakov, P.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Metal-Organic Coordination Polymers of Lanthanides(III) with Thienothiophendicarboxylate Ligands. J. Struct. Chem. 2021, 62, 1599–1606. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, X.; Shao, L.; Li, L.; Liu, Y.; Zhang, X.; Liu, J.; Meng, F.; Fu, Y. An ultra-high quantum yield Tb-MOF with phenolic hydroxyl as the recognition group for a highly selective and sensitive detection of Fe3+. J. Mater. Chem. C 2021, 9, 15840–15847. [Google Scholar] [CrossRef]
- Tan, B.; Zhuang, T.-H.; Velasco, E.; Xing, K.; Wu, Z.-F.; Huang, X.-Y. Syntheses, Structures, and Ratiometric Fluorescent Sensing Properties of a Series of Lanthanide Coordination Polymers. Cryst. Growth Des. 2021, 21, 6543–6551. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Samsonenko, D.G.; Fedin, V.P. Crystal structure of metal-organic frameworks based on terbium and 1,4-naphthalenedicarboxylic acid. J. Struct. Chem. 2020, 61, 1090–1096. [Google Scholar] [CrossRef]
- Abazari, R.; Ataei, F.; Morsali, A.; Slawin, A.M.Z.; Carpenter-Warren, C.L. A Luminescent Amine-Functionalized Metal–Organic Framework Conjugated with Folic Acid as a Targeted Biocompatible pH-Responsive Nanocarrier for Apoptosis Induction in Breast Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 45442–45454. [Google Scholar] [CrossRef]
- Saines, P.J.; Steinmann, M.; Tan, J.-C.; Yeung, H.H.-M.; Cheetham, A.K. Structural diversity and luminescent properties of lanthanide 2,2- and 2,3-dimethylsuccinate frameworks. CrystEngComm 2013, 15, 100–110. [Google Scholar] [CrossRef]
- Cui, P.-P.; Zhang, X.-D.; Zhao, Y.; Fu, A.-Y.; Sun, W.-Y. Synthesis, structure and adsorption properties of lanthanide–organic frameworks with pyridine-3,5-bis(phenyl-4-carboxylate). Dalton Trans. 2016, 45, 2591–2597. [Google Scholar] [CrossRef]
- Wang, X.Z.; Mao, X.Y.; Zhang, Z.Q.; Guo, R.; Zhang, Y.Y.; Zhu, N.J.; Wang, K.; Sun, P.P.; Huo, J.Z.; Wang, X.R.; et al. Solvothermal and Ultrasonic Preparation of Two Unique Cluster-Based Lu and Y Coordination Materials: Metal–Organic Framework-Based Ratiometric Fluorescent Biosensor for an Ornidazole and Ronidazole and Sensing Platform for a Biomarker of Amoeba Liver Abscess. Inorg. Chem. 2020, 59, 2910–2922. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).